Abstract
Purpose
Plant-based proteins may have the potential to improve glycaemic and gastrointestinal hormone responses to foods and beverages. The aim of this study was to investigate the effect of two doses of pea protein on postprandial glycaemic, insulinaemic, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) response following a high-carbohydrate beverage intake in healthy individuals.
Methods
In a single-blind, randomised, controlled, repeat measure, crossover design trial, thirty-one participants were randomly assigned to ingest 50 g glucose (Control), 50 g glucose with 25 g pea protein (Test 1) and 50 g glucose with 50 g pea protein (Test 2) on three separate days. Capillary blood samples (blood glucose and plasma insulin measurements) and venous blood samples (GIP and GLP-1 concentrations) were taken before each test and at fixed intervals for 180 min. The data were compared using repeated-measures ANOVA or the Friedman test.
Results
Glucose incremental Area under the Curve (iAUC180) was significantly lower (p < 0.001) after Test 2 compared with Control (− 53%), after Test 1 compared with Control (− 31%) and after Test 2 compared with Test 1 (−32%). Insulin iAUC 180 was significantly higher (p < 0.001) for Test 1 (+ 28%) and Test 2 (+ 40%) compared with Control and for Test 2 (+ 17%) compared with Test 1 (p = 0.003). GIP and GLP-1 release showed no clear difference between Control and Pea protein drinks.
Conclusion
The consumption of pea protein reduced postprandial glycaemia and stimulated insulin release in healthy adults with a dose–response effect, supporting its role in regulating glycaemic and insulinaemic responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing body of evidence supports the importance of the glycaemic response (GR) of foods and diets in the prevention and treatment of the major causes of morbidity and mortality in Western countries, including Type 2 diabetes, coronary heart disease and obesity [1,2,3,4,5]. In addition, low-GR foods have been associated with prolonged endurance during physical activity [6], improved insulin sensitivity [7] and increased colonic fermentation [8]. Randomised trials and epidemiological studies have shown that high-GR foods can increase the risk of insulin resistance and dyslipidaemia leading to the development of type 2 diabetes and cardiovascular diseases [4, 5, 9].
Insulin secretion is elicited primarily by the glycaemic carbohydrates present in food; however, studies have shown that there are other insulinotropic factors such as amino acids, fatty acids and gastrointestinal hormones [10, 11], including glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). GIP and GLP-1 are secreted from the K-cells and L-cells of the upper and lower intestine, respectively, into the blood stream in response to nutrient ingestion. Increases in GIP concentration are correlated with elevated intestinal glucose absorption [12,13,14], thus assessment of postprandial GIP response may be considered as evidence for the rate of glucose release from a food [14]. As GIP is implicated in liver fat accumulation and the development of impaired glucose tolerance, reducing postprandial GIP response may be a promising approach for the prevention and/or treatment of fatty liver and insulin resistance [15]. GLP-1 has numerous physiological actions including inhibition of gastric emptying, food intake and glucagon secretion, which control glycaemia [16, 17].
Pulses are low-glycaemic foods rich in protein and are reported to have significant health benefits, including weight management, improved gastrointestinal function and homeostasis and cardiovascular health [18]. Moreover, previous research has highlighted the potential of plant-based proteins, including pea protein, in improving the glycaemic and satiety response to foods and beverages [19, 20]. However, the results for glycaemic and insulinaemic response have not always demonstrated the same trend when different doses of pea protein were included with different test products [20, 21].
The aim of this study was to investigate the effect of two different doses of yellow pea protein powder (NUTRALYS® S85 Plus pea protein) on postprandial glycaemic, insulinaemic, GIP and GLP-1 response in healthy individuals. Adding smaller amounts of plant (pea) protein has a positive effect on postprandial glycaemic and satiety responses [19,20,21], thus the current study aimed to compare the effect of higher doses of pea protein. We hypothesised that when a Control (glucose) beverage is enriched with pea protein, postprandial glycaemic response would be reduced and subsequently insulin, GLP-1 and GIP release would be stimulated.
Materials and methods
Participants
Forty-five healthy male and female adults aged 19 to 55 years were recruited from the staff and student population at Oxford Brookes University and members of the public. Exclusion criteria were: pregnancy or lactating; < 18 or > 60 years of age; body mass index (BMI) ≥ 30 kg/m2; fasting blood glucose > 6.1 mmol/l; any known food allergy or intolerance; medical condition or medication known to affect glucose regulation or appetite and/or digestion and absorption of nutrients; known history of diabetes mellitus or the use of antihyperglycaemic drugs or insulin to treat diabetes and related conditions; major medical or surgical event requiring hospitalisation within the preceding three months; use of steroids, protease inhibitors or antipsychotics. In addition, participants were excluded from the study if they were unable to comply with the experimental procedures or did not follow testing safety guidelines.
Study design
This study was a single-blind, randomised, controlled, repeat measure, crossover design trial conducted at the Oxford Brookes Centre for Nutrition and Health. Participants were randomly assigned to test glucose (Control), glucose with 25 g pea protein (Test 1) and glucose with 50 g pea protein (Test 2) on three separate days.
The study was carried out in accordance with the declaration of Helsinki. Ethical approval was obtained from the University Research Ethics Committee (UREC) at Oxford Brookes University (UREC Registration No: 181259). Participants were given full details of the study protocol and the opportunity to ask questions. All participants gave written informed consent prior to participation. The study was retrospectively registered with Clinical Trials.Gov (NCT04610203).
Anthropometric measurements
Anthropometric measurements were made in the fasted state during the first session. Height was recorded to the nearest centimetre using a stadiometer (Seca Ltd, UK), with participants standing erect and without shoes. Body weight was recorded to the nearest 0.1 kg, with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated using the standard formula: weight (kg)/height (m)2. Body fat percentage was measured using a body composition analyser (Tanita BC-418 MA; Tanita UK Ltd).
Study protocol
On the day prior to a test, participants were asked to restrict their intake of alcohol and caffeine-containing drinks and to restrict their participation in intense physical activity. Participants were also told not to eat or drink after 21:00 the night before a test, although water was allowed in moderation. In addition, participants were asked to standardise and consume the same foods and drinks and quantities the day before each test and maintain the same physical activity the day before each test.
The Control (50 g glucose) was compared with Test 1 (50 g glucose + 25 g NUTRALYS® S85 Plus pea protein powder) and Test 2 (50 g glucose + 50 g NUTRALYS® S85 Plus pea protein powder). NUTRALYS® S85 Plus pea protein, obtained from the yellow pea (Pisum sativum) and designed for protein enrichment and food applications, was provided by Roquette Frères.
On the day of a test, the samples were each mixed with 250 ml water and consumed as beverages; they were all served with an additional 250 ml water. All beverages were tested once in random order on separate days, with at least a 7-day period between measurements to minimise carry over effects. Participants were studied in the morning before 10:00 after a 12-h overnight fast. Participants consumed the test products at a comfortable pace, within 15 min and remained sedentary during each session.
Blood samples were taken at 5 min and 0 min before consumption of the beverage and the baseline value taken as a mean of these two values. The beverage was consumed immediately after this and further blood samples were taken at different time points after starting to drink.
For blood glucose and plasma insulin measurements, blood was obtained by finger prick, using the Unistik®3 single-use lancing device (Owen Mumford), at 15, 30, 45, 60, 90, 120, 150 and 180 min. Blood glucose was measured using a photometric enzyme coupled assay system (HemoCue Glucose 201 DM analyser, HemoCue® Ltd, Sweden), which was calibrated daily using Control solution (GlucoTrol-NG) from the manufacturer. For plasma insulin, 350 μL of capillary blood was collected into chilled microvette® capillary blood collection tubes treated with di Potassium EDTA (CB 300 K2E; Sarstedt Ltd, Germany). The microvette® tubes were centrifuged at 4,000 rpm for 10 min and 150 μL of the supernatant plasma removed. Insulin concentrations in the plasma samples were determined by electrochemiluminescence immunoassay using an automated analyser (Cobas® E411; Roche diagnostics, Switzerland).
For the GIP and GLP-1 concentrations, 4 ml of venous blood was obtained via cannulation from the antecubital vein in the arm at 0, 30, 60, 90, 120 and 180 min. Blood was collected into chilled vacutainer® blood collection tubes treated with EDTA (K3E; Becton, Dickinson and Company, United States). DPP IV inhibitor (Sigma-Aldrich, UK) was added to each vacutainer® tube prior to blood collection. The vacutainer® tubes were centrifuged at 4,400 rpm for 10 min and 2 ml of the supernatant plasma subsequently removed. GIP concentrations and GLP-1 concentrations in the plasma samples were determined by enzyme-linked immunosorbent assay (ELISA) (RayBiotech, United States) and read on the EL × 800TM absorbance microplate reader (BioTek® Instruments, Inc., United States). Gen5TM software (BioTek® Instruments, Inc., United States) was used for the evaluation of results. Each sample was measured in duplicate and the mean of both measurements was taken for evaluation of results.
Statistical analysis
Sample size calculation was based on the published glycaemic response data of soup with added pea protein [21]. To detect a 72.7 mmol/l/min (SD 76.2) reduction in postprandial glucose iAUC with a two-sided α-level of 5% and a power of 90%, a sample size of at least 30 participants was necessary. To account for any dropouts, 45 participants were recruited for the current study.
The data were analysed using the IBM Statistical Package for the Social Sciences 25 (SPSS Inc., Chicago, Illinois). The data are presented as mean, standard deviation (SD) and standard error of the mean (SEM) values. Before statistical analysis, the normality of the data was tested using the Shapiro–Wilk statistic. For blood glucose, plasma insulin, plasma GIP and plasma GLP-1, the repeated measures ANOVA test (for normally distributed data) and non-parametric Friedman test (where data were not normally distributed) were used to compare concentrations at each time point, iAUC (at 60, 90 120 and 180 min), peak concentrations (blood glucose and plasma insulin only) and time of the peak concentrations (blood glucose and plasma insulin only) between the test products and glucose reference. Post hoc analyses were performed using the Bonferroni correction for parametric data and the Wilcoxon signed-rank test for non-parametric data.
Statistical significance was set at p < 0.05 for all tests, with the exception of the Wilcoxon signed-rank test (where required), which was conducted with a Bonferroni correction applied, resulting in a significance level set at p < 0.017.
Results
Out of the forty-five participants recruited, twelve withdrew from the study and two participants were excluded as they were either unable to comply with experimental procedures or no longer eligible for the study. Therefore, the complete GR, IR, GIP and GLP-1 data are reported for 31 participants. The physical characteristics of these participants are presented in Table 1.
Glycaemic and insulinaemic response
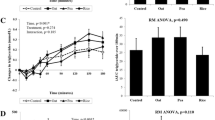
There was a significant difference in the change in blood glucose (A) and plasma insulin (B) from baseline between Control, Test 1 and Test 2 at various time points (Fig. 1). Table 2 shows the blood glucose and plasma insulin iAUC for the three beverages. Control blood glucose iAUC was significantly higher compared with both Test 1 and Test 2 (p < 0.001). In addition, blood glucose iAUC for Test 2 was significantly lower as compared to Test 1 (p < 0.05) suggesting a dose related effect. Compared with Control, there was 31% and 53% reduction in the mean glucose iAUC-180 for Test 1 and Test 2, respectively. There was a 32% reduction in the mean glucose iAUC-180 for Test 2 in comparison with Test 1.
Glycaemic (a) and insulinaemic (b) response curves for 50 g glucose, Test 1 (50 g glucose + 25 g NUTRALYS® S85 Plus pea protein) and Test 2 (50 g glucose + 50 g NUTRALYS® S85 Plus pea protein). The data are presented as mean and SEM (n 31). aSignificantly different from Glucose (repeated measures ANOVA: p < 0.05; Friedman test: p < 0.017); bSignificantly different from Test 1 (repeated measures ANOVA: p < 0.05; Friedman test: p < 0.017)
Insulin iAUC was significantly higher with Test 1 and Test 2 as compared to Control (p < 0.001). In addition, plasma insulin iAUC-180 for Test 2 was significantly higher as compared to Test 1 (p < 0.05) suggesting also a dose related effect. Compared with Test 1 and Test 2, there was 28% and 40% reduction in the mean insulin iAUC-180 for Control, respectively. There was a 17% reduction in the mean insulin iAUC-180 for Test 1 in comparison with Test 2.
There was a significant difference (p < 0.001) in the mean peak blood glucose between Control and Test 1, Control and Test 2 and Test 1 and Test 2 (Table 3). There was a significant difference in the mean peak plasma insulin between Control and Test 1 and Control and Test 2 (p < 0.001; Table 3).
GIP and GLP-1 response
GIP release was lower with pea protein and GLP-1 release was higher as compared to Control (Fig. 2). Table 2 shows the plasma GIP and GLP-1 iAUC for the three test beverages. There was no significant difference in the mean GIP iAUC between the three test beverages at any time point (p > 0.05). However, the peak plasma GIP was significantly different between the three test drinks (p < 0.05; Table 3). Pairwise comparisons showed significantly lower peak for Test 2 as compared to Control (p = 0.001). There was no significant difference in the peak time for plasma GIP between the three tests beverages at any time point (p > 0.05).
There was no significant difference (p > 0.05) in the mean GLP-1 iAUC between the three tests beverages at any time point (Table 2). Peak plasma GLP-1 for Test 1 and Test 2 were significantly higher than Control (p < 0.05; Table 3). The time of peak for Control was significantly earlier than for Test 1 and Test 2 (p < 0.05).
Discussion/conclusion
The aim of this study was to investigate the effect of two doses of NUTRALYS® pea protein (25 g and 50 g) on postprandial glycaemic, insulinaemic, GIP and GLP-1 responses in healthy individuals. It was hypothesised that a high-carbohydrate beverage enriched with pea protein would reduce postprandial glycaemic response and stimulate insulin, GLP-1 and GIP release as compared to a Control (glucose) beverage. Overall, the results of the current study support a role for pea protein in regulating glycaemic and insulinaemic response.
Past literature has shown that adding plant (pea) protein has a positive effect on postprandial glycaemic response [19,20,21,22]. Most studies have compared different plant proteins or smaller amounts (10–30 g) of pea protein, thus the current study aimed to compare the effect of a higher dose of pea protein. The results from the current study showed that both 25 g pea protein and 50 g pea protein produced a significantly lower glycaemic response when added to a Control (glucose) beverage; the glucose iAUC was significantly lower with 50 g pea protein (Test 2) compared with 25 g pea protein (Test 1), with a 41% reduction at iAUC-120. This is in contrast to the study of Re et al. [21] which found no significant difference in glucose AUC between 15 g pea protein and 30 g pea protein; however, this may be attributed to the different levels of carbohydrate in the test meals used in the Re et al. [21] study. The test soup used with 15 g pea protein had 28.7 g carbohydrate whereas the soup with 30 g pea protein consisted of only 12.1 g carbohydrate, which may have been inadequate to demonstrate the effect of the higher dose of pea protein [21]. Therefore, the current study is a direct comparison between test foods containing two different doses of pea protein in the presence of identical carbohydrate content.
It has been shown that incorporating protein into a carbohydrate food or beverage increases the insulinaemic response [22, 23] as proteins stimulate the release of insulin. The findings in the current study that the addition of pea protein to a Control (glucose) beverage significantly stimulated insulin release is consistent with previous research [20]. Moreover, 25 g and 50 g pea protein produced significantly different insulin responses at 120, 150 and 180 min, with 50 g resulting in higher plasma insulin concentrations as compared to the lower dose. Overstimulation of insulin by protein has been reported to be beneficial in insulin resistance [24]. However, long-term hyperinsulinemia may have detrimental effects on insulin sensitivity in healthy individuals [25]. Whilst a positive association between animal protein intake and insulin resistance has been reported previously due to high levels of branched chain amino acids, plant protein consumption has not been linked to insulin resistance [26]. Therefore, including pea protein with high glycaemic foods may be a safe and useful strategy to manage blood glucose levels.
Previous research has shown that GIP release was increased following the consumption of pea protein, consistent with a higher insulin release [27]; however, it may be noted that for the test food in that study, pea protein was provided with mixed meals [27] rather than with glucose. Moreover, the participants were individuals with type 2 diabetes, reported to have enhanced GIP response [28]. In contrast to this, the results of the current study in healthy individuals showed no differences in GIP response to 25 g and 50 g pea protein compared with Control. Moreover, the results showed an insignificant reduction in GIP release after consumption of pea protein, with 50 g pea protein producing the lowest response. This is in agreement with Kahleova et al. [29] who reported decreased postprandial GIP, yet increased insulin levels following a plant-based meal consisting of a tofu burger. Therefore, pea protein may exert metabolic effects similar to soy protein, which is the major plant-based protein used worldwide for food product development. Considering the inverse correlation between plasma GIP levels and insulin sensitivity [30], pea protein may be a promising ingredient to prevent the development of impaired glucose tolerance and insulin resistance.
Previous research has indicated that pea protein modulates GLP-1 levels and induces a plasma rise of GLP-1 [20, 21]. In the current study, consumption of 25 g and 50 g pea protein resulted in increased levels of GLP-1 compared with the Control, although these trends were not significant. This finding is consistent with the previous studies, which demonstrated an increase in GLP-1 concentrations with higher levels of pea protein [20, 21]. Furthermore, the postprandial increase in insulin secretion observed in this study can be attributed to the incretin effect of GLP-1 following plant protein consumption [29]. A significant increase in peak GLP-1 value after pea protein consumption can delay gastric emptying, increase satiety and inhibit glucagon secretion, further contributing to glycaemic control [16, 17].
It is well documented that protein stimulation of insulin secreting β-cells and the effects of protein on slowing gastric emptying may be the mechanisms responsible for the effects of pea protein on reducing postprandial glycaemia [19]. Pea protein used in this study is a fast protein (personal communication) with similar digestibility properties as whey protein [21], which has been associated with stimulation of plasma GLP-1 [30]. Although gastric emptying was not measured in this study, the faster absorption of amino acids from pea protein combined with the augmented GLP-1 response may be the mechanisms behind the dose-dependent insulinotropic effects seen in this study. The ability of higher doses of pea protein to modulate postprandial excursions of GIP following a high carbohydrate meal, as demonstrated in this study indicates potential for future therapeutic uses.
The glycaemic response of food depends on many factors, such as particle size, cooking and food processing, other food components (e.g., fat, protein, dietary fibre) and starch structure [32]. NUTRALYS® S85 Plus is a pea protein powder; while processing generally affects the starch in pulses and can alter their biological effects, previous research has shown that commercial processing of pulses to a powder form does not alter their low glycaemic characteristics [33], thus highlighting the potential role for pea protein powder in improving postprandial glycaemic and insulinaemic control. A recent review has highlighted regular high protein intake as a nutritional strategy to improve glycaemic control in older adults with pre-diabetes or type 2 diabetes [34]. Unlike the current study, the majority of the research so far have been using high protein from animal sources or soy with low carbohydrate/low caloric diets [34]. Therefore, it is pertinent to conduct long term studies using novel plant protein such as pea protein in individuals at risk of insulin resistance.
The main strengths of this study were the direct comparison of two different doses of pea protein showing a clear dose response impact and the use of glucose as the test beverage. Thus, carbohydrate levels were the same in all test beverages and there was no interference from other nutrients, such as fat and fibre. The main limitation of the study was the high variation seen in GIP and GLP-1 response, although this effect has been seen in other previous studies [20, 21]. For this reason, we did not see a statistically significant difference in the GLP-1 iAUC after pea protein even though they appeared to be lower than the Control.
Although the current study had sufficient statistical power to detect a significant difference in glycaemic response, a larger sample size is deemed necessary for detecting differences in the appetite hormone response. The present study measured the postprandial effect of pea protein for 3 h; however, extending the study duration beyond 180 min may provide an insight into any delayed effects of pea protein on blood glucose, plasma insulin and incretin hormones.
In conclusion, this study demonstrated that the addition of pea protein to a glucose beverage reduced postprandial glycaemia and stimulated insulin release with a dose–response effect, supporting a role for pea protein in regulating glycaemic and insulinaemic response. In addition, it highlights the use of pea protein in lowering glycaemic response to simple sugars without a disproportionate increase in GIP hormone levels. Unlike protein of animal origin that has been linked to insulin resistance, pea protein may therefore be a safer alternative to manage blood glucose levels. Considering the increasing popularity of plant proteins due to the environmental impact of whey protein and soy protein, future research could investigate the implications of long-term consumption of pea protein on metabolic markers.
References
Barclay AW, Petocz P, McMillan-Price J et al (2008) Glycemic index, glycemic load and chronic disease risk – a meta-analysis of observational studies. Am J Clin Nutr 87:627–637
Riccardi G, Rivellese AA, Giacco R (2008) Role of glycemic index and glycemic load in the healthy state, in prediabetes and diabetes. Am J Clin Nutr 87:269S-274S
Sloth B, Krog-Mikkelsen I, Flint A et al (2004) No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr 80:337–347
Jenkins DJA, Kendall CWC, McKeown-Eyssen G et al (2008) Effect of a low glycemic index or a high cereal fibre diet on type 2 diabetes A randomized trial. JAMA 300:2742–2753
Burani J, Longo PJ (2006) Low-glycemic index carbohydrates: an effective behavioral change for glycemic control and weight management in patients with type 1 and 2 diabetes. Diabetes Educ 32:78–88
Wu CL, Williams C (2006) A low glycemic index meal before exercise improves endurance running capacity in men. Int J Sport Nutr Exercise Metabol 16:510–527
Solomon TPJ, Haus JM, Kelly KR et al (2010) A low glycemic index diet combined with exercise reduced insulin resistance, postprandial hyperinsulinemia and glucose-dependent insulinotropic polypeptide responses in obese prediabetic humans. Am J Clin Nutr 92:1359–1368
Nilsson AC, Ostman EM, Granfeldt Y, Bjorck IME (2008) Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr 87:645–654
Hermansen MF, Eriksen NMB, Mortensen LS, Holm L, Hermansen K (2006) Can the glycemic index (GI) be used as a tool in the prevention and management of type 2 diabetes? Rev Diabet Stud 3:61–71
Collier GR, Greenberg GR, Wolever TM, Jenkins DJ (1988) The acute effect of fat on insulin secretion. J Clin Endocrinol Metab 66:323–326
Frid AH, Nilsson M, Holst JJ, Bjorck IME (2005) Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 82:69–75
Wachters-Hagedoorn RE, Priebe MG, Heimweg JA et al (2006) The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J Nutr 136:1511–1516
Eelderink C, Schepers M, Preston T, Vonk RJ, Oudhuis L, Priebe MG (2012) Slowly and rapidly digestible starchy foods can elicit a similar glycemic response because of differential tissue glucose uptake in healthy men. Am J Clin Nutr 96:1017–1024
Péronnet F, Meynier A, Sauvinet V et al (2015) Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: effect of starch digestibility. Eur J Clin Nutr 69:740–745
Keyhani-Nejad F, Irmler M, Isken F et al (2015) Nutritional strategy to prevent fatty liver and insulin resistance independent of obesity by reducing glucose-dependent insulinotropic polypeptide responses in mice. Diabetologia 58:374–383
Drucker D (2006) The biology of incretin hormones. Cell Metab 1:3153–3165
Holst J (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439
Dahl WJ, Foster LM, Tyler RT (2012) Review of the health benefits of peas (Pisum sativum L.). Br J Nutr 108:S3–S10
Mollard RC, Luhovyy BL, Smith C, Anderson GH (2014) Acute effects of pea protein and hull fibre alone and combined on blood glucose, appetite, and food intake in healthy young men - a randomized crossover trial. Appl Physiol Nutr Metab 39:1360–1365
Tan S-Y, Siow PC, Peh E, Henry CJ (2018) Influence of rice, pea and oat proteins in attenuating glycemic response of sugar-sweetened beverages. Eur J Nutr 57:2795–2803
Re R, Pombo S, Calame W, Lefranc-Millot C, Guérin-Deremaux L (2016) The satiating effect of NUTRALYS® pea protein leads to reduced energy intake in healthy humans. J Nutr Health Food Sci 4:1–10
Smith CE, Mollard RC, Luhovyy BL, Anderson GH (2012) The effect of yellow pea protein and fibre on short-term food intake, subjective appetite and glycaemic response in healthy young men. Br J Nutr 108:S74–S80
Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK (2007) Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr 86:1364–1368
Manders RJ, Little JP, Forbes SC, Candow DG (2012) Insulinotropic and muscle protein synthetic effects of branched-chain amino acids: potential therapy for type 2 diabetes and sarcopenia. Nutr 4:1664–1678
Rietman A, Schwarz J, Tome D, Kok FJ, Mensink M (2014) High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 68:973–979
Azemati B, Rajaram S, Jaceldo-Siegl K, Sabate J, Shavlik D, Fraser GE, Haddad EH (2017) Animal-protein intake is associated with insulin resistance in adventist health study 2 (AHS-2) calibration substudy participants: a cross-sectional analysis. Curr Dev Nutr 1(4):000299. https://doi.org/10.3945/cdn.116.000299
Markova M, Hornemann S, Sucher S, Wegner K, Pivovarova O, Rudovich N, Thomann R, Schneeweiss R, Rohn S, Pfeiffer AFH (2018) Rate of appearance of amino acids after a meal regulates insulin and glucagon secretion in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 108:279–291
Seino Y, Fukushima M, Yabe D (2010) GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig 1:8–23
Kahleova H, Tura A, Klementova M, Thieme L, Haluzik M, Pavlovicova R, Hill M, Pelikanova T (2019) A plant-based meal stimulated incretin and insulin secretion more than an energy- and macronutrient-matched standard meal in type 2 diabetes: a randomized crossover study. Nutrients 11:486
Smith K, Davies KAB, Stevenson EJ, West DJ (2020) The clinical application of mealtime whey protein for the treatment of postprandial hyperglycaemia for people with type 2 diabetes: a long whey to go. Front Nutr 7:587843
Pfeiffer AFH, Keyhani-Nejad F (2018) High glycemic index metabolic damage – a pivotal role of GIP and GLP-1. Trends Endocrinol Metab 29:289–299
Vosloo MC (2005) Some factors affecting the digestion of glycaemic carbohydrates and the blood glucose response. J Family Ecol Consumer Sci 33:1–9
Anderson GH, Liu Y, Smith CE, Liu TT, Nunez MF, Mollard RC, Luhovyy BL (2014) The acute effect of commercially available pulse powders on postprandial glycaemic response in healthy young men. Br J Nutr 112:1966–1973
Beaudry KM, Devries MC (2019) Nutritional strategies to combat type 2 diabetes in aging adults: the importance of protein. Front Nutr 6:138. https://doi.org/10.3389/fnut.2019.00138
Acknowledgments
We would like to thank all participants in this study. We would like to thank Nasim Soleymani Majd for her assistance with blood analysis and Jonathan Tammam for reviewing one of the drafts. This study was supported by a grant from Roquette Frères, France.
Funding
This study was supported by funding from Roquette Frères, France.
Author information
Authors and Affiliations
Contributions
HL, PST, EA, LG-D and CL-M contributed to the development of the study protocol. Material preparation and data collection were performed by PST, IA, AS and TM. Data analysis was performed by PST, IA and HL. The first draft of the manuscript was written by HL. PST, LG-D and CL-M revised the subsequent drafts of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
L Guérin-Deremaux and C Lefranc-Millot are employees of Roquette Frères. Roquette Frères did not play a part in the execution of the study or analysis of the results.
Ethics approval
The study was carried out in accordance with the declaration of World Medical Association Declaration of Helsinki. Ethical approval was obtained from the University Research Ethics Committee (UREC) at Oxford Brookes University (UREC Registration No: 181259).
Informed consent
Participants were given full details of the study protocol and the opportunity to ask questions. All participants gave written informed consent prior to participation.
Rights and permissions
About this article
Cite this article
Thondre, P.S., Achebe, I., Sampson, A. et al. Co-ingestion of NUTRALYS® pea protein and a high-carbohydrate beverage influences the glycaemic, insulinaemic, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) responses: preliminary results of a randomised controlled trial. Eur J Nutr 60, 3085–3093 (2021). https://doi.org/10.1007/s00394-021-02481-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02481-8