Abstract
Purpose
The objective of the study was to evaluate the anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047, and illustrate the potential functional mechanism about the alleviation of high fat and high fructose diet (HFFD) induced obesity and related metabolic abnormalities.
Methods
C57BL/6J mice were subjected to a standard or HFFD with or without supplementation of L. rhamnosus LS-8 and L. crustorum MN047 for 10 weeks. Obesity related metabolic indices including glucose tolerance, insulin resistance, serum lipid, liver function, hormones and inflammatory cytokines were assessed by standard protocols. For the monitoring of inflammatory response and lipid metabolism, transcriptional levels were profiled in liver and/or adipose tissues. Furthermore, gut microbiota composition analyses in the fecal samples were performed using 16S rRNA gene sequencing, and gut microbial metabolites, including lipopolysaccharide (LPS) and short-chain fatty acids (SCFAs), were also tested for the assessment of the relationship between gut microbiota variation and inflammatory response.
Results
Administration with L. rhamnosus LS-8 and L. crustorum MN047 significantly mitigated body weight gain and insulin resistance, and inflammatory response (TNF-α, IL-1β and IL-6 levels in serum and corresponding mRNA levels in adipose tissues) was significantly inhibited in these two strains-treated mice. Moreover, L. rhamnosus LS-8 and L. crustorum MN047 could partially normalized mRNA expression levels involved in lipid metabolism including Pparγ, Srebp-1c, CD36, Fabp2 and FAS. In addition, these two strains manipulated gut microbiota by decreasing the abundance of Bacteroides and Desulfovibrio and increasing that of Lactobacillus and Bifidobacterium, which in turn raised the levels of feces SCFAs and lowered the levels of circulating LPS.
Conclusion
These results indicated that L. rhamnosus LS-8 and L. crustorum MN047 supplementation possessed the anti-obesity effect on the HFFD fed mice by alleviating inflammatory response and regulating gut microbiota, which further suggested that these two probiotics can be considered as an alternative dietary supplement in combination with the preventive and therapeutic strategies against obesity and related complications.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide explosion of obesity and obesity-related metabolic syndrome, such as insulin resistance [1] and type 2 diabetes mellitus [2], has become a serious public health concern in the whole world. Over several decades, there has been a global shift in modern diets toward a high-energy diet that is rich in fats and/or sugars, especially in the western diet, which has been associated with high prevalence of overweight and metabolic syndrome [3]. At present, several diet pills are available commercially, such as orlistat and lorcaserin. However, adverse effects and little prospect of long-term benefit by using these drugs have greatly limited the clinical applications [4, 5]. Therefore, it is necessary to exploit more effective and better anti-obesity dietary supplement substance from natural biological sources for the application alone or combination with the preventive and therapeutic strategies to prevent or treat obesity and obesity-related disease.
Obesity is characterized by an excess accumulation of adipose tissues, which causes the increase of chronic low-grade inflammatory response [6, 7]. However, numerous literatures also reported that inflammation may play a causative role in the generation of insulin resistance and diabetes [6, 8]. Previous studies also suggested that adipose tissues macrophage infiltration can increase the secretion of some pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, which interfere with the insulin-signaling pathway in peripheral tissues and facilitate the development of insulin resistance [9,10,11]. In addition, the overproduction of chemokine monocyte chemotactic protein-1 (MCP-1) was also proven to impair insulin sensitivity and led to the development of metabolic syndrome [12, 13]. Hence, the inflammatory response might be one of the key point for the development of obesity, therefore, which can be seen as the target for the screening of anti-obesity supplementations.
Compelling evidences support that high fat diet (HFD) or high fat and high fructose diet (HFFD) can profoundly, rapidly and sustainably alter intestinal microbiota communities in as little as 24–28 h in mice or humans [14, 15], which may lead to the occurrence of obesity-related metabolic diseases through alteration of energy metabolism, immune and chronic low-grade inflammatory responses [16]. Metabolic products from gut microbiota have both positive and negative effects on host health. For example, short-chain fatty acids (SCFAs), a major metabolite in the colon, are an important energy source for the host and helpful in regulating lipid metabolism, immunity and adipocyte development [17, 18]. But lipopolysaccharide (LPS), a major constituent of the Gram-negative bacterial outer membrane, has been proved an inducement to trigger insulin resistance [19]. Therefore, in recent years, growing attention has been paid to the possible role of the gut microbiota as a novel potential contributor in reducing or increasing the prevalence of obesity and obesity related complications [4, 20].
As an important part of gut microbiota, probiotics are gradually being proven to be beneficial in preventing and/or treating obesity. For example, intervention of Lactobacillus gasseri SBT2055 in obese adults has been shown to reduce abdominal adiposity and improve metabolic disorder [21]. In addition, administration with Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in HFD-induced obese mice was able to alleviate weight gain and fat accumulation [22]. More recently, supplementation of Lactobacillus rhamnosus GG displayed an obvious inhibitory effect on dyslipidemia in HFD-induced obese mice [23]. It is deduced that the anti-obesity effects of probiotics mainly consists of three parts: (1) modulation of gut microbiota by influencing pathogen colonization through competing with pathogens for attachment sites and nutrients, and producing antimicrobial substances; (2) increasing the integrity of epithelial barrier by modulating mucus properties and influencing the turnover of epithelial cells; (3) inhibition of the inflammatory response by producing SCFAs. Despite these findings, there are still limitations in using Lactobacillus as preventive and therapeutic agents for obesity and metabolic syndrome, because the microorganisms exist species-specific effects that may further lead to different mechanistic actions. Therefore, it is necessary to pay more attention to the screening of Lactobacillus with anti-obesity effect and the functional mechanism of this activity.
Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 are two novel probiotics screened from traditional fermented foods, and both of them had been proved in our previous studies to possess potential health benefits by producing novel antimicrobial substance, especially for L. crustorum MN047 which can secrete multiple novel bacteriocins [24, 25]. Since different bacteriocins possess different antimicrobial spectrum and action mechanism, it was hypothesized that supplementation of these two probiotics could reshape a new balance of gut microbiota. In addition, to our knowledge no previous study has focused on examining the anti-obesity ability of L. crustorum. To investigate the anti-obesity effects of the proposed two strains, as well as the modulation function of the glucose and lipid metabolism, inflammatory response and gut microbiota, the present study was conducted.
Thus, in the present study, the anti-obesity effects of L. rhamnosus LS-8 and L. crustorum MN047 were investigated, in addition, the modulations of serum lipid, hormones, liver function, systemic insulin resistance, hepatic lipid accumulation and epididymal fat expansion were also determined. To illustrate the mechanism of anti-obesity, the influences of these two strains on the inflammatory response, gut microbiota and its metabolites of HFFD-induced mice were systematically analyzed, as well as the relationship of the parameters. The results of the present study will provide new knowledge about the action mode of anti-obesity effects of L. rhamnosus LS-8 and L. crustorum on HFFD-induced mice, which also promotes the development of the proposed strains as function food for the prevention or therapy of obesity and obesity-related complications in the future.
Materials and methods
Preparation of L. rhamnosus LS-8 and L. crustorum MN047
Lactobacillus rhamnosus LS-8 and L. crustorum MN047 were isolated from fermented milk and homemade koumiss of Xinjiang Autonomous Region, China, respectively [24, 25]. Both of them were cultured in MRS medium at 37 °C for 16 h. Cells were harvested by centrifugation at 6500g for 5 min at 4 °C, and then the collected cells were washed twice and adjusted to 5 × 109 CFU/mL with sterile saline and used for gavage feeding.
Animals and diets
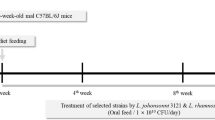
Eight-week-old C57BL/6J specific pathogen-free male mice were purchased from Xi’an Jiaotong University Health Science Center (Xi’an, China) and housed in standard polycarbonate cages (four mice per cage) under a controlled environment (temperature 22 ± 2 °C and humidity 55 ± 5% with 12 h light–dark cycle). All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4, and experimental procedures were approved by the Animal Ethics Committee of Xi’an Jiaotong University.
After acclimatization for one week, a total of seventy-two male mice were randomly divided into six groups (n = 12/group) as follows: C group fed with standard diet (AIN-93M); CLS group fed with standard diet and administration with L. rhamnosus LS-8; CMN group fed with standard diet and administration with L. crustorum MN047; M group fed with high-fat diet (45% kcal from fat, TP23100, purchased from Trophic Animal Feed High-Tech Co., Ltd. Nantong, China) and 10% w/v fructose (99%, F108331, purchased from Aladdin Biochemical Technology Co., Ltd. Shanghai, China) in drinking water (HFFD); MLS group fed with HFFD and administration with L. rhamnosus LS-8; MMN group fed with HFFD and administration with L. crustorum MN047. The compositions of experimental diets were shown in Table S1. All the probiotics administration mice (mice in CLS group, CMN group, MLS group and MMN group) were gavaged with 200 μL prepared bacterial suspension (1 × 109 CFU/day per mouse), and other mice (mice in C group and M group) were gavaged with the same volume of saline during the same period. Body weight, food and water intake of the mice were monitored weekly throughout the duration of the study. At the end of the 10-week experimental, the 12 h-fasted mice were euthanized with 10% chloral hydrate (300 mg/kg body weight, intraperitoneal injection). Blood samples were collected into 2 mL of centrifuge tubes by eyeball picking method, and centrifuged at 3000g for 10 min at 4 °C after standing for 30 min at room temperature, and then the serum was taken out slowly and stored in sterile EP tubes at − 80 °C for further analysis. Liver, kidney, spleen, epididymal and inguinal fat tissues were collected, washed with ice-cold saline, and weighed after drying the surface water by filter paper, and then stored at − 80 °C until use.
Oral glucose tolerance test and insulin tolerance test
Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were performed as described in Balakumar et al. [26] with slight modifications. Briefly, for OGTT analysis, mice were fasted overnight and then orally administered glucose solution (2 g/kg body weight) at 8th week. Blood samples were collected from the tail vein and glucose levels were measured using a glucometer (Sannuo Biological Transmission Co., Ltd. Changsha, China) prior to glucose intake, and at 15, 30, 60, 90 and 120 min after glucose administration. For ITT, the mice were fasted 6 h and then were intraperitoneally injected with insulin (0.5 U/kg body weight, novolin R, Novo Nordisk) at 9th week. Glucose levels were determined at 0, 15, 30, 60, 90 and 120 min after insulin injection. The total glucose areas under the curve (AUCglucose) represented the magnitude of the glucose response and were calculated using the trapezoidal rule.
Blood serum analysis
The serum insulin, leptin, adiponectin, LPS, TNF-α, IL-1β and IL-6 levels were tested by ELISA (Xin le Biotechnology Co., Ltd. Shanghai, China). Serum total alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), blood urea nitrogen (BUN), creatinine (CRE), C-reactive protein (CRP), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were analyzed using a biochemical automatic analyzer (Hitachi High Technologies Corporation, Tokyo, Japan). The insulin resistance index (HOMA-IR) was calculated using the following formula: HOMA-IR = fasting insulin levels × fasting blood glucose/22.5 [27].
Histological analysis
After euthanizing of mice, the liver and epididymal fat tissues were subsequently fixed in 4% paraformaldehyde overnight at 4 °C, and then embedded in paraffin for hematoxylin and eosin (H&E) staining analyses. The stained area was viewed and photographed with an Olympus microscope (Olympus Corporation, Tokyo, Japan) under the objective of 20×. Histological sections of liver and epididymal fat tissues were evaluated and analyzed by a pathologist, and the sizes of adipocytes were calculated using float morphology in the Image J software (developed by Wayne Rasband from NIH, USA).
Short-chain fatty acid analysis
The concentrations of SCFAs (acetic acid, propionic acid, isobutyric, butyric acid, isovaleric and valeric acid) analyses were performed as described by Zhao et al. [28] with minor modifications. Briefly, approximately 200 mg of fecal samples were homogenized with 2 mL of distilled water and centrifuged at 10,000g for 10 min at 4 °C. The supernatant was filtered through a 0.22 µm nylon filter (EMD Millipore). Taken 1 mL of filtrate and acidified by adding 200 µL 50% H2SO4 (v/v). After vortexing and standing for 5 min, 1 mL of diethyl ether was added, and the mixed solution was incubated for 30 min at 4 °C. After centrifuging at 10,000g for 10 min at 4 °C, the organic phase was filtered through a 0.22 µm nylon filter and collected for gas chromatography analysis. SCFAs were determined with gas chromatography (GC-2014C, Shimadzu Corporation, Japan), which equipped with a RTX-wax column (30 m × 0.25 µm × 0.25 µm) and a flame ionization detector. The initial temperature was 50 °C, which was maintained for 1 min and raised to 120 °C at 15 °C/min, and then increased to 170 °C at 5 °C/min, finally, increased to 240 °C at 15 °C/min and held at this temperature for 3 min. The injector and the detector temperature were 250 °C and 270 °C, respectively.
Quantitative PCR analysis
Total RNA was extracted from liver and epididymal fat tissues using the RNAiso Plus reagent (TaKaRa, Dalian, China) according to the manufacturer’s instruction. The total RNA quality was determined using a Micro-Spectrophotometer Nano-200 (Hangzhou Allsheng Instruments, Korea) with the ratios of 260 nm/280 nm (1.9–2.1) and 260 nm/230 nm (2.0–2.5), and 2 μL of total RNA (0.5–0.6 mg/mL) was used to synthesize cDNA (20 μL of total reaction volume) using the FastKing RT Kit (With gDNase) (TIANGEN, Beijing, China). Two μL of the diluted cDNA (1:10) was performed quantitative PCR (qPCR) by using SYBR Green BioEasy Master mix (BIOER, Hangzhou, China) and a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). Cycle conditions: 1 min at 95 °C, followed by 39 cycles of incubation at 95 °C for 15 s, 60 °C for 15 s, and then 72 °C for 30 s. The sequences of the primers used for RT-qPCR are shown in Table 1. Relative mRNA expression level of target genes were normalized using the mRNA level of the housekeeping gene β-actin in each sample and the data were analyzed according to the 2−ΔΔCt method.
Fecal microbiota analysis
Total bacterial DNA of each feces sample was extracted using the PowerSoil® DNA Isolation Kit (MO BIO Laboratories) according to the manufacturer’s protocol. The V3–V4 regions of the bacterial 16S rRNA gene were amplified with the common primers (forward primer, 5′-ACTCCTACGGGAGGCAGCA-3′; reverse primer, 5′-GGACTACHVGGGTWTCTAAT-3′) combined with adapter sequences and barcode sequences after evaluation of DNA quality and quantity by the ratios of 260 nm/280 nm (1.8–2.0) and 260 nm/230 nm (2.0–2.5). Two steps of PCR amplification were performed, and the first round PCR was operated using High-Fidelity DNA Polymerase (Q5, NEB) under the following thermal cycling conditions: denaturation at 95 °C for 5 min, followed by 15 cycles at 95 °C for 1 min, 50 °C for 1 min and 72 °C for 1 min, with a final extension at 72 °C for 7 min. The PCR products from the first step were purified through VAHTSTM DNA Clean Beads. And the second round PCR was then performed under the following thermal cycling conditions: an initial denaturation at 98 °C for 30 s, followed by 10 cycles at 98 °C for 10 s, 65 °C for 30 s and 72 °C for 30 s, and a final extension at 72 °C for 5 min. Finally, all PCR products were quantified by Quant-iT™ dsDNA HS Reagent and pooled together. High-throughput sequencing analysis of bacterial rRNA genes was performed on the purified, pooled sample using the Illumina Hiseq 2500 platform (2 × 250 paired ends) at Biomarker Technologies Corporation, Beijing, China.
After sequencing, raw sequence reads were filtered with the QIIME pipeline to obtain high-quality clean sequences. Paired-end reads were first merged using FLASH (version 1.2.7) with default parameters [29]. Chimeric sequences were then identified and removed by UCHIME (version 4.2) software. Remaining sequences with similarity > 97% were classified into an operational taxonomic unit (OTU) using USEARCH software (version 10.0) and finally classified as species and genus level using Ribosomal Database Project (RDP) Classifier (version 2.2) [30]. Alpha-diversity indices (ACE, Chao 1, Simpson and Shannon) were calculated with the Mothur package [31]. The weighted and unweighted principal coordinate analyses (PCoA) and unweighted pair group method with arithmetic mean (UGMA) clustering were performed with the QIIME software. Linear discriminant analysis (LDA) effect size (LEfSe) was used to detect significant changes in relative abundance of microbial taxa among different groups. Briefly, LEfSe first identifies features that are significantly different among biological classes using the non-parametric factorial Kruskal–Wallis ran-sum test, and then LEfSe utilizes linear discriminant analysis (LDA) to estimate the effect of each differentially abundant feature.
Statistical analysis
All experimental data sets were tested for normality using Anderson–Darling test (Minitab statistical software, v. 16.2.3), and the p value of all data sets was greater than 0.05, indicating that all data sets were normally distributed. Data were presented as the mean ± standard deviation (SD) of at least three independent experiments. Significant differences between mean values were determined by Duncan’s multiple range test with SPSS V20 software (IBM, Armonk, NY, USA). A value of p < 0.05 was regarded as statistically significant.
Results
Effects of L. rhamnosus LS-8 and L. crustorum MN047 on body weight and tissues weight in HFFD-fed mice
During the experimental period, the body weight, food and energy intake of mice in six groups were recorded. Although HFFD-fed groups (M group, MLS group and MMN group) led to lower food intake compared to standard diet fed groups (C group, CLS group and CMN group), the total energy intake had no difference among all groups (Table 2). At 0th week, the body weight showed no significant difference in all groups, but body weight and weight gain in the M group were significantly increased compared to the C group at 10th week, while these alterations were obviously ameliorated by the administration with L. rhamnosus LS-8 and L. crustorum MN047 (Table 2). In contrast, supplementation of L. rhamnosus LS-8 and L. crustorum MN047 in standard diet fed mice did not affect the body weight and weight gain at the end of feeding period compared to the C group. Therefore, the food efficiency ratio in the M group was higher than the C group, while it could be significantly alleviated by supplementation of L. rhamnosus LS-8 and L. crustorum MN047 (Table 2). Moreover, compared to the C group, oral L. rhamnosus LS-8 and L. crustorum MN047 in standard diet fed mice did not influence the average weight of epididymal and inguinal fat, liver and kidney tissues (Table 2); however, which significantly increased in the M group and dramatically decreased after L. rhamnosus LS-8 and L. crustorum MN047 administration (Table 2).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 on metabolic parameters in HFFD-fed mice
For the purpose to investigate the effect of L. rhamnosus LS-8 and L. crustorum MN047 on amelioration of systemic insulin resistance induced by HFFD feeding, glucose tolerance and insulin tolerance tests were performed. As shown in Fig. 1a–c, it was clearly that an impaired glucose tolerance in the M group compared with the C group was observed, which was clearly verified by a higher area under the curve (Fig. 1c), while L. rhamnosus LS-8 and L. crustorum MN047 supplementation significantly reversed this abnormity (Fig. 1a–c). In addition, insulin tolerance test was also conducted to determine the sensitivity of insulin receptors by measuring blood glucose levels before and after insulin injection. It was obviously found that the mice in the M group exhibited distinct insulin tolerance compared to the C group (Fig. 1d–f), while insulin tolerance in HFFD-fed mice was significantly ameliorated by supplementation of L. rhamnosus LS-8 and L. crustorum MN047. Furthermore, glucose levels after a 12-h fast were 53.67% higher in the M group compared with the C group (Fig. 1g). However, it was interesting to find that the fasting blood glucose levels decreased 31.94% and 29.84% after the intervention of L. rhamnosus LS-8 and L. crustorum MN047 in HFFD-fed mice compared with the M group, respectively (Fig. 1g). Moreover, overnight fasting insulin levels in the M group were 58.12% higher than the C group, while L. rhamnosus LS-8 and L. crustorum MN047 administration in HFFD-fed mice both significantly prevented these changes by an 18.17% and 21.91% reduction, respectively (Fig. 1h). The changes in fasting glucose and insulin levels were further determined by HOMA-IR index. Results showed that the value of HOMA-IR index in the M group was approximately 2.6-fold higher than the C group (Fig. 1i), while the value of HOMA-IR in the MLS group and MMN group both showed an apparent reduction compared to the M group (Fig. 1i). Meanwhile, supplementation of L. rhamnosus LS-8 and L. crustorum MN047 in standard diet fed mice did not affect the values of HOMA-IR compared to the C group (Fig. 1i).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on oral glucose tolerance test, insulin tolerance test, insulin and homeostasis model of insulin resistance (HOMA-IR) in HFFD-fed mice. Oral glucose tolerance test results of administration with aL. rhamnosus LS-8 and bL. crustorum MN047, respectively, and c area under curve (AUC) analyses for glucose tolerance test at week 8; Insulin tolerance test results of administration with dL. rhamnosus LS-8 and eL. crustorum MN047, respectively, and f AUC analyses for insulin tolerance tests at week 9; g Fasting glucose level, h fasting insulin level, and i HOMA-IR at week 10. Bars represent the mean ± SD (n = 8 mice/group), bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
The serum lipid levels were also tested at the end of the experimental period to analysis the improvement of lipid homeostasis caused by intervention of L. rhamnosus LS-8 and L. crustorum MN047 in HFFD-fed mice. It indicated that the serum contents of TC, TG and LDL-C in the M group were dramatically increased compared with the C group, while both of these alterations were significantly ameliorated by L. rhamnosus LS-8 and L. crustorum MN047 administration (Table 3). In addition, it also needed to mentioned that the levels of HDL-C in all HFFD-fed groups (M group, MLS group and MMN group) were significantly higher than that in standard diet fed groups (C group, CLS group and CMN group) (Table 3).
The levels of γ-GT, AST and ALT were tested in serum to evaluate the prevention of HFFD-induced liver function injury caused by the supplementation of L. rhamnosus LS-8 and L. crustorum MN047. Table 3 revealed that γ-GT, AST and ALT were significantly elevated in the M group, while these alterations were obviously reversed by treatment with L. rhamnosus LS-8 and L. crustorum MN047. Interestingly, the levels of γ-GT in the MLS group and MMN group were even significantly lower than that in the C group (Table 3). In addition, the levels of serum CRE and BUN were also investigated to verify the amelioration of HFFD-induced kidney function injury after intervention of the proposed strains. It demonstrated that serum CRE levels were obviously raised in HFFD-induced obesity mice (Table 3), and which showed a significant reduction after treatment with L. rhamnosus LS-8 and L. crustorum MN047 (Table 3). For the levels of serum BUN, except for MMN group had an obvious decrease compared to the M group, there was no significant difference between the other groups (C group, M group, MLS group, CLS group and CMN group) (Table 3). In addition, the concentration of serum CRP was also determined and it was markedly elevated compared to the C group, while both L. rhamnosus LS-8 and L. crustorum MN047 administration could ameliorate these changes (Table 3).
The analysis of lipid-associated cytokines (such as serum adiponectin and leptin) indicated that HFFD feeding led to the decreasing of serum adiponectin level and increasing of serum leptin level, and which were both inhibited or reversed by the supplementation of L. rhamnosus LS-8 and L. crustorum MN047 as shown in Fig. 2a, b. It was also found that the intervention of L. rhamnosus LS-8 and L. crustorum MN047 could relieve HFFD-induced inflammatory response. In detail, the serum pro-inflammatory factors (TNF-α, IL-1β and IL-6) in the M group were markedly increased when compared to the C group (Fig. 2c–e). On the contrary, the increasing trend of these pro-inflammatory factors in HFFD-fed mice were apparently down-regulated by the administration with L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 2c–e). Interestingly, the levels of IL-1β in the CMN group and MMN group were both extremely lower than the C group (Fig. 2d), and the ability of L. crustorum MN047 in preventing the elevation of IL-6 was better than L. rhamnosus LS-8 (Fig. 2e). In addition, the serum LPS levels, which had been proved to be an inducement to trigger insulin resistance by stimulating systemic inflammatory response [21], significantly higher in the M group than that in the C group, while it were distinctly decreased after administration with L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 2f).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on serum cytokines in HFFD-fed mice. a Adiponectin; b leptin; c TNF-α; d IL-1β; e IL-6; and f LPS. Bars represent the mean ± SD (n = 8 mice/group), bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
Effect of L. rhamnosus LS-8 and L. crustorum MN047 on liver and epididymal fat histology in HFFD-fed mice
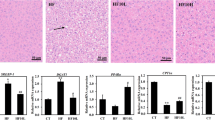
At the end of the experimental period, the effects of L. rhamnosus LS-8 and L. crustorum MN047 intervention on liver and epididymal fat histology were evaluated. Compared to the C group, HFFD feeding induced prominent diffuse macro-vesicular steatosis in the liver tissue (Fig. 3a). Interestingly, the supplementation of L. rhamnosus LS-8 and L. crustorum MN047 significantly reversed the formation of hepatic steatosis (Fig. 3a). In addition, epididymal fat histology analysis also indicated that the average adipocyte size in the M group was distinctly increased compared to the C group, while the mean value of adipocyte cell size was markedly reduced by treatment with L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 3b, c).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on liver and epididymal fat tissues histological in HFFD-fed mice under the objective of ×20. a Representative H&E staining of liver tissue (scale bar 50 μm); b representative H&E staining of epididymal fat tissue (scale bar 50 μm); c average cell size of epididymal fat. Bars represent the mean ± SD, bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
Effect of L. rhamnosus LS-8 and L. crustorum MN047 on liver and epididymal fat gene expressions
To further understand the underlying molecular mechanisms about the inhibitory effect of L. rhamnosus LS-8 and L. crustorum MN047 on hepatic lipid accumulation, the mRNA levels related with lipid metabolism were assessed, including peroxisome proliferator-activated receptor gamma (Pparγ), sterol regulatory element-binding protein 1 (Srebp-1c), platelet glycoprotein 4 (CD36) and fatty acid-binding protein (Fabp2). Results indicated that the mRNA expressions of hepatic lipogenic genes (Pparγ and Srebp-1c) were higher in the HFFD-fed mice than that in the C group mice (Fig. 4a), while the expression of these genes were significantly reduced via supplementation of L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 4a). Moreover, the expressions of fatty acid metabolic genes, including CD36 and Fabp2, were also dramatically increased in the M group compared to the C group and showed a down-regulated expression in L. rhamnosus LS-8 and L. crustorum MN047 treatment group (Fig. 4a).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on liver and epididymal fat tissues gene expressions in HFFD-fed mice. a mRNA levels of Pparγ, CD36, Srebp-1c and Fabp2 in the liver tissue; b mRNA levels of FAS, Pparγ, CD36 and Fabp2 in the epididymal fat tissue; c mRNA levels of TNF-α, IL-1β, IL-6 and MCP-1 in the epididymal fat tissue. Bars represent the mean ± SD (n = 5 mice/group), bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
The prevention effects of supplementation of L. rhamnosus LS-8 and L. crustorum MN047 on the change of genes expression (related to lipid metabolism and inflammatory response) in white fat tissue were investigated by the total RNA analysis in epididymal fat. Compared to the C group, the expressions of lipid metabolism-related genes, including fatty acid synthase (FAS), Pparγ, CD36 and Fabp2, were significantly increased in HFFD-fed mice, while these alterations were obviously ameliorated by the treatment of L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 4b). Moreover, the expression of some pro-inflammatory genes, including TNF-α, IL-1β, IL-6 and MCP-1 also showed an increase trend in HFFD-fed mice, which were also significantly reduced by supplementation of L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 4c).
Effect of L. rhamnosus LS-8 and L. crustorum MN047 on the SCFAs levels in mice feces
It was reported that SCFAs produced in colon, such as acetic acid, propionic acid, isobutyric, butyric acid, isovaleric and valeric acid, showed multiple beneficial effects on metabolic syndrome, therefore, the effects of L. rhamnosus LS-8 and L. crustorum MN047 on SCFAs levels in HFFD-fed mice were analyzed. From Fig. 5a–f, it was observed that acetic acid, propionic acid and butyric acid were the main colonic SCFAs metabolites in all groups, and the concentration of acetic acid in each group was the highest among the six detected SCFAs (Fig. 5a–f). Furthermore, it was interesting to find that except for the CLS group, the concentration of acetic acid in all other groups were significantly decreased compared to the C group (Fig. 5a). For the concentration of propionic acid, the M group was lower than the C group (Fig. 5b), and interestingly, no matter whether with HFFD feeding or not, the treatment with L. crustorum MN047 dramatically increased the concentration of propionic acid, and it was even significantly higher than that in the C group (Fig. 5b). Similarly, the level of butyric acid was significantly decreased in HFFD-fed mice, and which was significantly reversed by administration with L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 5c). Although the concentration of isobutyric, isovaleric and valeric acid were extremely lower than the SFCAs mentioned above, a distinct decreasing in HFFD-fed mice compared to the C group and obviously elevated by supplementation of L. rhamnosus LS-8 and L. crustorum MN047 were also found (Fig. 5d–f).
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on the concentration of SCFAs in mice feces after 10 weeks of HFFD feeding. a Acetic acid; b propionic acid; c butyric acid; d isobutyric; e valeric acid; and f isovaleric. Bars represent the mean ± SD (n = 8 mice/group), bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
Effect of L. rhamnosus LS-8 and L. crustorum MN047 on gut microbiota
16S rRNA gene sequencing analysis were conducted for the investigation of effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on gut microbiota. A total of 1,909,884 clean sequences were obtained after the sequences were quality-filtered with the QIIME pipeline and an average of 63,663 clean sequences per sample were used for the downstream analysis. For alpha diversity analysis, Simpson, Shannon, Ace and Chao indexes were used to assess community diversity and richness. Except for the Simpson indexes were significantly elevated in HFFD-fed mice, another three indexes (Shannon, Ace and Chao) were markedly decreased as compared to the standard diet fed mice, which suggested that HFFD feeding induced a decreasing in the alpha diversity of gut microbiota (Fig. 6a). Although both supplementation of L. rhamnosus LS-8 and L. crustorum MN047 in HFFD-fed mice had no significant difference in the alpha diversity compared to the M group, it was also found that these alterations were improved by L. rhamnosus LS-8 but were aggravated by L. crustorum MN047 (Fig. 6a). Correspondingly, the alpha diversity of the MLS group was significantly better than that of the MMN group (Fig. 6a). In addition, analysis by UPGMA clustering (Fig. 6e), as well as the unweighted and weighted UniFrac distance based PCoA (Fig. 6f, g) showed a distinctive microbiota profiles between the standard diet fed groups (C, CLS and CMN group) and the HFFD-fed groups (M, MLS and MMN group), which indicates these two probiotics supplementation almost did not lead to any significant recovery of beta diversity in the gut microbiota of HFFD-fed mice.
Effects of L. rhamnosus LS-8 and L. crustorum MN047 administration on gut microbiota structure in HFFD-fed mice. a Alpha diversity of the microbial community indicated by the Simpson, Shannon, Ace and Chao indexes; b gut microbiota composition at phylum level; c The relative abundance of (i) Firmicutes, (ii) Bacteroides and (iii) Proteobacteria; d gut microbiota composition at genus level; e weighted UPGMA of all samples; f, g Plots of unweighted and weighted UniFrac-based PCoA, respectively. Bars represent the mean ± SD (n = 5 simples/group), bars with different letters indicate significant differences (p < 0.05) by Duncan tests. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
A closer look at the microbial community revealed the influence of probiotics supplementation at both phylum and genus levels. At phylum level, Firmicutes, Bacteroidetes and Proteobacteria dominated the gut microbiota in the all groups (Fig. 6b). Specifically, HFFD feeding induced a distinct elevation in the relative abundance of Firmicutes (from 31.9 to 47.3%) and an obvious decreasing in Bacteroidetes (from 53.7 to 23.3%) compared with the C group, which also resulted in the increasing of the Firmicutes to Bacteroidetes ratio (from 0.596 to 2.030) in HFFD-fed mice (Fig. 6c). However, supplementation of L. rhamnosus LS-8 and L. crustorum MN047 did not prevent these changes in HFFD-fed mice compared to the M group, and the ratio of these two phyla were even higher than that in the M group (Firmicutes/Bacteroidetes, from 2.030 to 5.276 and 13.267, respectively) (Fig. 6c). In contrast, the abundance of Proteobacteria was significantly elevated in the M group when compared with the C group (from 8.1 to 22.2%), which was strikingly ameliorated (from 22.2 to 18.5% and 15.6%, respectively) by the administration with L. rhamnosus LS-8 and L. crustorum MN047 in HFFD-fed mice (Fig. 6c). In addition, at the genus level, 30 most abundant genera were selected for the further analysis (Fig. 6d and Table S2). There were 17 genera which had significant changes (increased or decreased) in the M group compared with the C group, and there were 10 genera significantly reversed via the supplementation of L. rhamnosus LS-8 and/or L. crustorum MN047. Specifically, compared with the C group, the genera of Lactobacillus, uncultured_bacterium_f_Erysipelotrichaceae, Olsenella and uncultured_bacterium_f_Bacteroidales_S24-7_group were decreased in the M group. The results also indicated that the changes of other three genera were only prevented by L. crustorum MN047, except for the abundance of Lactobacillus increasing in both administration of L. rhamnosus LS-8 and L. crustorum MN047. Conversely, the genera Desulfovibrio, Blautia, Catenibacterium, Bacteroides, Candidatus_Saccharimonas and Faecalibaculum were significantly increased in the M group compared to the C group. And except for the abundance of Blautia only significantly decreased by L. rhamnosus LS-8 supplementation, other five genera were significantly reversed by both treatment with L. rhamnosus LS-8 and L. crustorum MN047. Interestingly, although some beneficial genera had no significant difference between the M group and C group, the abundance of them was apparently elevated by probiotics supplementation, such as Bifidobacterium, Anaerotruncus, Turicibacter, Ruminiclostridium_9 and Lachnospiraceae_NK4A136_group, etc.
To identify the specific phylotypes that were significantly altered in response to probiotics supplementation, all effective sequences of the sample were analyzed using the LEfSe method. Taxonomy cladogram obtained from phylum to species indicated that microbiota in the Deltaproteobacteria class, Desulfovibrionales order, Desulfovibrionaceae family, Desulfovibrio genus and uncultured_bacterium_g_Desulfovibrio species were enriched in HFFD-fed mice, whereas Bacteroidetes phylum, Bacteroidia class, Bacteroidales order, Bacteroidales_S24_7_group family, uncultured_bacterium_f_Bacteroidales_S24_7_group genus and uncultured_bacterium_f_Bacteroidales_S24_7_group species were enriched in the C group (Fig. 7a). These results were also shown in the LDA score plot (Fig. 7b). In addition, with the same method, it was found that the Firmicutes phylum, Bacilli class, Lactobacillales order, Lactobacillaceae family, Lactobacillus genus were enriched in both supplementation of L. rhamnosus LS-8 and L. crustorum MN047 when compared with the M group (Fig. 7c–f).
LEfSe analysis results. Only the taxa with LDA score higher than 3.5 are shown; a, b Cladogram showing the phylogenetic relationships of bacteria taxa and LDA scores between the C and the M group. c, d Cladogram showing the phylogenetic relationships of bacteria taxa and LDA scores between the M and the MLS group. e, f Cladogram showing the phylogenetic relationships of bacteria taxa and LDA scores between the M and the MMN group. Yellow dots indicate no statistical significance. C: mice fed with standard diet, CLS: mice fed with standard diet and Lactobacillus rhamnosus LS-8, CMN: mice fed with standard diet and Lactobacillus crustorum MN047, M: mice fed with HFFD, MLS: mice fed with HFFD and Lactobacillus rhamnosus LS-8, MMN: mice fed with HFFD and Lactobacillus crustorum MN047
Discussion
It has been reported that excessive intake of fructose and saturated fats may cause obesity-associated metabolic syndrome and intestinal microbiota disorder [15, 32]. Although there were some evidences suggested that some Lactobacillus may have potentially beneficial effects on anti-obesity, the applications of specific Lactobacillus strains for anti-obesity were still limited as the anti-obesity mechanisms were not fully understood. Thus, the objective of this study was to evaluate the potential anti-obesity effects of two new strains (L. rhamnosus LS-8 and L. crustorum MN047) on HFFD-fed mice. Results indicated that consumption of HFFD throughout the experimental period significantly increased body weight gain and the levels of serum TG, TC and LDL-c, accelerated hepatic lipid accumulation, epididymal fat expansion and led to liver function (γ-GT, AST, ALT) injury, which were consistent with that reported by Liu et al. [33]. It was found that the levels of HDL-C in all the HFFD-fed groups (M group, MLS group and MMN group) were significantly higher than that in the standard diet fed groups (C group, CLS group and CMN group), which was in accordance with the recent reports by Lee et al. [34] and Pothuraju et al. [35], and it might be caused by the stress reaction. In addition, kidney function (serum CRE and BUN) injury was also found in the M group, which was similar to the HFD and STZ-induced diabetic rats [36]. Fortunately, by the supplementation of L. rhamnosus LS-8 and L. crustorum MN047 during the HFFD feeding period, these adverse changes were remarkably ameliorated. It was also found that although the total energy intake among all groups were not significantly different, the body weight gain was significantly suppressed by both L. rhamnosus LS-8 and L. crustorum MN047 supplementation in HFFD-fed mice, which indicated that energy intake was not the only determinant for obesity development [10, 33].
Leptin, a specific secreted protein from adipose tissues, mainly used to regulate food intake and energy expenditure by the central nervous system [37]. Leptin deficiency mice (ob/ob mice) shown hyperphagia, obesity and insulin resistance, and which was proved to be reversed by supplementation with leptin [38]. However, in the present study, the increasing of leptin levels were not accompanied with the expected anorectic responses in HFFD-induced obese mice, nevertheless, which was reversed by administration with L. rhamnosus LS-8 and L. crustorum MN047. This phenomenon was similar to the previous literature [33] and related to the occurrence of leptin resistance. Grunfeld et al. [39] found that the levels of leptin in the infected host were increased in response to pro-inflammatory stimulation, such as TNF and LPS. Therefore, it was speculate that the high levels of leptin in the M group might be mainly caused by the high secretion/expression of pro-inflammatory cytokine (TNF-α, IL-1β, IL-6, MCP-1), as well as the high production of pro-inflammatory substance (LPS) in HFFD-induced obese mice. Therefore, after the supplementation of the proposed strains, the modulation of gut microbiota and its metabolites (SCFAs) would lead to the amelioration of inflammatory response, and which then led to the decreasing of leptin levels and even obesity related reactions. For other hormones, such as adiponectin and insulin, it also played an important role in obesity. For example, adiponectin was a smaller numbers of anti-inflammatory factors secreted by adipose tissues, and the function of adiponectin was to stimulate autophagy and reduce oxidative stress, which would further improve insulin sensitivity [40]. Moreover, the secretion of adiponectin by adipocytes was inhibited by pro-inflammatory factors [41]. Consistent with these reports, in the present study, the obese mice induced by HFFD feeding had lower serum adiponectin levels, and accompanied with higher pro-inflammatory factors (TNF-α, IL-1β, IL-6 and LPS) levels and insulin resistance, and it also found that these changes were distinctly ameliorated by administration with L. rhamnosus LS-8 and L. crustorum MN047. Hence, it was clearly that the level of the inflammatory response was closely related to the secretion of leptin and adiponectin, and which were also modulated by the intervention of the proposed strains via action on the gut microbiota that had significant influence on the inflammatory response. Therefore, the results indicated that the gut microbiota can be act as an important checkpoint for the inflammatory response and further lead to the amelioration of obesity.
Chronic low-grade inflammation is closely related to obesity and metabolic disorder. It has been previously reported that chronic inflammation plays a crucial role in the development of obesity-related insulin resistance [6], and an inflammatory program is activated early in adipose expansion, and then, during chronic obesity, which would permanently lead the immune system to a pro-inflammatory phenotype [8]. Obviously, it can be seen that an increasing of some pro-inflammatory factors including TNF-α, IL-1β, IL-6 in HFFD-fed mice serum (Fig. 2c–e), and the expression of these inflammatory genes mentioned above were also significantly elevated in obese mice epididymal adipose tissues, which was consistent with the previous reports [10, 33]. What’s more, these alterations were significantly reversed by the administration with L. rhamnosus LS-8 and L. crustorum MN04. Strikingly, the levels of serum IL-1β in L. crustorum MN04 intervention mice were significantly lower than the C group, suggesting that L. crustorum MN04 was more effective than L. rhamnosus LS-8 in inhibiting the secretion of IL-1β. Moreover, MCP-1, as a cytokine produced in adipose tissues, was an important chemokine for macrophage recruitment and responsible for the beginning of macrophages infiltration into adipose tissues [42, 43]. The overexpression of MCP-1 in adipose tissues was finally led to the development of insulin resistance and some other obesity-related complications, such as atherosclerosis [12, 13]. In the present study, the expression of MCP-1 was remarkably increased in HFFD-induced obese mice, which was similar to the HFD-induced obese mice [1]. While these alterations were prevented by the treatment with L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 4c). In addition, LPS as a biomarker of Gram-negative bacteria has been proved as an inducement to trigger insulin resistance [19] for the effects on stimulating the release of pro-inflammatory cytokines, and disruption of the metabolism function, such as insulin function and lipid metabolism [20, 44]. Furthermore, LPS was also regarded as a biomarker of intestinal dysbiosis for the increasing of gut permeability [32, 45]. The present study found that the higher levels of LPS in HFFD-induced obese mice was significantly inhibited by supplementation of L. rhamnosus LS-8 and L. crustorum MN047 (Fig. 2f), which was similar to the results that HFD-induced obese mice had extremely higher circulatory levels of LPS than lean mice [26, 46]. Some studies also found that the abundance of LPS-producing bacteria such as Proteobacteria, Bacteroides, Enterobacterium, Escherichia coli, Salmonella sp. would be enriched in the obese individuals [47,48,49]. Therefore, the serum LPS levels were remarkably decreased in these two probiotics supplementation groups (MLS and MMN) was probably due to the lower abundance of LPS-producing bacteria (Proteobacteria and Bacteroides) caused by these two strains (Fig. 7 and Table S2). All these results implied that the administration with these two probiotics could ameliorate intestinal dysbiosis, and which further led to the decreasing of inflammatory response and the amelioration of obesity related metabolic abnormalities.
Meanwhile, growing literature reported that high fat and/or high fructose diet consumption led to gut microbiota dysbiosis, which further promoted an elevation in systemic LPS levels [50, 51]. However, no complete consensus reached on the composition of intestinal microbiota in obese and lean individuals up to now. Therefore, 16S rRNA gene sequencing analysis was conducted to illustrate the variation of the gut microbiota in mice during the HFFD feeding process, and the results showed that both L. rhamnosus LS-8 and L. crustorum MN047 administration had significant effects on the abundance of some specific species, although no extremely significant difference in the alpha and beta diversity. Contrary to some findings that the increasing tendency of Firmicutes/Bacteroidetes in HFFD or HFD fed mice could be inhibited by probiotics intervention [2, 52], it showed that the ratio of Firmicutes and Bacteroidetes was even higher than that in the M group after L. rhamnosus LS-8 and L. crustorum MN047 supplementation. It need to be emphasized that the ratio of Firmicutes and Bacteroidetes has not been used as a biomarker of obesity so far, and the results indicated that the change of Firmicutes/Bacteroidetes might be different in the obesity mice when administration with Lactobacillus. Proteobacteria mainly consist of Gram-negative bacteria and its amount showed a positive correlation with serum LPS levels [53]. Compared with the M group, a decreasing of fecal Proteobacteria in the MLS group and MMN group were observed, which was consistent with the results of serum LPS levels among the corresponding groups. In addition, at the genus level, it needed to be mentioned that some harmful genera were elevated in HFFD induce obese mice, but which were reversed by L. rhamnosus LS-8 and L. crustorum MN047 administration. For example, a higher abundance of Desulfovibrio and Bacteroides in obese mice was observed, which would inhibit the mitochondrial respiration of colonic epithelial cells and trigger inflammatory response and insulin resistance by producing hydrogen sulfide and LPS, respectively [49, 54], nevertheless, the supplementation of L. rhamnosus LS-8 or L. crustorum MN04 not only reduced the abundance of the proposed harmful genera, but also promoted the increasing of the beneficial genera, such as Lactobacillus, Bifidobacterium and Lachnospiraceae NK4A136 group, all of which were related to SCFAs-producing [47, 55]. As SCFAs were the important energy sources for the host and helpful in regulating lipid metabolism, immunity and adipocyte development [17, 18], the increasing of the SCFAs level in gut would be beneficial for the amelioration of inflammatory response and other obesity related metabolic abnormalities. Therefore, similar with other related studies [4, 56, 57], the significantly increasing of the levels of SCFAs (Fig. 5a–d) showed a high consistency with the alleviation of inflammatory response, glucose tolerance and insulin resistance mentioned in each group, furthermore, which further indicated that the gut microbiota and its metabolites were the potential targets for the prevent or cure of obesity.
Meanwhile, the influence on the expression of host genes (such as Pparγ, CD36, Fabp2, Srebp-1c, Fas, etc.), which were closely related with lipid metabolism, was also important for the amelioration of metabolic syndrome. In the present study, it was found that the mRNA levels of these lipid metabolism-related genes, including PPAR-γ, CD36, Fabp2, Srebp-1c, FAS, were extremely higher in the hepatic and/or adipose tissues of mice in the M group than that in the C group, but all these up-regulations were reversed by L. rhamnosus LS-8 and L. crustorum MN047 supplementation (Fig. 4a, b), which was also corresponding to the results of recovering morphology of fatty liver and epididymal fat expansion, as well as the improving insulin sensitivity in these two probiotics administration groups. Although it was not sure whether the supplementation of the proposed probiotics directly influenced on the expression of these key genes, it would like to believe that the modulation of the gut microbiota and its metabolites can lead to the decreasing of the inflammatory response and further to ameliorate the obesity abnormalities, such as lipid accumulation, inulin resistant and even the expression of some crucial genes. In addition, there was an important point to be mentioned that β-actin might not be the best housekeeping gene for the analysis of adipose tissue related genes, and if the investigation has a highly requirements for the quantification and accuracy of the test, it is necessary to select more appropriate housekeeping gene as internal reference for RT-qPCR analysis by using geNorm, NormFinder or BestKeeper, etc.
The above results indicated that the L. rhamnosus LS-8 and L. crustorum MN047 supplementation possessed a good anti-obesity on HFFD fed mice by the combined action of gut microbiota modulation, inflammatory response amelioration, as well as glucose and lipid metabolism regulation. Therefore, the action mode of the proposed strains can be deduced as the following three aspects: (1) gut microbiota were manipulated firstly by these two probiotics supplementation, resulting in a decreasing of harmful bacteria abundance (such as LPS-producing bacteria, Proteobacteria and Bacteroides) and an increasing of beneficial bacteria abundance (such as SCFAs-producing bacteria, Lactobacillus, Bifidobacterium and Lachnospiraceae NK4A136 group), and then which in turn raised the levels of SCFAs and lowered the levels of LPS; (2) inflammatory response was then ameliorated by inhibiting the secretion/expression of pro-inflammatory factors (TNF-α, IL-1β, IL-6 and MCP-1), which mainly regulated by the related metabolites (inflammatory stimulus, LPS; and inflammatory inhibitor, SCFAs) of gut microbiota; (3) with the attenuated inflammatory response, the expression of lipid metabolism-related genes including Pparγ, Srebp-1c, CD36, Fabp2 and FAS were further normalized, as well as the abnormal sugar and lipid metabolism (TG, TC and LDL-c, glucose tolerance and insulin resistance). Based on the proposed three action modes, the obesity caused by HFFD in mice was significantly alleviated by the intervention of L. rhamnosus LS-8 and L. crustorum MN047, which not only enriched the function of probiotics, but also provided a new insight for the prevention and therapy of obesity.
Conclusion
The present study indicated that L. rhamnosus LS-8 and L. crustorum MN047 possessed good anti-obesity effect on the HFFD-induced mice, as well as the alleviation of inflammatory response, lipid metabolic and other obesity related abnormalities. Based on the analysis of cytokines secretion, related gene expression, gut microbiota and its metabolites (LPS and SCFAs), the anti-obesity action mode of the proposed two strains was concluded as a process that the gut microbiota and its metabolites were firstly modulated by the oral intervention of these strains, and then which resulted in the attenuation of the inflammatory response, finally the lipid metabolism, expression of related genes, secretion of hormones and other obesity related abnormalities were significantly alleviated. The results of the present study provided new knowledge about the action mode of anti-obesity effects of L. crustorum on HFFD-induced mice, which also promotes the development of the proposed strains as function food for the prevention or therapy of obesity and obesity-related complications. In addition, the synergistic effect of these two strains on anti-obesity and the molecule mechanism of the regulation on inflammatory response of the gut microbiota or its metabolites would be further studied in the future.
References
Shang H, Sun J, Chen YQ (2016) Clostridium Butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS One 11(4):e0154373. https://doi.org/10.1371/journal.pone.0154373
Li X, Xu Q, Jiang T, Fang S, Wang G, Zhao J, Zhang H, Chen W (2016) A comparative study of the antidiabetic effects exerted by live and dead multi-strain probiotics in the type 2 diabetes model of mice. Food Funct 7(12):4851–4860. https://doi.org/10.1039/C6FO01147K
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes. Diabetes Care 33(11):2477–2483. https://doi.org/10.2337/dc10-1079
Pan Y-Y, Zeng F, Guo W-L, Li T-T, Jia R-B, Huang Z-R, Lv X-C, Zhang J, Liu B (2018) Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. https://doi.org/10.1039/C8FO01116H
Kang JG, Park C-Y (2012) Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J 36(1):13–25. https://doi.org/10.4093/dmj.2012.36.1.13
Xu HY, Barnes GT, Yang Q, Tan Q, Yang DS, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig 112(12):1821–1830. https://doi.org/10.1172/jci200319451
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132(6):2169–2180. https://doi.org/10.1053/j.gastro.2007.03.059
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Investig 127(1):1–4. https://doi.org/10.1172/jci92035
Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM (2012) Increased macrophage migration into adipose tissue in obese mice. Diabetes 61(2):346–354. https://doi.org/10.2337/db11-0860
Roselli M, Finamore A, Brasili E, Rami R, Nobili F, Orsi C, Zambrini AV, Mengheri E (2018) Beneficial effects of a selected probiotic mixture administered to high fat-fed mice before and after the development of obesity. J Funct Foods 45:321–329. https://doi.org/10.1016/j.jff.2018.03.039
Hsieh FC, Lan CC, Huang TY, Chen KW, Chai CY, Chen WT, Fang AH, Chen YH, Wu CS (2016) Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct 7(5):2374–2388. https://doi.org/10.1039/c5fo01396h
Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T (2006) Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281(36):26602–26614. https://doi.org/10.1074/jbc.M601284200
Bianconi V, Sahebkar A, Atkin SL, Pirro M (2018) The regulation and importance of monocyte chemoattractant protein-1. Curr Opin Hematol 25(1):44–51. https://doi.org/10.1097/moh.0000000000000389
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559. https://doi.org/10.1038/nature12820
Martinez-Guryn K, Hubert N, Frazier K, Urlass S, Musch MW, Ojeda P, Pierre JF, Miyoshi J, Sontag TJ, Cham CM, Reardon CA, Leone V, Chang EB (2018) Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23(4):458. https://doi.org/10.1016/j.chom.2018.03.011
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336(6086):1262–1267. https://doi.org/10.1126/science.1223813
Engevik MA, Versalovic J (2017) Biochemical features of beneficial microbes: foundations for therapeutic microbiology. Microbiol Spectr 5(5):35. https://doi.org/10.1128/microbiolspec.BAD-0012-2016
Delzenne NM, Cani PD (2011) Interaction between obesity and the gut microbiota: relevance in nutrition. In: Cousins RJ, Bier DM, Bowman BA (eds) Annual review of nutrition, vol 31. Annual Reviews, Palo Alto, pp 15–31. https://doi.org/10.1146/annurev-nutr-072610-145146
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7):1761–1772. https://doi.org/10.2337/db06-1491
Mei XR, Zhang XY, Wang ZG, Gao ZY, Liu G, Hu HL, Zou L, Li XL (2016) Insulin sensitivity-enhancing activity of phlorizin is associated with lipopolysaccharide decrease and gut microbiota changes in obese and type 2 diabetes (db/db) mice. J Agric Food Chem 64(40):7502–7511. https://doi.org/10.1021/acs.jafc.6b03474
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64(6):636–643. https://doi.org/10.1038/ejcn.2010.19
Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. https://doi.org/10.1371/journal.pone.0059470
Kim B, Park KY, Ji Y, Park S, Holzapfel W, Hyun CK (2016) Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem Biophys Res Commun 473(2):530–536. https://doi.org/10.1016/j.bbrc.2016.03.107
Zhang LH, Wang L, Yi LH, Wang X, Zhang Y, Liu JY, Guo X, Liu L, Shao CE, Lu X (2017) A novel antimicrobial substance produced by Lactobacillus rhamnous LS-8. Food Control 73:754–760. https://doi.org/10.1016/j.foodcont.2016.09.028
Yi L, Luo L, Lü X (2018) Efficient exploitation of multiple novel bacteriocins by combination of complete genome and peptidome. Front Microbiol 9:1567. https://doi.org/10.3389/fmicb.2018.01567
Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, Mohan V, Balasubramanyam M (2018) Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr 57(1):279–295. https://doi.org/10.1007/s00394-016-1317-7
Haffner SM, Greenberg AS, Weston WM, Chen HZ, Williams K, Freed MI (2002) Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 106(6):679–684. https://doi.org/10.1161/01.cir.0000025403.20953.23
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359(6380):1151–1156. https://doi.org/10.1126/science.aao5774
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. https://doi.org/10.1128/aem.00062-07
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-Source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541. https://doi.org/10.1128/aem.01541-09
Zubiría MG, Gambaro SE, Rey MA, Carasi P, Serradell MDLÁ, Giovambattista A (2017) Deleterious metabolic effects of high fructose intake: the preventive effect of Lactobacillus kefiri administration. Nutrients 9(5):470. https://doi.org/10.3390/nu9050470
Liu ZG, Qiao QL, Sun YL, Chen YW, Ren B, Liu XB (2017) Sesamol ameliorates diet-induced obesity in C57BL/6J mice and suppresses adipogenesis in 3T3-L1 cells via regulating mitochondria-lipid metabolism. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600717
Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI (2018) Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mrna levels associated with glucose and lipid metabolism. Nutrients 10(5):643. https://doi.org/10.3390/nu10050643
Pothuraju R, Sharma RK, Kumar KP, Chagalamarri J, Jangra S, Bhakri G, De S (2016) Anti-obesity effect of milk fermented by Lactobacillus plantarum NCDC 625 alone and in combination with herbs on high fat diet fed C57BL/6J mice. Benef Microbes 7(3):1. https://doi.org/10.3920/BM2015.0083
Li C, Ding Q, Nie SP, Zhang YS, Xiong T, Xie MY (2014) Carrot juice fermented with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. J Agric Food Chem 62(49):11884. https://doi.org/10.1021/jf503681r
Pan WW, Myers MG (2018) Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 19(2):95–105. https://doi.org/10.1038/nrn.2017.168
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395(6704):763–770. https://doi.org/10.1038/27376
Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, Friedman J, Feingold KR (1996) Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters—a role for leptin in the anorexia of infection. J Clin Investig 97(9):2152–2157. https://doi.org/10.1172/jci118653
Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu AM, Sweeney G (2015) Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 64(1):36–48. https://doi.org/10.2337/db14-0267
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56(4):901–911. https://doi.org/10.2337/db06-0911
Cranford TL, Enos RT, Velazquez KT, McClellan JL, Davis JM, Singh UP, Nagarkatti M, Nagarkatti PS, Robinson CM, Murphy EA (2016) Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int J Obes 40(5):844–851. https://doi.org/10.1038/ijo.2015.244
Torres S, Fabersani E, Marquez A, Gauffin-Cano P (2018) Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur J Nutr. https://doi.org/10.1007/s00394-018-1790-2
Zhu J, Tang HY, Zhang ZH, Zhang Y, Qiu CF, Zhang L, Huang PE, Li F (2017) Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol 43:236–242. https://doi.org/10.1016/j.intimp.2016.12.020
Cani PD, Delzenne NM (2011) The gut microbiome as therapeutic target. Pharmacol Ther 130(2):202–212. https://doi.org/10.1016/j.pharmthera.2011.01.012
Moya-Pérez A, Neef A, Sanz Y (2015) Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One. https://doi.org/10.1371/journal.pone.0126976
Li KK, Tian PJ, Wang SD, Lei P, Qu L, Huang JP, Shan YJ, Li BL (2017) Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J Funct Foods 38:561–570. https://doi.org/10.1016/j.jff.2017.09.049
Singh DP, Khare P, Bijalwan V, Baboota RK, Singh J, Kondepudi KK, Chopra K, Bishnoi M (2017) Coadministration of isomalto-oligosaccharides augments metabolic health benefits of cinnamaldehyde in high fat diet fed mice. BioFactors 43(6):821–835. https://doi.org/10.1002/biof.1381
Kang C, Wang B, Kaliannan K, Wang XL, Lang HD, Hui SC, Huang L, Zhang Y, Zhou M, Chen MT, Mi MT (2017) Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio 8(3):14. https://doi.org/10.1128/mBio.00470-17
Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, Fernandez-Real JM (2012) Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes 36(11):1442–1449. https://doi.org/10.1038/ijo.2011.256
Hersoug LG, Moller P, Loft S (2016) Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev 17(4):297–312. https://doi.org/10.1111/obr.12370
Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA (2014) Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity 22(3):763–771. https://doi.org/10.1002/oby.20632
Thiennimitr P, Yasom S, Tunapong W, Chunchai T, Wanchai K, Pongchaidecha A, Lungkaphin A, Sirilun S, Chaiyasut C, Chattipakorn N (2018) Lactobacillus paracasei HII01, xylooligosaccharides and synbiotics reduced gut disturbance in obese rats. Nutrition 54:40–47. https://doi.org/10.1016/j.nut.2018.03.005
Linden DR (2014) hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal 20(5):818–830. https://doi.org/10.1089/ars.2013.5312
Floch MH (2010) The effect of probiotics on host metabolism the microbiota and fermentation. J Clin Gastroenterol 44:S19–S21. https://doi.org/10.1097/MCG.0b013e3181dd4fb7
Chen YT, Lin YC, Lin JS, Yang NS, Chen MJ (2018) Sugary kefir strain Lactobacillus mali APS1 ameliorated hepatic steatosis by regulation of SIRT-1/Nrf-2 and gut microbiota in rats. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700903
Liang Y, Lin C, Zhang Y, Deng Y, Liu C, Yang Q (2018) Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology 26(4):1051–1055
Acknowledgements
This work was financially supported by Special Fund for Agro-scientific Research in the Public Interest [Grant No. 201503135].
Author information
Authors and Affiliations
Contributions
TW and XL designed the study and wrote the manuscript; HY and YL performed the experiments; XL and XW analyzed the data; YS and YY interpreted the results of experiments; BL and YZ prepared figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Ethical standards
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4, and experimental procedures were approved by the Animal Ethics Committee of Xi’an Jiaotong University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Yan, H., Lu, Y. et al. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur J Nutr 59, 2709–2728 (2020). https://doi.org/10.1007/s00394-019-02117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02117-y