Abstract
Purpose
Despite longstanding voluntary salt iodisation in Switzerland, data suggest inadequate iodine intake in vulnerable population groups. In response, the salt iodine concentration was increased from 20 to 25 mg/kg and we assessed the impact on iodine status.
Methods
We conducted a cross-sectional national study in school-age children (n = 731), women of reproductive age (n = 353) and pregnant women (n = 363). We measured urinary iodine concentration (UIC) and urinary sodium concentration (UNaC) in spot urine samples. The current median UIC was compared with national data from 1999, 2004 and 2009. We measured TSH, total T4 and thyroglobulin (Tg) on dried blood spot samples collected in women.
Results
The median UIC (bootstrapped 95% CI) was 137 µg/L (131, 143 µg/L) in school children, 88 µg/L (72, 103 µg/L) in women of reproductive age and 140 µg/L (124, 159 µg/L) in pregnant women. Compared to 2009, the median UIC increased modestly in school children (P < 0.001), but did not significantly change in pregnant women (P = 0.417). Estimated sodium intake exceeded the recommendations in all population groups. The prevalence of thyroid disorders in women was low, but Tg was elevated in 13% of the pregnant women.
Conclusion
Iodine intake is overall adequate in Swiss school-age children, but only borderline sufficient in pregnant and non-pregnant women, despite high salt intakes and satisfactory household coverage with iodized salt. Our findings suggest increasing the concentration of iodine in salt may not improve iodine intakes in women if iodised salt is not widely used in processed foods.
Registration
This trial was registered at clinicaltrials.gov as NCT02312466.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine fortification of salt is the most effective strategy to correct and prevent iodine deficiency [1,2,3]. Switzerland was historically affected by moderate to severe iodine deficiency, with high goitre rates in certain areas, and one of the first countries worldwide to introduce iodised salt in 1922 [4,5,6]. Iodised salt has been available nationwide since 1952 [4]. The level of iodine fortification was increased stepwise from 3.75 to 7.5 mg/kg in 1962, to 15 mg/kg in 1980 and to 20 mg/kg in 1998, and goitre due to iodine deficiency has successfully been eliminated [4, 7]. The legislation stipulates voluntary iodine fortification, i.e. both iodised and non-iodised salt must be available, and the current fortification range is regulated at 20–40 mg iodine/kg [8]. One main salt producer distributes salt in the country and changes to the actual salt fortification level are rapidly implemented.
The Swiss voluntary salt iodisation strategy has been regarded an international model with its cautious approach and the well-working public private partnership between the government bodies, academia and salt industry. More than 80% of the households use iodised salt and the iodine intake is adequate in school-age children, as we showed in periodic national urinary iodine concentration (UIC) surveys conducted in 1999, 2004 and 2009 [7, 9, 10]. However, in the same studies we observed concurrent borderline deficient iodine intakes in pregnant women [7, 10]. Poor iodine nutrition has also been reported in national studies conducted in adults [11, 12], lactating women [10] and infants [10, 13]. Thus, although Switzerland may be considered an iodine-sufficient country based on data in 6–12-year-old children, iodine intakes appear to be low in vulnerable population groups. This situation is similar in many other countries worldwide, particularly in Europe [14,15,16]. In an attempt to improve the overall iodine intake in Switzerland, the iodine concentration in salt was increased from 20 to 25 mg/kg in January 2014.
We conducted a cross-sectional national study in 2015 with the objective to assess the current iodine status in school-age children, women of reproductive age and pregnant women. We performed a longitudinal analysis of UIC in school-age children and pregnant women over the years 1999, 2004, 2009 and 2015, and evaluated the impact of increasing the salt iodine concentration by 5 mg/kg.
Subjects and methods
Study design
We conducted a cross-sectional study in April 2015–January 2016 and aimed to obtain a representative national sample of primary school children (6–12 years), women of reproductive age (18–44 years) and pregnant women (18–44 years). The study design was consistent with the previous national iodine status studies conducted in 1999, 2004 and 2009 [7, 9, 10, 17].
Following WHO recommendations for iodine surveys [1], we applied a two-stage probability proportionate-to-size (PPS) cluster sampling, using the current census data from the Swiss Federal Office of Statistics in which Switzerland is divided into five geographic regions. Each of the regions are divided into three strata listing communities with different population size (< 10′000, 10′000–99′999 and > 99′999 inhabitants). The number of clusters in the study was balanced proportional to the population size by region and community size. We selected one school and one obstetric/prenatal care clinic per cluster. For school children, we aimed for 30 clusters including 30 subjects per cluster, and for the women of reproductive age and pregnant women, we aimed for 20 clusters each including 35 subjects.
School-age children
We contacted 581 randomly selected primary schools. Out of the 349 schools that replied, 31 initially agreed to participate and 29 schools finally participated (one private school and 28 public schools). In each school, 2–11 classes (depending on class size and response rate) were randomly selected, and all children in the classes who consented were enrolled. The response rate was 34% and we enrolled additional classes until we reached the intended sample size for the cluster.
Women
We contacted 346 obstetric/prenatal care clinics/hospitals throughout Switzerland by email and 29 clinics/hospitals agreed to participate. Eleven clinics withdrew after the study start without enrolling any subjects. Of the remaining 18 units, 14 enrolled between 20 and 26 women per population group and 4 included < 20 women per group.
Subjects
The inclusion criteria for all subjects were: (1) residence in Switzerland for ≥ 12 months; (2) general good health as assessed by no reported treatment for chronic disease, gastrointestinal or metabolic disorders; (3) no family history of thyroid disease; (4) no exposure to iodine-containing contrast agent or medication within the last year. Women of reproductive age were non-pregnant and non-lactating. Additional inclusion criteria for pregnant women were a healthy and singleton pregnancy.
The sample size of the study was determined to assess the median UIC with 5% precision (95% CI) [18], using data on the intra- and inter-individual variability of UIC in the study population. A previous study reported an intra- and inter-individual variability of 38 and 55% for UIC in Swiss women and recommended a sample size of 473 to accurately estimate median UIC with 5% precision [19]. To account for the cluster survey design [1], we aimed to enrol 900 school-age children, 700 women of reproductive age and 700 pregnant women. We over-sampled school children in the Italian part of Switzerland (4.5 times, i.e. 90 children from three schools) to compare the iodine status in Ticino with the rest of the country. We hypothesised that the iodine intake in Ticino may be lower than in the other parts of the country due to consumption of non-iodised salt entering the market from Italy.
We obtained ethical permission for the study from the Ethics Committee of the Canton Zurich (KEK-ZH-Nr. 2014-0692). Written informed consent was obtained from the parents of the participating school-age children and all participating women. An oral consent was additionally obtained from all children in a semi-private setting on the day of sampling. All data were collected anonymously and registered only by subject number, location, age and sex. The study was registered at clinicaltrials.gov as NCT02312466.
Study procedures
The sample collection period was April 2015–November 2015 for school children and April 2015–Jan 2016 for women. Subject recruitment was done with the help of the teachers in the selected classes. Teachers were informed in detail about the study procedures. They received an overview of the most important points to communicate to the children and their parents. The teachers distributed a written information sheet addressed to the parents outlining the goals and procedures of the study and a shorter version of the information for the children themselves, plus a consent form to be signed by the parents. Children who brought back the signed written informed consent were eligible to participate in the study.
Women were recruited by the supervising physician in respective participating obstetric/prenatal care clinics. The physicians were informed about the study procedures and instructed the subjects. Eligible subjects were provided with the written study information and were given sufficient time to read and take a decision. Written informed consent was obtained before sampling was initiated. The participants received no compensation for their participation.
A questionnaire was administered to all participants. It was adapted to each population group specifically and was used to assess the inclusion criteria, use of iodised salt in the household, consumption of processed foods containing iodised salt and foods rich in native iodine as well as consumption of iodine-containing dietary supplements. All women were asked about education, cigarette smoking, number of births and number of children living with the women at home.
Height and weight were measured in school-age children and women of reproductive age using standard anthropometric techniques [20]. For the measurements, subjects removed their shoes, emptied their pockets, and wore light indoor clothing. Body weight was measured to the nearest 0.1 kg using a digital scale. Height was measured to the nearest 0.1 cm using a portable stadiometer in the children and a fixed stadiometer in the clinics.
We collected a casual “spot” urine sample from all subjects for the measurements of UIC, urinary creatinine concentration (UCC) and urinary sodium concentration (UNaC). A repeat spot urine sample was obtained in a randomly selected subgroup of 30% of the subjects. We aimed to collect the second repeat spot urine sample within a week of the first urine sample. Urine samples were collected at various times of the day, but not from the first morning void. The subjects were given a plastic cup and were asked to provide ~ 20 mL of fresh midstream urine. All urine samples were aliquoted (2.0 mL) and frozen at − 20 °C until analysis.
Women provided a dried blood spot (DBS) sample for determination of thyroid stimulating hormone (TSH), total thyroxine (TT4) and thyroglobulin (Tg). Blood drops (50 µL) were collected by a finger prick and directly collected onto filtre paper cards (IDBS-226, Perkin Elmer, CT, USA). The DBS cards were dried at room temperature, placed in sealed plastic bags, and stored at 4 °C until analysis or frozen at − 20 °C.
We collected household salt samples from a random subsample of 30% of participating school-age children for measurement of salt iodine concentration. The subjects were asked to bring a 40 g salt sample (two table spoons) from their homes in provided clean plastic containers. The salt samples were stored in closed containers and stored in a cool and dry place until analysis.
Biochemical analysis
Urinary iodine concentration
We analysed UIC with the use of the Sandell–Kolthoff method [21]. Our laboratory participates in the programme to ensure the quality of UIC procedures (EQUIP, U.S. Centers for Disease Control and Prevention, Atlanta GA, USA). Four external control samples (range 10–400 µg/L) are measured quarterly and our results are compared to ICP-MS measured at U.S. CDC (Atlanta GA, USA, R2 = 0.99: data available on request). External quality control was ensured by measuring two control urine samples that were added to each plate analysed. The CV was 6% at 64 µg/L (n = 70) and 5% at 198 µg/L (n = 70). Adequate iodine status was defined as median UIC ≥ 100 µg/L for school-age children and women of reproductive age, and as median UIC ≥ 150 µg/L for pregnant women [1].
Urinary creatinine concentration
The UCC was analysed manually using the modified Jaffé method [22]. We measured a subset of the samples (n = 130) using an automated system (Beckman Coulter, SYNCHRON® system) and the agreement was satisfactory (R = 0.99, P < 0.001).
Urinary sodium concentration
The UNaC was determined in the spot urine samples using flame atomic absorption spectrometry (AA240FS; Varian Inc., Tectron, Agilient Technology USA). Results were verified by measurement of certified reference material, i.e. Seronorm Trace Elements Urine Levels (Sero, Norway). Between-run precision was 6.7% at 1680 g/L, 5.6% at 2340 g/L and 6.4% at 3980 g/L.
Salt iodine concentration
The salt iodine concentration was analysed with the use of the Sandell–Kolthoff method [21]. Urine control samples (same as for UIC) were used as external controls for the analysis: The CV was 7% at 65 µg/L (n = 16) and 3% at 194 µg/L (n = 16). The salt iodine concentration was defined as low at < 15 mg/kg, adequate at 15–40 mg/kg and high at > 40 mg/kg [1].
Thyroid function tests
We analysed TSH and TT4 in all DBS samples with the use of an automated time-resolved fluoroimmunoassay method (GSP 2021-0010; PerkinElmer, Turku, Finland) and related GSP Neonatal TSH/T4 kit (PerkinElmer, Turku, Finland). Kit-specific DBS controls were used for the analysis. The CV for TSH was 10% at 15 mU/L (n = 31) and 10% (n = 31) at 63 mU/L. For TT4, the CV was 14% at 42 nmol/L (n = 34) and 10% (n = 34) at 102 nmol/L. Age-, kit- and laboratory-specific reference ranges were used to calculate the prevalence of thyroid dysfunction for school children and women of reproductive age (TSH: 0.1–3.7 mU/L; TT4: 65–165 nmol/L).
For pregnant women, we used trimester- and pregnancy week-specific reference ranges. We used the normal reference values defined for DBS-TSH in non-pregnant adults (0.1–3.7 mIU/L) for pregnant women in the second and third trimesters. We lowered the upper limit of our DBS-TSH assay by 18% to 3.0 mIU/L in the first trimester, as recommended by the American Thyroid Association [23]. For DBS-TT4 in pregnant women in weeks 1–6, we applied the assay-specific normal reference range for non-pregnant adults of 65–165 nmol/L. We then increased the non-pregnant upper reference limit by 5% per week, beginning with week 7 (week 7, 65.0–173.3 nmol/L; week 8, 65.0–181.5 nmol/L; week 9, 65.0–189.8 nmol/L; week 10, 65.0–198.0 nmol/L; week 11, 65.0–206.3 nmol/L; week 12, 65–214.5 nmol/L; week 13, 65.0–222.8 nmol/L; week 14, 65.0–231.0 nmol/L; week 15, 65-239.3 nmol/L) [23, 24]. Starting at week 16, we multiplied the non-pregnant adult reference range by 1.5 and used the resulting range of 97.5–247.5 nmol/L.
Tg was measured on the DBS samples with a DBS-Tg enzyme-linked immunosorbent assay (ELISA) [25]. Serum control samples (Liquicheck Tumor Marker Control; Bio-Rad, Hercules, CA, USA) were used as standards for the DBS-Tg assays. In-house DBS samples were used for quality control and the CVs were 28% at 21 µg/L (n = 9) and 15% at 65 µg/L (n = 12). Assay-specific reference ranges for DBS-Tg were used for school-age children (4–40 µg/L [26]) and pregnant women (0.3–43.5 µg/L [27]). No reference ranges are available for this DBS-Tg assay for women of reproductive age.
Thyroid autoimmunity and the presence of Tg antibodies (TgAb) may confound the individual assessment of Tg in clinical monitoring of thyroid disorders. We measured DBS-TgAb concentrations in a subsample (n = 255) of the samples collected in pregnant women using a serum enzyme-linked immunosorbent assay (TgAb enzyme-linked immunosorbent assay, version 2; RSR, Cardiff, UK) adapted in our laboratory for DBS [25]. The inter-assay CV was 13.8% at 150 ± 50 U/mL and 9.9% at 520 ± 150 U/mL. The manufacturer cut-off for TgAb positivity is ≥ 65 U/mL.
Statistical analysis
We used Excel 2010 (Microsoft, Redmond, WA, USA) and SPSS version 23 (IBM, Armonk, NY, USA) for data processing and analysis.
The primary outcome of the study was UIC. Secondary outcomes were UNaC and thyroid function parameters measured on DBS (TSH, TT4 and Tg) in the women. We calculated the UIC:UCC ratio and estimated the daily urinary iodine excretion (UIE) for each subject. For children, we used the following formula [28]:
For women, we used the following formula,
To obtain the estimated daily iodine intake, we assumed 92% of consumed iodine is excreted in the urine and divided the estimated daily excretion by 0.92 [31, 32]. The estimated daily sodium excretion and sodium intake were calculated in the same way as for iodine. We estimated the habitual iodine intake and the prevalence of inadequate iodine intake using the EAR cut-point method [11, 33, 34]. We used the estimated average requirement (EAR) and upper level (UL) established by the US IOM [32]. The EARs for iodine for girls and boys aged 4–8 years and 9–13 years are 65 µg/day and 73 µg/day, respectively. The ULs for iodine for girls and boys aged 4–8 years and 9–13 years are 300 µg/day and 600 µg/day, respectively. The EAR and UL for non-pregnant and non-lactating women (19–70 years) are 95 µg/day and 1100 µg/day, respectively. We used the personal computer version of Software for Intake Distribution Estimation (version 1.0, 2003; available from the Department of Statistics, Iowa State University) and the supporting documentation to estimate usual intake distributions [35, 36]. Details of the method known as the Iowa State University method are discussed in detail elsewhere [33, 37]. An estimate of variance from the subsample in which duplicate urine samples were collected was applied to the entire population. The software produces an empirical estimate of the usual nutrient intake of each EAR/UL age and sex subgroup, estimates adjusted percentiles, and calculates the prevalence of inadequate or excessive intake from the subgroup using the EAR/UL cut-point method. Per definition, a prevalence of 2.5% below the EAR and 2.5% above the UL is acceptable in a population with adequate iodine intake [34].
We assessed normality by testing the distributions of continuous variables against a normal distribution with the Kolmogorov–Smirnov test and graphically by evaluating histograms and Q–Q plots. Normally distributed data were expressed as mean ± SD (95% CI). Non-normally distributed data were log-transformed and if a parameter met the assumption of normal distribution it was presented as geometric mean (SD). Median (IQR) was used for data that remained skewed after transformation. Nonparametric 95% confidence intervals (CI) around the median were obtained using the bootstrap technique (n = 1000). No outliers were removed from the descriptive data, apart from one UIC outlier for school-age children (UIC > 2500 µg/L).
Wilcoxson signed rank test was used to test differences in measured parameters between the first and second urine samples. For continuous variables, group differences between two categories were tested by using the Mann–Whitney U test and for more than two categories we used the Kruskal–Wallis ANOVA test followed by Mann–Whitney post hoc tests and Bonferroni correction of the significance level (e.g. longitudinal comparison of UIC in school-age children and pregnant women). We used Pearson chi-square test to evaluate differences between the trimesters for categorical data. Associations between two variables were assessed using the Kendall’s tau correlation coefficient (R). The association between estimated iodine and sodium intakes was assessed by Pearson correlation using log data. Iodine intakes > 4000 ug/day (n = 3) and sodium intakes > 15000 mg/day (n = 3) were excluded from the analysis for women of reproductive age. Statistical significance was set at P < 0.05.
Results
School-age children
We enrolled 754 school-age children from 29 schools: 23 children did not meet the inclusion criteria or did not provide a urine sample and were excluded from the data analysis. The final study population is comprised of 731 children, representing approximately 1 in 760 children in the age group from 6 to 12 years in Switzerland. General characteristics of the children are shown in Table 1. All regions were well represented, except for the northeast region where only 41% of the intended numbers of children were recruited. Communities with a population > 99′999 inhabitants were under-represented: only 1 out of 5 intended subjects were obtained.
We obtained 193 salt samples collected from a random subsample of the households of the participating children. The measured salt iodine concentration in the collected salt samples (n = 193) was adequate (15–40 mg/kg) in 82.9% and low (5–15 mg/kg) in 4.7%. No iodine (< 5 mg/kg) was detected in 12.4% of salt samples, but none of the samples had iodine concentrations exceeding 40 mg/kg. The median iodine concentration in iodised salt samples (≥ 5 mg/kg) was 23.9 mg/kg (IQR: 21.8, 25.4 mg/kg) (n = 169). We did not observe higher proportion of non-iodized salt samples or different salt iodine concentration in Ticino (n = 37) compared to the other regions combined (P = 0.670).
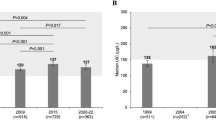
The overall median UIC in school-age children was 137 µg/L (bootstrapped 95% CI: 131, 143 µg/L, n = 729) (Table 2), higher than the median UIC of 120 µg/L (bootstrapped 95% CI: 116, 124 µg/L) obtained in 2009 (n = 916, P < 0.001, Fig. 1a). Only 5.7% of the children had a UIC < 50 µg/L for the first spot urine sample. UIC did not correlate with age (P = 0.335) or household salt iodine concentration (P = 0.642), but boys had marginally higher median UIC than girls (median = 143 µg/L vs. 131 µg/L; P = 0.046).
Median (boostrapped 95% CI) UIC in school-age children (a) and pregnant women (b) by year [7, 9, 10]. UIC urinary iodine concentration. The shaded areas indicate optimal iodine nutrition according to WHO UIC thresholds [1, 55]. Kruskal–Wallis ANOVA followed by Mann–Whitney post hoc tests with Bonferroni correction was used to test differences in median UIC between years. Values with different superscript letters differed (P < 0.001)
The median UIC in children was ≥ 100 µg/L in all five of the geographic regions of Switzerland, ranging from 128 µg/L to 163 µg/L. The highest median UIC of 163 µg/L (IQR: 109, 228 µg/L) was observed in Ticino (n = 142), higher than the median UIC of 133 µg/L (IQR: 98, 179 µg/L) of the other four regions combined (n = 589) (P < 0.001). However, the overall national median UIC was unaffected by the data obtained in Ticino (P = 0.141). We observed no differences in median UIC between communities with a population of < 10′000 inhabitants (n = 472) compared to communities with a population between 10′000 and 99′999 inhabitants (n = 239: P = 0.367). The only school obtained in communities with > 99′999 was excluded from the comparison.
The median daily iodine intake was estimated separately for two age groups in accordance with the dietary intake recommendations [32] and by adjusting for intra-individual variability. The estimated iodine intake was inadequate (below the EAR) in 10% of the children: 11.5% for 6–8 years old and 8.8% for 9–12 years old (Table 2). No children had excessive intakes exceeding the UL. The median estimated daily sodium intake was 2394 mg/day (IQR: 2059, 2770 mg/day, Table 2). The estimated iodine intake was positively associated with the estimated sodium intake (R = 0.407, P < 0.001, Fig. 2a).
Women of reproductive age
A total sample of 361 women from 18 obstetricians/gynaecologists participated in the study. We excluded eight subjects as they did not fulfil the inclusion criteria and the final sample size entailed 353 women of reproductive age (Table 1). The northeast region had only one participating clinic and was under sampled.
Ninety-two percent of the women reported using iodised salt in their homes, but only 1.4% consumed iodine-containing dietary multivitamin supplements (Table 1). The median UIC in women of reproductive age was 88 µg/L (bootstrapped 95% CI: 72, 103 µg/L, n = 345), borderline below the WHO threshold of 100 µg/L indicating iodine sufficiency (Table 2). The UIC distribution of the first urine sample was strongly skewed and the proportion of UIC < 50 µg/L was 28%. After adjusting the iodine intake for intra-individual variability, the median iodine intake was estimated to be 163 µg/day (IQR: 117, 241 µg/L, n = 330, Table 2). The prevalence of inadequate iodine intake was 12.4% and only 0.4% of the women had estimated iodine intakes above the upper intake level.
The median UNaC was 2018 mg/L (IQR: 1045, 3199 mg/L) and estimated median daily sodium intakes was 3566 mg/day IQR: (2850, 4407 mg/day, Table 2). We observed a positive correlation between the estimated iodine and sodium intakes (R = 0.328, P < 0.001, Fig. 2b).
TSH, TT4 and Tg concentrations of the women are presented in Table 3. The prevalence of subclinical and overt thyroid disorders was low, comparable to a typical euthyroid population (Table 3). No reference values have been established for DBS-Tg, but when applying the reference ranges for pregnant women the prevalence of elevated Tg was 16.3%. We observed no association between UIC and any of the thyroid parameters (data not shown).
Pregnant women
A total sample of 375 pregnant women from 18 clinics participated. We excluded 12 subjects as they did not fulfil the inclusion criteria and the final sample size was 363 pregnant women. The median UIC in pregnant women was 140 µg/L (bootstrapped 95% CI: 124, 159 µg/L, n = 359), statistically not below the WHO threshold of 150 µg/L indicating inadequate iodine intake during pregnancy. The median UIC (bootstrapped 95% CI) of women in the first trimester of pregnancy (n = 60) was 110 µg/L (75, 153 µg/L), in the second trimester (n = 163) it was 128 µg/L (106, 174 µg/L), and for women in the third trimester (n = 130) it was 171 µg/L (146, 216 µg/L, P = 0.055). The proportion of UIC < 50 µg/L was 18% and the proportion > 1000 µg/L was 3%.
Eighty-five percent of the women reported using iodised salt in their homes. Iodine-containing prenatal multivitamin supplements (containing 150 to 220 µg iodine/day) were consumed by 37% of the pregnant women and the rates were similar for all three trimesters (P = 0.943). The median UIC did not differ between supplement users and non-users (P = 0.404). The median UNaC in pregnant women was comparable to that in women of reproductive age (1872 vs. 2018 mg/L, P = 0.207).
We observed a statistical difference in the median UIC in pregnant women over the years from 1999 to 2015 in the overall longitudinal analysis (P < 0.001), but the difference was only significant for the median UIC in 2004 (post hoc analysis). We did not find an explanation for the high median UIC in 2004, but it is possible that some of the collected urine samples were contaminated with iodine from urinary glucose test dip sticks used by the participating clinics in that study [9, 38]. The median UIC in 2015 was not lower than the median of 162 µg/L (bootstrapped 95% CI: 144, 177 µg/L, n = 648) observed in 2009 (P = 0.417, Fig. 1b).
The median TSH and geometric mean TT4 represent an overall euthyroid population, and the prevalence of subclinical hypothyroidism, subclinical hyperthyroidism and isolated maternal hypothyroxinaemia was low at 0.9%, 0.2% and 4.8%, respectively (Table 3). TSH did not differ between trimester (P = 0.764), but as expected TT4 was lower in the first trimester (P < 0.001).
The median DBS-Tg concentration was high at 23.8 µg/L (IQR: 15.5, 35.3 µg/L), comparable to the median Tg in women of reproductive age (P = 0.968). We observed no difference in the median Tg concentration between the trimesters (P = 0.833). The prevalence of elevated DBS-Tg was 13.3%. The Tg concentration was lower in women taking iodine-containing supplements (n = 126), compared to non-users (n = 219) (median Tg 22.6 vs. 24.6 µg/L, P = 0.013). We also observed lower TT4 concentration in women taking iodine-containing supplements (n = 129), compared to non-users (n = 220) (median TT4 130.5 vs. 139.7 nmol/L, P = 0.011).
Discussion
The iodine intake in Switzerland is overall adequate in school-age children, but only borderline sufficient in women of reproductive age and pregnant women using the WHO thresholds for the median UIC. At the same time, 10% of children and 12% of women have inadequate habitual iodine intakes, i.e. intakes below the EAR.
Iodine deficiency in a population is defined as a median UIC < 100 µg/L (< 150 µg/L for pregnant women) [39], i.e. at an intake level below which the risk of goitre increases (< 100 µg/L in school-age children [40]; <100 µg/day, i.e UIC < 60–70 µg/L in adults [41, 42]). An alternative method to assess adequate iodine intakes from UIC was recently proposed [11], based on the established EAR cut-point method [34]. This method defines adequate iodine intake as when 97% of individuals in a population meet the EAR. The intake level required to maintain adequate habitual iodine intake is higher than the minimum intake needed to prevent goitre. Optimal iodine nutrition is achieved when both criteria are fulfilled [3, 11].
We observed a modest improvement of the median UIC in children in the current study compared to the three previous national studies, which in part may be attributed to the increased salt iodine content from 20 mg/kg to 25 mg/kg. Yet, the iodine intake remained low in women of reproductive age and pregnant women [10,11,12] and a 5 mg/kg increase of the salt iodine content did not appear to benefit women with higher dietary iodine requirements. We recently showed that salt iodine fortification at 25 mg/kg can be sufficient to meet the dietary iodine requirements of all population groups if the coverage of iodised salt is high [3]. However, the median UIC in Swiss children and women is only half of that observed in populations with mandatory iodisation of all edible salt [3].
Iodised salt is likely the primary source of iodine in the Swiss diet [12]. The use of iodised salt in the households remains high (> 80%) and satisfactory. However, foods produced or cooked outside the households, i.e. ready-made foods obtained from a store or a restaurant, are the main sources of salt in a western diet (70–80% of the total amount of salt consumed) [43]. The use of iodised salt in such foods is critical to achieve optimal iodine status. Data on the proportion of food products produced and/or sold with iodised salt in Switzerland are limited, but the coverage appears incomplete: only 61% of the food grade salt sold in 2017 was iodised (Personal communication, Swiss Saltworks AG, 2018). The estimated median iodine intake in women of reproductive age in our study was 163 µg/day (Table 2). If all salt would be iodized the intake would increase to ≥ 220 µg/day. Universal salt iodisation, i.e. iodisation of all food grade salt, remains the golden standard [1], yet its implementation is challenging. National policies and legislation for iodised salt differ across countries [44] and many European food producers choose to use non-iodised salt to facilitate export and import of their products. The consequence is inadequate iodine nutrition in women of reproductive age and pregnant women [15, 16]. In Switzerland an overall increase in iodine intake of approximately 25 µg/day is likely sufficient to achieve optimal iodine nutrition.
The sodium intake in the Swiss population is well above the national (1.5 g/day [45]) and international recommendations (2 g/day [46]). We estimate a sodium intake of 3.5 g/day in women (corresponding to a salt intake of 8.9 g/day), consistent with a previous national study [47]. Not surprisingly, the sodium intakes exceed the recommendations also in children, in agreement with a recent local study [48]. The Swiss nutrition strategy for 2017–24 set out to reduce the overall sodium intake [49]. A successful salt reduction may increase the risk of emerging iodine deficiency in the Swiss population, if the overall coverage of iodised salt is not improved. The salt iodisation strategy must, therefore, be integrated with the salt reduction policy and be adapted to the actual salt intakes [50, 51].
The contribution of iodine from different dietary sources cannot directly be quantified by our data. However, Fig. 2 suggests that a substantial proportion of the daily iodine intake may come from sources other than salt and partially compensate for the incomplete coverage of iodized salt. Cow’s milk and dairy products are important sources of dietary iodine in the Swiss population, particularly in children [52, 53]. We estimate that daily consumption of one glass (0.3 L) of Swiss milk (with a median milk iodine concentration of ≈ 87 µg/L) would contribute ≈ 25 µg iodine [53], i.e. a quarter of the recommended daily iodine intake for children [1, 32]. Subgroups not consuming dairy products may have particular low iodine intakes and be at high risk of iodine deficiency, as previously shown for vegans [54].
Is the borderline low iodine intake a health concern? Our data suggest an increased thyroid activity in women, possibly due to the borderline low iodine intake. The median Tg concentrations in women were higher than typically seen in iodine-sufficient populations and 13% of the pregnant women had elevated Tg concentrations [3, 27]. Tg increases in iodine deficiency [27, 55, 56], but the potential significance is uncertain. The thyroid gland in healthy individuals is generally able to adapt to mildly iodine-deficient intakes [57, 58] and this is supported by the low prevalence of hypothyroidism in our study. However, to maintain euthyroidism in the face of chronic low iodine intake requires increased thyroid activity; over the long term this may increase risk of multifocal autonomous growth and thyroid nodules [57, 59]. Long-term iodine sufficiency normalises thyroid activity, reduces the incidence of thyroid nodules and autonomy, and prevents thyroid disorders in the general population [58, 60, 61].
Representative data on thyroid disorders in Switzerland are limited [62]. The risk of a transient increased incidence of hyperthyroidism at further improvements of the iodine intake into the adequate intake range is likely low [58, 60, 61], considering that the iodine intake is only borderline inadequate and has been stable since the 1980s [63]. A small increase of subclinical hypothyroidism associated with increased risk of mild thyroid autoimmunity (with antibodies at a low titre) has been observed after improved iodine status, but the risk is uncertain [58, 64, 65]. Studies evaluating the impact of iodine fortification on thyroid disorders have been conducted in populations with mild-to-moderate iodine deficiency, but long-term studies are needed to evaluate the benefits and risks associated with correcting borderline inadequate intakes. Importantly, there is low risk of excessive iodine intakes at current salt intake and the present level of salt iodine fortification [3, 66].
Iodine supplementation of 150 µg iodine daily during pregnancy is recommended in several countries [23, 67, 68] and supported by WHO when the coverage of iodised salt is incomplete [69]. In the U.S., 62% of prenatal vitamins contain iodine [70], whereas in Europe most prenatal vitamin supplements do not. In Switzerland, iodine supplementation is not specifically recommended, but approximately half of the various prescribed prenatal supplement brands contain iodine, yet the most commonly used product does not. The consumption of iodine-containing prenatal multivitamin supplements during pregnancy increased from 15% in 2009 to 41% in 2015 [10]; however, the median UIC in 2015 was not higher in users compared to non-users, possibly because of the low sample size. Controlled studies evaluating the impact of iodine supplementation on thyroid function of the mother and long-term health effects of the child are limited [71, 72]. A recent randomised controlled trial of prenatal iodine supplementation in mildly deficient pregnant women observed improved iodine status during pregnancy, but no long-term benefit on child development [73]. Furthermore, iodine supplementation is generally begun at the end of the first trimester and may miss the critical time-window of rapid thyroid and brain development that occurs during the first trimester. Thus, the priority should be an overall improvement of the iodine intake in the population at large, covering women of reproductive age and thereby ensuring adequate iodine nutrition in early pregnancy as well as lactating women with low breastmilk concentrations [10].
Strengths of our study include: (1) longitudinal iodine status assessment using data from four national studies conducted over 16 years, which all used recommended and consistent study design [1]; (2) parallel assessment of three population groups identifying gaps in the effectiveness of the salt iodization policy; (3) assessment of both UIC and thyroid function; (4) data on the prevalence of inadequate iodine intake recognising disparities in habitual iodine intakes not detected by the overall median UIC. We recognise that the sample sizes were lower than intended, due to the poor response rate. However, previous studies did not report differences between regions or community size [10,11,12] and we still consider the data representative.
In conclusion, despite longstanding and successful voluntary salt iodisation in Switzerland, the strategy currently falls short to achieve optimal iodine nutrition in women, despite high salt intakes and high household coverage with iodized salt. Our findings suggest increasing the concentration of iodine in salt may not improve the overall iodine intake if iodised salt is not widely used in processed foods. Thus, effective strategies to improve the use of iodised salt in processed food production are warranted.
Abbreviations
- DBS:
-
Dried blood spot
- EAR:
-
Estimated average requirement
- Tg:
-
Thyroglobulin
- TSH:
-
Thyroid stimulating hormone
- TT4:
-
Total thyroxine
- UCC:
-
Urinary creatinine concentration
- UIC:
-
Urinary iodine concentration
- UIE:
-
Urinary iodine excretion
- UNaC:
-
Urinary sodium concentration
- UL:
-
Upper level
References
WHO, UNICEF, ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers, 3rd edn. World Health Organization, Geneva
Aburto N, Abudou M, Candeias V, Wu T (2014) Effect and safety of salt iodization to prevent iodine deficiency disorders: a systematic review with meta-analyses. WHO eLibrary of Evidence for Nutrition Actions (eLENA). World Health Organization, Geneva
Dold S, Zimmermann MB, Jukic T, Kusic Z, Jia Q, Sang Z, Quirino A, San Luis TOL, Fingerhut R, Kupka R, Timmer A, Garrett GG, Andersson M (2018) Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: a cross-sectional multicenter study. J Nutr 148(4):587–598
Bürgi H, Supersaxo Z, Selz B (1990) Iodine deficiency diseases in Switzerland one hundred years after Theodor Kocher’s survey: a historical review with some new goitre prevalence data. Acta Endocrinol (Copenh) 123(6):577–590
Zimmermann MB (2008) Research on iodine deficiency and goiter in the 19th and early 20th centuries. J Nutr 138(11):2060–2063
Bürgi H, Andersson M (2013) History and current epidemiology of iodine nutrition in Switzerland. In: Federal Commission for Nutrition. Iodine supply in Switzerland: current status and recommendations. Expert report of the FCN. Federal Office of Public Health, Zurich. https://www.eek.admin.ch/eek/en/home/pub/jodversorgung-in-der-schweiz-.html. Accessed 9 Oct 2018
Hess SY, Zimmermann MB, Torresani T, Burgi H, Hurrell RF (2001) Monitoring the adequacy of salt iodization in Switzerland: a national study of school children and pregnant women. Eur J Clin Nutr 55(3):162–166
EDI (2018) Verordnung des EDI über den Zusatz von Vitaminen, Mineralstoffen und sonstigen Stoffen. In: Lebensmitteln (817.022.32) (in German), vom 16. Dezember 2016 (Stand am 6. Februar 2018). Eidgenössisches Department des Innern (EDI) (Federal Department of Home Affairs), Bern
Zimmermann MB, Aeberli I, Torresani T, Burgi H (2005) Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study. Am J Clin Nutr 82(2):388–392
Andersson M, Aeberli I, Wust N, Piacenza AM, Bucher T, Henschen I, Haldimann M, Zimmermann MB (2010) The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J Clin Endocrinol Metab 95(12):5217–5224
Zimmermann MB, Andersson M (2012) Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70(10):553–570
Haldimann M, Bochud M, Burnier M, Paccaud F, Dudler V (2015) Prevalence of iodine inadequacy in Switzerland assessed by the estimated average requirement cut-point method in relation to the impact of iodized salt. Public Health Nutr 18(8):1333–1342
Dorey CM, Zimmermann MB (2008) Reference values for spot urinary iodine concentrations in iodine-sufficient newborns using a new pad collection method. Thyroid 18(3):347–352
Gizak M, Gorstein J, Andersson M (2017) Epidemiology of iodine deficiency. In: Pearce E (ed) Iodine deficiency disorders and their eradication. Springer, New York, pp 29–43
IGN (2017) Global scorecard of iodine nutrition in 2017 in the general population and in pregnant women. Iodine Global Network. http://www.ign.org/cm_data/IGN_Global_Scorecard_AllPop_and_PW_May2017.pdf. Accessed 24 Aug 2018
Zimmermann MB, Gizak M, Abbott K, Andersson M, Lazarus JH (2015) Iodine deficiency in pregnant women in Europe. Lancet Diabetes Endocrinol 3(9):672–674
Hess SY, Zimmermann MB (2000) Thyroid volumes in a national sample of iodine-sufficient swiss school children: comparison with the World Health Organization/International Council for the control of iodine deficiency disorders normative thyroid volume criteria. Eur J Endocrinol 142(6):599–603
Fraser CG, Harris EK (1989) Generation and application of data on biological variation in clinical-chemistry. Crit Rev Cl Lab Sci 27(5):409–437
König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB (2011) Ten repeat collections for urinary iodine from spot samples or 24-h samples are needed to reliably estimate individual iodine status in women. J Nutr 141(11):2049–2054
WHO (1995) Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organization, Geneva
Pino S, Fang SL, Braverman LE (1998) Ammonium persulfate: a new and safe method for measuring urinary iodine by ammonium persulfate oxidation. Exp Clin Endocrinol Diabetes 106(Suppl 3):S22–S27
Vasiliades J (1976) Reaction of alkaline sodium picrate with creatinine: I. Kinetics and mechanism of formation of the mono-creatinine picric acid complex. Clin Chem 22(10):1664–1671
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S (2017) 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389
Weeke J, Dybkjaer L, Granlie K, Jensen SE, Kjaerulff E, Laurberg P, Magnusson B (1982) A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol (Copenh) 101(4):531–537
Stinca S, Andersson M, Erhardt J, Zimmermann MB (2015) Development and validation of a new low-cost enzyme-linked immunoassay for serum and dried blood spot thyroglobulin. Thyroid 25(12):1297–1305
Zimmermann MB, de Benoist B, Corigliano S, Jooste PL, Molinari L, Moosa K, Pretell EA, Al-Dallal ZS, Wei Y, Zu-Pei C, Torresani T (2006) Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 91(12):4881–4887
Stinca S, Andersson M, Weibel S, Herter-Aeberli I, Fingerhut R, Gowachirapant S, Hess SY, Jaiswal N, Jukic T, Kusic Z, Mabapa NS, Nepal AK, San Luis TO, Zhen JQ, Zimmermann MB (2017) Dried blood spot thyroglobulin as a biomarker of iodine status in pregnant women. J Clin Endocrinol Metab 102(1):23–32
Montenegro-Bethancourt G, Johner SA, Stehle P, Neubert A, Remer T (2015) Iodine status assessment in children: Spot urine iodine concentration reasonably reflects true twenty-four-hour iodine excretion only when scaled to creatinine. Thyroid 25(6):688–697
Remer T, Neubert A, Maser-Gluth C (2002) Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr 75(3):561–569
Kesteloot H, Joossens JV (1996) On the determinants of the creatinine clearance: a population study. J Hum Hypertens 10(4):245–249
Jahreis G, Hausmann W, Kiessling G, Franke K, Leiterer M (2001) Bioavailability of iodine from normal diets rich in dairy products–results of balance studies in women. Exp Clin Endocrinol Diabetes 109(3):163–167
Food, Board N, Institute of Medicine (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. National Academy Press, Washington DC
Carriquiry AL (1999) Assessing the prevalence of nutrient inadequacy. Public Health Nutr 2(1):23–33
Food, Board N, Institute of Medicine (2000) Dietary reference intakes: applications in dietary assessment. National Academy Press, Washington DC
Dodd KW (1996) A User’s guide to C-SIDE: software for intake distribution estimation version 1.0. CARD Technical Report 96–TR31. Ames (IA): Center for Agricultural and Rural Development, Iowa State University
Dodd KW (1996) A technical guide to C-SIDE. Dietary assessment research series report 9. CARD technical report 96-TR32. Ames (IA): Center for Agricultural and Rural Development, Iowa State University
Guenther PM, Kott PS, Carriquiry AL (1997) Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr 127(6):1106–1112
Pearce EN, Lazarus JH, Smyth PP, He X, Smith DF, Pino S, Braverman LE (2009) Urine test strips as a source of iodine contamination. Thyroid 19(8):919
World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders (2007) Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers, 3rd edn. World Health Organization, Geneva
Delange F, Benker G, Caron P, Eber O, Ott W, Peter F, Podoba J, Simescu M, Szybinsky Z, Vertongen F, Vitti P, Wiersinga W, Zamrazil V (1997) Thyroid volume and urinary iodine in European schoolchildren: standardization of values for assessment of iodine deficiency. Eur J Endocrinol 136(2):180–187
Ascoli W, Arroyave G (1970) Epidemiologia el bocio ende´mico en Centro Ame´rica. Relacio´n entre prevalencia y excrecio´n urinaria de yodo (Epidemiology of endemic goiter in Central America. Association between prevalence and urinary iodine excretion). Archives of Latinoamer Nutrition 20:309–320
Langer P (1980) Eastern and southeastern Europe. In: Stanbury JB, Hetzel BS (eds) Endemic goiter and endemic cretinism. Wiley, New York, pp 141–153
Brown IJ, Tzoulaki I, Candeias V, Elliott P (2009) Salt intakes around the world: implications for public health. Int J Epidemiol 38(3):791–813
The EUthyroid Consortium (2018) The Krakow declaration on iodine. Tasks and responsibilities for prevention programs targeting iodine deficiency disorders. Eur Thyroid J 7(4):201–204
Strohm D, Bechthold A, Ellinger S, Leschik-Bonnet E, Stehle P, Heseker H, German Nutr Soc DGE (2018) Revised reference values for the intake of sodium and chloride. Ann Nutr Metab 72(1):12–17
WHO (2012) Guideline: sodium intake for adults and children. World Health Organization, Geneva
Chappuis A, Bochud M, Glatz N, Vuistiner P, Paccaud F, Burnier M (2011) Swiss survey on salt intake: main results. Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Suisse, Lausanne
Rios-Leyvraz M, Bovet P, Bochud M, Genin B, Russo M, Rossier MF, Tabin R, Chiolero A (2018) Estimation of salt intake and excretion in children in one region of Switzerland: a cross-sectional study. Eur J Nutr. https://doi.org/10.1007/s00394-018-1845-4
Federal Department of Home Affairs (2017) Swiss nutrition policy 2017–2024. Federal Food Safety and Veterinary Office, Bern
WHO (2014) Salt reduction and iodine fortification strategies in public health: Report of a joint technical meeting. World Health Organization, Geneva
Webster J, Land MA, Christoforou A, Eastman CJ, Zimmerman M, Campbell NR, Neal BC (2014) Reducing dietary salt intake and preventing iodine deficiency: towards a common public health agenda. Med J Aust 201(9):507–508
van der Reijden OL, Zimmermann MB, Galetti V (2017) Iodine in dairy milk: Sources, concentrations and importance to human health. Best Pract Res Clin Endocrinol Metab 31(4):385–395
van der Reijden OL, Galetti V, Hulmann M, Krzystek A, Haldimann M, Schlegel P, Manzocchi E, Berard J, Kreuzer M, Zimmermann MB, Herter-Aeberli I (2018) The main determinants of iodine in cows’ milk in Switzerland are farm type, season and teat dipping. Br J Nutr 119(5):559–569
Schupbach R, Wegmuller R, Berguerand C, Bui M, Herter-Aeberli I (2017) Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr 56(1):283–293
Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, Jukic T, Kartono D, Kusic Z, Pretell E, San Luis TO Jr, Untoro J, Timmer A (2013) Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 98(3):1271–1280
Ma ZF, Skeaff SA (2014) Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 24(8):1195–1209
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carle A (2010) Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 24(1):13–27
Zimmermann MB, Boelaert K (2015) Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3(4):286–295
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism Lancet 388(10047):906–918
Baltisberger BL, Minder CE, Burgi H (1995) Decrease of incidence of toxic nodular goitre in a region of Switzerland after full correction of mild iodine deficiency. Eur J Endocrinol 132(5):546–549
Petersen M, Knudsen N, Carle A, Andersen S, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Thuesen BH, Pedersen IB (2018) Thyrotoxicosis after iodine fortification. A 21-year Danish population-based study. Clin Endocrinol (Oxf) 89(3):360–366
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE (2018) Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 14(5):301–316
Burgi H, Kohler M, Morselli B (1998) Thyrotoxicosis incidence in Switzerland and benefit of improved iodine supply. Lancet 352(9133):1034
Pedersen IB, Knudsen N, Carle A, Vejbjerg P, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Laurberg P (2011) A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 75(1):120–126
Petersen M, Knudsen N, Carle A, Andersen S, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Thuesen BH, Pedersen IB (2018) Increased incidence rate of hypothyroidism after iodine fortification in Denmark. A 20 year prospective population-based study. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-01993
Farebrother J, Zimmermann MB, Andersson M (2019) Excessive iodine intake: sources, assessment and effects on thyroid function. Ann NY Acad Sci. https://doi.org/10.1111/nyas.14041
National Health and Medical Research Council (NHMRC) (2010) Iodine supplementation for pregnant and breastfeeding women. In: NHMRC Public Statement, January 2010
Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B (2014) 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 3(2):76–94
Andersson M, de Benoist B, Delange F, Zupan J (2007) Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr 10(12A):1606–1611
Lee SY, Stagnaro-Green A, MacKay D, Wong AW, Pearce EN (2017) Iodine contents in prenatal vitamins in the United States. Thyroid 27(8):1101–1102
Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB (2016) Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr 104(Suppl 3):918S–923S
Harding KB, Peña-Rosas JP, Webster AC, Yap CMY, Payne BA, Ota E, De-Regil LM (2017) Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Systematic Rev 3:CD011761
Gowachirapant S, Jaiswal N, Melse-Boonstra A, Galetti V, Stinca S, Mackenzie I, Thomas S, Thomas T, Winichagoon P, Srinivasan K, Zimmermann MB (2017) Effect of iodine supplementation in pregnant women on child neurodevelopment: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 5(11):853–863
Acknowledgements
The study was funded by the Federal Food Safety and Veterinary Office (FSVO), Bern, Switzerland, and ETH Zurich, Zurich, Switzerland. We would like to thank the subjects for their participation and, teachers, doctors and nurses for assistance with subject recruitment and sample collection. We thank ETH students Leonie Arns, Friederike Becker, Matthias Buchli, Simon Hartung, Lisa Mazzolini, Laura Salvioni, Elisabeth Schlunke, Sara Stinca, Alexandra Thoma and Lea Wildeisen for assistance with the study and support with laboratory analysis. We also thank Leonie Arns for conducting the statistical analysis of the prevalence of inadequate iodine intake and Stefan Trachsel for providing salt sales data from the Swiss Saltworks AG.
Author information
Authors and Affiliations
Contributions
The author’s responsibilities were as follows—MA, IHA, MBZ: designed the research and wrote the study protocol; MA, IHA: supervised the study; SH: assisted with study coordination and conducted biochemical analysis of TSH, TT4 and Tg; RF: supervised the measurement of TSH and TT4; MA: conducted the statistical analysis and wrote the paper. MA had primary responsibility for final content. All authors read, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest statement.
Rights and permissions
About this article
Cite this article
Andersson, M., Hunziker, S., Fingerhut, R. et al. Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr 59, 581–593 (2020). https://doi.org/10.1007/s00394-019-01927-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01927-4