Abstract
Background
Diet and inflammation have been suggested to be important risk factors for lung cancer. We examined the ability of the dietary inflammatory index (DII®) to predict lung cancer in the Singapore Chinese Health Study (SCHS). The DII is a diet quality index based on the literature linking foods and nutrients with inflammatory biomarkers.
Patients and methods
Using data from the SCHS for 60,232 participants, including 1851 lung cancer cases, we investigated the associations of baseline DII scores calculated from a food frequency questionnaire with risk of developing lung cancer over an average of 17.6 years of follow-up. Hazard ratios (HR) were estimated using Cox regression, adjusting for smoking status and other risk factors.
Results
After excluding cancers diagnosed in the first 2 years of follow-up, the DII was non-significantly associated with risk of lung cancer (HRQ5vsQ1 = 1.13; 95% CI 0.94–1.35; P-trend = 0.24) after adjusting for age, dialect group, sex, interview year, education, body mass index, total calorie intake, physical activity and various smoking variables. In stratified analysis, stronger, statistically significant associations were evident in current smokers (HR 1.44; 95% CI 1.11–1.86; Ptrend = 0.03, P for interaction = 0.003) and in male ever-smokers (HRQ5vsQ1 = 1.37; 95% CI 1.07–1.77; P-trend = 0.03).
Conclusion
A pro-inflammatory diet, as shown by higher DII scores, is associated with an elevated risk of lung cancer for subjects with a history of smoking. Public health measures should be adopted to promote consumption of a healthy, anti-inflammatory diet to reduce the risk of lung cancer, especially in current and former smokers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, lung cancer is the most commonly diagnosed cancer, the most common cause of cancer death for men, and is the leading cause of cancer death in developed countries [1]. In Singapore, lung cancer is the second most commonly diagnosed cancer in men and the third most common cancer in women [2]. In both sexes, it is the most common cause of cancer death [3].
Chronic inflammation—which is characterized by the continuous presence of inflammatory cytokines in circulation and in the tissues—is known to play a key role in the development of lung cancer [4]. There have been several studies reporting positive associations between biomarkers of inflammation and lung cancer risk [5, 6]. Within the screening arm of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), a nested case–control study found that elevated serum high-sensitivity C-reactive protein (hs-CRP) was associated with increased risk of incident lung cancer [5, 6].
Smoking is a well-established risk factor for lung cancer [7]. Cigarette smoke has been shown to induce a pro-inflammatory state, which may contribute to the aetiology of lung cancer [8, 9]. Apart from smoking, there is growing evidence that specific dietary components influence both inflammation [10, 11] and lung cancer [12]. The Mediterranean dietary pattern, which is high in fruits, vegetables, olive oil, whole grains, and fish, and low in red meat and butter, with moderate alcohol intake, has been associated with lower levels of inflammation [13]. By contrast, the Western-type diet, which is high in red meat, high-fat dairy products, and refined grains, has been associated with higher levels of CRP, IL-6 and fibrinogen [14]. A meta-analysis of 37 studies of 20,075 lung cancer cases indicated that intake of vegetables and fruits may have a protective effect on lung cancer, the pooled RR were 0.74 (95% CI 0.67, 0.82) for vegetables and 0.80 (95% CI 0.74, 0.88) for fruits [12]. Previously, results from the Singapore Chinese Health Study, showed fried meat intake to be strongly associated with lung cancer; compared with the lowest tertile of fried meat intake, the HRs (95% CIs) for the second and third tertiles were 1.43 (0.98, 2.08) and 1.51 (1.03, 2.22), respectively [15].
The dietary inflammatory index (DII®) is a literature-derived, population-based dietary index score designed to assess the inflammatory potential of a given individual’s diet [16]. The DII was found to predict changes in C-reactive protein (CRP) in the Seasonal Variation in Blood Cholesterol Study [17, 18]. Subsequently, the DII has been used in several studies from around the world to test the effect of diet-associated inflammation on inflammation markers such as CRP, interleukin (IL)-6, and tumor necrosis factor and (TNF)-α-R2 [18,19,20,21,22,23,24,25]. In the Seasonal Variation of Blood Cholesterol Study, higher DII scores were associated with higher levels of circulating hs-CRP > 3 mg/l (OR 1.08; 95% CI 1.01, 1.16, P = 0.035 for the 24HR subset; and OR 1.10; 95% CI 1.02, 119, P = 0·015 for the 7-Day Dietary Recall) [18]; in the Women’s Health Initiative, the DII was associated with four inflammation biomarkers with beta estimates comparing the highest with lowest DII quintiles as follows: interleukin-6: 1.26 (1.15–1.38), Ptrend < 0.0001; tumor necrosis factor alpha receptor 2: 81.43 (19.15–143.71), Ptrend = 0.004; dichotomized hs-CRP (odds ratio for higher vs lower hs-CRP): 1.30 (0.97–1.67), Ptrend = 0.34; and the combined inflammatory biomarker score: 0.26 (0.12–0.40), Ptrend = 0.0001 [19]. The DII has been associated with various cancers, including colorectal [26, 27], pancreatic [28], hepatocellular [29], and others [30,31,32]. DII scores in relation to risk of lung cancer have been examined in Western populations before [33, 34], but never in an Asian population, which has distinct dietary patterns.
Using data from the Singapore Chinese Health Study (SCHS), we examined the association between the DII and risk of developing lung cancer. Our working hypothesis is that increasing inflammatory potential of diet is positively associated with lung cancer. We further hypothesized that a pro-inflammatory DII score has a stronger effect on lung cancer in ever smokers than never smokers because the former have been shown to be sensitized for inflammatory response by smoking [35], and smoking has been shown to be associated with a broad range of alterations in systemic immune and inflammation marker levels among older, long-term smokers [8]. Also, higher levels of circulating inflammatory markers have been associated with greater risk of lung cancer among smokers compared to risk estimates among non-smokers [36]. Additionally, chronic inflammation is a well-known risk factor for squamous cell cancers [37] and the DII has been shown to be associated with risk of several squamous cell cancers of the upper aerodigestive tract including esophageal squamous cell cancer [38, 39], nasopharyngeal cancer [40] and oral cancers [41]. We hypothesized that DII will be strongly associated with squamous cell cancer of the lung.
Materials and methods
Study population
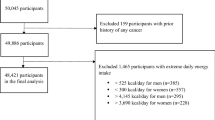
This analysis is based on the Singapore Chinese Health Study (SCHS), a population-based cohort study that enrolled 35,303 Chinese women and 27,954 Chinese men aged 45–74 years at the time of enrollment between April 1993 and December 1998. The participants were recruited from among Hokkiens and Cantonese, the two major dialect groups of Chinese in Singapore, who mostly originated from Fujian and Guangdong provinces in Southern China, respectively. The Institutional Review Boards at the National University of Singapore and the University of Pittsburgh approved the study and informed consent was obtained from each study participant. Further details of SCHS design have been published previously [42].
At recruitment, an in-person interview was conducted at the home of the subject by a trained interviewer using a structured questionnaire. The questionnaire requested information on demographics, body weight and height, lifetime use of tobacco (cigarettes and water pipe), current physical activity, menstrual/reproductive history (women only), occupational exposure, medical history, and family history of cancer. Information on current diet was assessed via a 165-item semi-quantitative food frequency questionnaire (FFQ) that had been validated against a series of 24-h dietary recall interviews [42] and selected biomarker studies [43] conducted on random subsets of cohort participants. The questionnaire also asked for intake of each dietary supplement with frequency and dosage: vitamins A, C and E, beta-carotene, calcium, selenium and zinc. Body mass index was calculated as the current weight in kilograms divided by the square of height in meters.
Dietary assessment
The FFQ was administered during the baseline interview. For each of food and fruit items, the respondents were required to select from eight frequency categories (ranging from “never or hardly ever” to “two or more times a day”) and were provided with photographs to choose from three portion sizes (small, medium and large). Nine frequency categories (from “never or hardly ever” to “6 or more times a day”) without portion size categories were used for non-alcoholic beverages while 6 frequency categories (the same as food items) and 4 portion sizes (approximately one drink per portion) for each of 4 alcoholic beverage types. Subsequently, the FFQ had been validated by two 24-h recalls, one weekday and one weekend, among 810 participants who were randomly selected from the cohort participants. Results showed that correlation coefficients for most calorie-adjusted nutrients ranged from 0.30 to 0.70 [42].
Dietary inflammatory index (DII®)
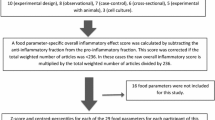
FFQ-derived dietary data were used to calculate DII scores for each cohort participant. Briefly, the self-reported intake for each food parameter included in the DII was linked to a database that contained estimates of the mean and the standard deviation for a global reference database representing 11 countries from around the world. A z score for each parameter was then computed by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing”, this value was then converted to a centered (on zero) proportion (by taking the proportion ranking of the z score, multiplying by 2 and subtracting 1). The centered proportion score for each food parameter for each subject was then multiplied by the corresponding food parameter effect score to obtain a food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for each subject. SCHS had data on 36 of the 45 food parameters studied for DII development [16]. These included energy, carbohydrate, protein, fat, alcohol, fibre, cholesterol, saturated fatty acid, mono- and poly-unsaturated fatty acid, omega 3 fat (eicosapentaenoic acid, docosahexaenoic acid from fish and other seafood; and alpha-linolenic acid from grains, cooking oils; and legumes and soy), omega 6 fat (linoleic acid and arachidonic acid from cooking fat/oil, grain products and legumes), niacin, thiamin, riboflavin, vitamin B12,vitamin B6, iron, magnesium, zinc, selenium, vitamin A, vitamin C, vitamin E, folic acid, beta carotene, garlic, onion, caffeine, tea, anthocyanidins, flavan-3-ol, flavones, flavonol, flavanone and isoflavones. Out of these 36 food parameters, 7 nutrients (zinc, selenium, vitamin A, C, E, folic acid and beta-carotene) were derived from both food items and supplements. The 9 missing food parameters were trans-fat, vitamin D, ginger, turmeric, saffron, eugenol, thyme oregano, pepper and rosemary. Steps involved in calculating the DII score are described in Fig. 1. In addition, energy-adjusted DII (E-DII) was calculated wherein all the food parameters were adjusted for energy using the residual approach [44]. E-DII did not include total energy intake as a separate component.
Cohort follow-up and case ascertainment
Identification of incident lung cancer cases among cohort members was accomplished by annual record linkage of all cohort participants with the database of the population-based Singapore Cancer Registry (C34 of the International Classification of Diseases, ICD-O-2). The vital status was determined by a similar record linkage analysis with the Singapore Registry of Births and Deaths. To date, only 47 (< 0.1%) cohort participants were known to be lost to follow-up due to migration out of Singapore. As of December 31, 2015, 2,008 cohort participants who were free of cancer at baseline developed lung cancer.
Statistical analyses
Individuals who had cancer at baseline identified by either self-report or via linkage with the nationwide Singapore Cancer Registry (n = 1936) were excluded from the analysis. In addition, to avoid potential impact of non-symptomatic underlying disease on dietary habit, we excluded 157 lung cancer cases that occurred within the first 2 years after baseline interview in our main analysis. Consequently, a total of 60,232 participants, including 1851 lung cancer cases, were included for this analysis.
DII quintile cut points (Q) were obtained from the entire eligible sample; the lowest quintile (Q1) being the referent category. The χ2 test and the t test were used to compare the distributions of selected variables across DII quintile groups.
Person-years of follow-up were calculated from the date of baseline interview to the date of diagnosis of lung cancer, death, migration, or 31 December 2015, whichever occurred first. Cox proportional hazard regression modeling was employed for calculation of hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were used to assess the risk of lung cancer associated with higher quintiles of DII scores compared with the lowest quintile. Tests for linear trends were conducted by treating the quintiles of DII as an ordinal variable in the Cox model. To test PH assumption, a time-varying covariate, an interaction between DII and the event time was included Cox regression model and found to be valid. In all analyses, we adjusted for smoking by including covariate terms for average number of cigarettes smoked per day (never smokers, 1–12, 13–22, or 23+), number of years of smoking (never smokers, 1–19, 20–39, or 40+), and number of years since last smoked for quitters (< 1, 1–4, 5–19 or 20+). Other potential confounders included in the multivariable Cox proportional hazards models were age at baseline, sex, dialect group (Hokkiens or Cantonese), level of education (no formal education, primary school, secondary or higher education), body mass index (< 20, 20–< 24, 24–< 28, or ≥ 28 kg/m2), physical activity and total energy intake.
Stratified analyses were carried out by smoking status, sex and histological subtype. The potential modifying effect of smoking and sex on the association between DII score and lung cancer risk was evaluated by including a product term between DII score and smoking status and/or sex in the multivariable Cox regression models. We also conducted sensitivity analysis for the association between DII and lung cancer risk by including lung cancer cases and associated person-years of observations occurring within the first 2 years post-enrolment into the cohort. The DII was coded as ordinal values (1, 2, 3, 4, and 5) of quintile variables to assess the linear trends of the DII-lung cancer association. In addition, parallel analyses were conducted between E-DII and lung cancer risk. Statistical analyses were carried out using SAS® software version 9.3 (SAS Institute, Cary, NC, USA). All P values reported are two sided, and those values that were less than 0.05 were considered statistically significant.
Results
Of the 1851 patients diagnosed with lung cancer 2 or more years after baseline interview, 1599 (86%) were histopathologically confirmed, while the remaining 252 (14%) were diagnosed based on radiography or computer-assisted tomography evidence. Among the histopathologically confirmed cases, 734 (45.9%) were adenocarcinomas, 336 (21.0%) were squamous cell cancers, 169 (10.6%) were small cell cancers, and 360 (22.5%) were other cell types. The mean age (standard deviation) at cancer diagnosis of all case patients was 72.5 (7.8) years. The median time interval between baseline interview and lung cancer diagnosis was 12.1 years, ranging from 2 to 22.4 years.
The mean DII score and the corresponding standard deviation (SD) in this study are 0.57 ± 2.32. Baseline characteristics of subjects across quintiles of DII are provided in Table 1. Subjects in the highest quintile (representing the most pro-inflammatory diet) were older, more likely to be females, current smokers, non-drinkers, have no formal education, lower BMI, fewer number of cigarettes/day, lower energy intake, lower physical activity and more years of smoking.
Table 2 shows HRs of lung cancer per quintiles of DII from the two multivariable model fits: Model 1, with adjustment for age, dialect group, sex, interview year, education, body mass index, physical activity and total energy intake, and Model 2, with additional adjustment for number of cigarettes per day, number of years of smoking and number of year since quitting smoking for former smokers. Results from model 1 showed that participants in the highest quintile of DII had a statistically significant 50% increased risk of lung cancer compared to those in the lowest quintile (HR 1.50; 95% CI 1.25–1.80, P trend < 0.001). Further adjustment for smoking (model 2) attenuated the DII-lung cancer risk association, which became statistically non-significant (HR 1.13; 95% CI 0.94–1.35; P trend=0.24). Additional adjustment with intake of dietary supplements (yes or no) did not significantly change the results (data not shown).
Table 3 shows multivariable-adjusted HRs of lung cancer in relation to DII quintile in never, former and current smokers. Among current smokers, the highest quintile of DII was associated with a statistically significant 44% increased risk of developing lung cancer compared to their counterparts in the lowest quintile (HRQ5vsQ1 = 1.44; 95% CI 1.11–1.86; Ptrend = 0.03) whereas no association in former smokers and a statistically borderline significant inverse association in never smokers (HRQ5vsQ1 = 0.74; 95% CI 0.53–1.03; P trend = 0.07) were observed. The interaction between DII and smoking status (current, former, and never smokers) on lung cancer risk was statistically significant (P value for interaction = 0.003).
We also conducted analyses for men and women separately (Table 4). The risk was higher among men (HRQ5vsQ1 = 1.29; 95% CI 1.03–1.61; Ptrend = 0.06). Further stratification by smoking status showed that among men who are smokers, higher DII scores were strongly associated with increased risk of lung cancer (HRQ5vsQ1 = 1.37; 95% CI 1.07–1.77; P trend = 0.03). However, there was no statistically significant difference in the DII-lung cancer risk associations between men and women.
We also conducted analyses by histological subtype of lung cancer cases (Table 5). The risk estimates for lung cancer subtypes varied across different DII levels, but there was no statistically significant association for any of the four subtype groups of lung cancer examined because of small numbers of cancer cases in each of the four cancer subtypes. Significantly elevated HRs for lung squamous cell carcinoma were associated with quintiles 2, 3 and 4 of DII, but no significant trend (P trend = 0.41). Further stratification by smoking status did not materially change the association between DII score levels and the risk of lung cancer subtypes (data not shown). Parallel analyses for energy-adjusted DII using the residual model yielded similar results for overall and subgroups of study participants (Supplementary Tables 1, 2, 3 and 4).
Discussion
In this prospective study, we did not observe a significant association between DII and lung cancer risk in the overall population. However, we did observe that a pro-inflammatory diet, as shown by higher DII scores, was associated with a statistically significantly elevated risk of lung cancer among current male smokers, and among men who had a history of smoking. This finding is consistent with studies showing that biomarkers of inflammation were prospectively associated with lung cancer risk [5, 6, 45].
While there have been several studies assessing the association of the DII and incidence and mortality of various cancer sites, there are only two other studies on lung cancer. One was conducted in Italy as part of the Continuous Observation of Smoking Subjects (COSMOS) study of lung cancer [34]. The COSMOS cohort members were all heavy smokers screened annually using CT scanning. Univariate analysis showed that DII scores were positively associated with risk of lung cancer development. After adjustment for age, sex, smoking intensity and duration, years of smoking cessation and asbestos exposure the HR was only slightly changed. The Melbourne Collaborative Cohort study in Australia reported a statistically significant positive association between DII score and risk of lung cancer in current smokers [HRQ4vsQ1 = 1.70 (1.02, 2.82); Ptrend = 0.008] [33]. Like these results, in our study, we observed significant findings between DII and lung cancer only among current smokers. The present study was the first prospective investigation for DII score in relation to risk of developing lung cancer in an Asian population. Although their dietary and cultural habits are distinct from those of Italian and Australian populations, the findings on DII and lung cancer risk are consistent with those of previous studies, suggesting a potential common biological mechanism for the observed relation between DII and lung cancer among smokers. We report higher prevalence of current smoking among participants in the highest quintile of DII and BMI has been shown to be low among smokers [46], hence this could explain the reason for observing an inverse trend of BMI across quintiles of DII.
Even though we observed a strong association between the DII and lung squamous cell cancer, we did not observe a significant trend. Additionally, we did not observe a significant association among women; this could be due to fewer numbers (8.7%) of Chinese women who ever smoked cigarettes, resulting in lower statistical power for the present analysis.
One of the possible mechanisms for this positive association of the DII with lung cancer might be through the excess production of proto-oncogenic cytokines such as IL-6, IL-8, platelet-derived growth factor, and vascular endothelial growth factor in the tumor microenvironment, which are then responsible for carcinogenic activities like anti-apoptosis, tumor angiogenesis and metastasis [47]. Hypoxia, a common state in inflamed tissues [48, 49], is associated with DNA damage, induces tumorigenic factors [50]. Finally, tissue vasculature is a vital part of its microenvironment, supplying oxygen, nutrients, and growth factors to rapidly dividing cells and providing a mechanism for metastatic spread [51]. Smoking, by itself, is a major risk factor for lung cancer [52] and it creates a pro-inflammatory state [53]; hence, as we have observed in this study and in the Australian study, the risk for lung cancer among smokers becomes accentuated when combined with a pro-inflammatory diet. There also is a possibility of residual confounding from smoking on the DII and lung cancer risk, as it has been shown in previous studies that smokers, especially male smokers, consume less healthy diets that are rich in pro-inflammatory components such as sugar and saturated fat and low in anti-inflammatory components such as vegetables and fruits, than non-smokers [54]. In a recent study conducted in Luxembourg, smoking status was shown to be inversely associated with overall diet quality as measured through the Diet Quality Index-International (DQI-I), Recommendation Compliance Index (RCI), Dietary Approach to Stop Hypertension (DASH) score, Energy Density Score (EDS), Dietary Diversity Score (DDS), Recommended Food Score (RFS), non-Recommended Food Score (non-RFS), and the DII [55]. We also observed stronger association between DII scores and lung cancer risk among males. Previously, it has been shown that women consume healthier diets rich in fruits and vegetables compared to men [56]. So, there could be other factors that influence development of lung cancer in women. This needs further exploration.
Another interesting finding from our study is the null association or suggestion of an inverse association between DII and lung cancer among never smokers. The exact reason or mechanism for this observation is not clear. Because there is little information available on the descriptive epidemiology of lung cancer in never smokers, there could be other etiological factors such as exposure to radon, and other indoor air pollutants and polycyclic aromatic hydrocarbons that may play an important role in the development of lung cancer [57, 58] and some level of “inflammation” may countervail processes involved in lung carcinogenesis [59]. This aspect must be studied in more detail in future studies.
The DII is different from other dietary indices that have been used to describe patterns of intake, virtually all of which fall into three main categories: (1) those derived from specific dietary prescriptions based on some external standard (e.g., Healthy Eating Index), which was derived from the adherence to the US Dietary guidelines [60]; (2) those derived from findings within particular study populations (e.g., computing a pattern using principal component analysis [61]); (3) those that link to cultural patterns of dietary intake (e.g., the Mediterranean diet) [62].
Previous studies have been conducted to examine various other dietary components, patterns and indices in relation to lung cancer [63,64,65]. A recently conducted systematic review and meta-analyses including eight observational studies suggested that a healthy dietary pattern characterized by a high intake of vegetables, fruits, white meat, fish and whole-grain bread and a low intake of red meat, fat and refined grains is associated with a lower lung cancer risk [63]. Fruits; green leafy vegetables containing high levels of important nutrients including vitamins A, B1 and B2, flavonoids, and zinc; and fish and nuts high in omega-3 fatty acids are all anti-inflammatory components of DII and, therefore, help to reduce DII scores [16]. On the other hand, red meat, high-fat dairy and refined grains are rich in proteins, carbohydrates, saturated fats which are the pro-inflammatory components of the DII. Thus, the consumption of more pro-inflammatory, less anti-inflammatory dietary components leads to increased DII score [16], which has been found to be associated with increased risk of lung cancer [66].
Strengths of this study include minimal loss to follow-up, presence of a large number of lung cancer cases, and the ability to control for important potential confounders that were measured as part of study protocol. The main weaknesses relate to the dietary assessment based on a single FFQ at baseline, which is known to suffer from measurement error; though not differentially related to cancer diagnosis because this was a prospective study that also excluded lung cancer cases diagnosed within 2 years of baseline. Additionally, it is possible that people could change their dietary habits over time. However, we would like to note that previous studies have reported that dietary pattern classification is moderately stable over long periods of time during adulthood [67]. However, because the association was strong for older subjects it should be considered that both the conditions related to aging and those related to diet can mutually interact. Although we included 36 of the 45 items for the computation of DII score, the remaining nine items were rarely consumed in our study population. However, any error resulting from imprecision would most likely be nondifferential, thus leading to underestimation of the association between the DII and lung cancer risk. Other covariates also were self-reported and may have resulted in some misclassification and residual confounding. Additionally, the application of an index developed specifically to determine the inflammatory potential of diet cannot be easily translated into a different dietary pattern (e.g., including different foods, ingredients, preparations). However, in the past, the DII has been shown to be moderately strongly correlated with other indices and dietary patterns like Alternate Healthy Eating Index [68, 69] and Mediterranean Diet Score [70, 71].
In conclusion, our findings support the hypothesis that consumption of a pro-inflammatory diet as shown by higher DII scores increases the risk of lung cancer among current smokers. Public health measures should be adopted to promote consumption of a healthy, anti-inflammatory diet to reduce the risk of lung cancer, especially in smokers.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Singapore Cancer Registry, Interim Annual Report (Trends in Cancer Incidence in Singapore 2010–2014)
Lim WY, Tan CS, Loy EY, Omkar Prasad R, Seow A, Chia KS (2014) Lung cancer incidence in Singapore: ethnic and gender differences. Lung Cancer 84(1):23–30. https://doi.org/10.1016/j.lungcan.2014.01.007
Orozco-Morales M, Soca-Chafre G, Barrios-Bernal P, Hernandez-Pedro N, Arrieta O (2016) Interplay between cellular and molecular inflammatory mediators in lung cancer. Mediators Inflamm 2016:3494608. https://doi.org/10.1155/2016/3494608
Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, Chanock SJ, Goedert JJ, Engels EA (2010) C-reactive protein and risk of lung cancer. J Clin Oncol 28(16):2719–2726. https://doi.org/10.1200/JCO.2009.27.0454
Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK (2013) Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105(24):1871–1880. https://doi.org/10.1093/jnci/djt309
Monographs on the evaluation of carcinogenic risks to humans (2004) International Agency for Research on Cancer (IARC) volume 83 (Tobacco smoke and involuntary smoking, Lyon, France)
Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, Kitahara CM, Furr M, Li Y, Kemp TJ, Goedert JJ, Chang CM, Engels EA, Caporaso NE, Pinto LA, Hildesheim A, Chaturvedi AK (2014) Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju294
Arnson Y, Shoenfeld Y, Amital H (2010) Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 34(3):J258–J265. https://doi.org/10.1016/j.jaut.2009.12.003
de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M (2011) A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia 54(11):2755–2767
Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA (2011) Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 8(10):2868–2875
Wang M, Qin S, Zhang T, Song X, Zhang S (2015) The effect of fruit and vegetable intake on the development of lung cancer: a meta-analysis of 32 publications and 20,414 cases. Eur J Clin Nutr 69(11):1184–1192. https://doi.org/10.1038/ejcn.2015.64
Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E (2006) Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 145(1):1–11
Johansson-Persson A, Ulmius M, Cloetens L, Karhu T, Herzig KH, Onning G (2013) A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr 7:7
Butler LM, Montague JA, Koh WP, Wang R, Yu MC, Yuan JM (2013) Fried meat intake is a risk factor for lung adenocarcinoma in a prospective cohort of Chinese men and women in Singapore. Carcinogenesis 34(8):1794–1799. https://doi.org/10.1093/carcin/bgt113
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696. https://doi.org/10.1017/S1368980013002115
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR (2009) A new dietary inflammatory index predicts interval changes in high-sensitivity c-reactive protein. J Nutr 139(12):2365–2372
Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR (2014) A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 17(8):1825–1833. https://doi.org/10.1017/S1368980013002565
Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR (2015) Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol 25(6):398–405. https://doi.org/10.1016/j.annepidem.2015.03.009
Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, Michels N, Arouca A, Gonzalez-Gil E, Frederic G, Gonzalez-Gross M, Castillo MJ, Manios Y, Kersting M, Gunter MJ, De Henauw S, Antonios K, Widhalm K, Molnar D, Moreno L, Huybrechts I (2017) Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Res. https://doi.org/10.1002/mnfr.201600707
Shivappa N, Wirth MD, Hurley TG, Hebert JR (2017) Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600630
Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Davoodi SH, Sadeghi M (2017) Association between Maternal Dietary Inflammatory Index (DII) and Abortion in Iranian Women and Validation of DII with serum concentration of inflammatory factors: case–control study. Appl Physiol Nutr Metab 42 (5):511–516. https://doi.org/10.1139/apnm-2016-0274
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR (2015) Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy J Brit Soc Allergy Clin Immunol 45(1):177–183. https://doi.org/10.1111/cea.12323
Wirth MD, Shivappa N, Davis L, Hurley TG, Ortaglia A, Drayton R, Blair SN, Hebert JR (2017) Construct validation of the dietary inflammatory index among African Americans. J Nutr Health Aging 21(5):487–491. https://doi.org/10.1007/s12603-016-0775-1
Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR (2014) Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med Am Coll Occup Environ Med 56(9):986–989. https://doi.org/10.1097/JOM.0000000000000213
Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, Hebert JR (2015) Inflammatory potential of diet and risk of colorectal cancer: a case–control study from Italy. Br J Nutr 114(1):152–158. https://doi.org/10.1017/S0007114515001828
Shivappa N, Godos J, Hébert J, Wirth M, Piuri G, Speciani A, Grosso G (2017) Dietary Inflammatory index and colorectal cancer risk—a meta-analysis. Nutrients 9(9):1043
Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR (2014) Dietary inflammatory index and risk of pancreatic cancer in an Italian case–control study. Br J Nutr. https://doi.org/10.1017/S0007114514003626
Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La Vecchia C, Serraino D (2016) Inflammatory potential of diet and risk for hepatocellular cancer in a case–control study from Italy. Br J Nutr 115(2):324–331. https://doi.org/10.1017/S0007114515004419
Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hebert JR (2016) Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201600592
Shivappa N, Hébert JR, Rosato V, Rossi M, Libra M, Montella M, Serraino D, La Vecchia C dietary inflammatory index and risk of Bladder Cancer in a Large italian case–control study. Urology. https://doi.org/10.1016/j.urology.2016.09.026
Shivappa N, Hebert JR, Rosato V, Serraino D, La Vecchia C (2016) Inflammatory potential of diet and risk of laryngeal cancer in a case–control study from Italy. Cancer Causes Control 27(8):1027–1034. https://doi.org/10.1007/s10552-016-0781-y
Hodge AM, Bassett JK, Shivappa N, Hebert JR, English DR, Giles GG, Severi G (2016) Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control 27(7):907–917. https://doi.org/10.1007/s10552-016-0770-1
Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P (2016) Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr 55(3):1069–1079. https://doi.org/10.1007/s00394-015-0920-3
Robbins CS, Pouladi MA, Fattouh R, Dawe DE, Vujicic N, Richards CD, Jordana M, Inman MD, Stampfli MR (2005) Mainstream cigarette smoke exposure attenuates airway immune inflammatory responses to surrogate and common environmental allergens in mice, despite evidence of increased systemic sensitization. J Immunol 175(5):2834–2842
Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, Kemp TJ, Caporaso NE, Pinto LA, Chaturvedi AK (2015) Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv199
Gajanan G, Bohra C, Nanjappa S, Greene JN (2016) Chronic infections leading to squamous cell carcinoma from chronic inflammation: a case report and review of all causes. Infect Dis Clin Pract 24(3):133–137. https://doi.org/10.1097/ipc.0000000000000349
Shivappa N, Hebert JR, Rashidkhani B (2015) Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Iran. Nutr Cancer 67(8):1253–1259. https://doi.org/10.1080/01635581.2015.1082108
Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR (2015) Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Italy. Cancer Causes Control 26(10):1439–1447. https://doi.org/10.1007/s10552-015-0636-y
Shivappa N, Hebert JR, Zucchetto A, Montella M, Libra M, Garavello W, Rossi M, La Vecchia C, Serraino D (2016) Increased risk of nasopharyngeal carcinoma with increasing levels of diet-associated inflammation in an Italian case–control study. Nutr Cancer 68(7):1123–1130. https://doi.org/10.1080/01635581.2016.1216137
Shivappa N, Hebert JR, Rosato V, Garavello W, Serraino D, La Vecchia C (2017) Inflammatory potential of diet and risk of oral and pharyngeal cancer in a large case–control study from Italy. Int J Cancer 141(3):471–479. https://doi.org/10.1002/ijc.30711
Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC (2001) Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 39(2):187–195. https://doi.org/10.1207/S15327914nc392_5
Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC (1998) Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomark Prev 7(9):775–781
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4 Suppl):1220S–1228S. https://doi.org/10.1093/ajcn/65.4.1220S (discussion 1229S–1231S)
Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC (2011) Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 103(14):1112–1122. https://doi.org/10.1093/jnci/djr216
Plurphanswat N, Rodu B (2014) The association of smoking and demographic characteristics on body mass index and obesity among adults in the US, 1999–2012. BMC Obes 1(1):18. https://doi.org/10.1186/s40608-014-0018-0
Pries R, Wollenberg B (2006) Cytokines in head and neck cancer. Cytokine Growth Factor Rev 17(3):141–146. https://doi.org/10.1016/j.cytogfr.2006.02.001
Milagro FI, Mansego ML, De Miguel C, Martinez JA (2013) Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med 34(4):782–812. https://doi.org/10.1016/j.mam.2012.06.010
Tili E, Michaille JJ, Croce CM (2013) MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev 253:167–184. https://doi.org/10.1111/imr.12050
Fosslien E (2000) Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci 30(1):3–21
Bonomi M, Patsias A, Posner M, Sikora A (2014) The role of inflammation in head and neck cancer. Adv Exp Med Biol 816:107–127. https://doi.org/10.1007/978-3-0348-0837-8_5
Schwartz AG, Cote ML (2016) Epidemiology of lung cancer. Adv Exp Med Biol 893:21–41. https://doi.org/10.1007/978-3-319-24223-1_2
Ohsawa M, Okayama A, Nakamura M, Onoda T, Kato K, Itai K, Yoshida Y, Ogawa A, Kawamura K, Hiramori K (2005) CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long-term smoking cessation in male smokers. Prev Med 41(2):651–656. https://doi.org/10.1016/j.ypmed.2005.02.002
Palaniappan U, Starkey LJ, O’Loughlin J, Gray-Donald K (2001) Fruit and vegetable consumption is lower and saturated fat intake is higher among Canadians reporting smoking. J Nutr 131(7):1952–1958
Alkerwi A, Baydarlioglu B, Sauvageot N, Stranges S, Lemmens P, Shivappa N, Hebert JR (2017) Smoking status is inversely associated with overall diet quality: findings from the ORISCAV-LUX study. Clin Nutr 36(5):1275–1282. https://doi.org/10.1016/j.clnu.2016.08.013
Kiefer Ingrid R, Theres MK (June 2005) Eating and dieting differences in men and women. J Men’s Health Gender 2(2):194–201
Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM (2009) Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 15(18):5626–5645. https://doi.org/10.1158/1078-0432.CCR-09-0376
Yuan JM, Butler LM, Gao YT, Murphy SE, Carmella SG, Wang R, Nelson HH, Hecht SS (2014) Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis 35(2):339–345. https://doi.org/10.1093/carcin/bgt352
Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90:1–50. https://doi.org/10.1016/S0065-2776(06)90001-7
Kennedy ET, Ohls J, Carlson S, Fleming K (1995) The healthy eating index: design and applications. J Am Diet Assoc 95(10):1103–1108
Jackson M, Tulloch-Reid M, Walker S, McFarlane-Anderson N, Bennett F, Francis D, Coard K (2013) Dietary patterns as predictors of prostate cancer in Jamaican men. Nutr Cancer 65(3):367–374
Sjogren P, Becker W, Warensjo E, Olsson E, Byberg L, Gustafsson IB, Karlstrom B, Cederholm T (2010) Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr 92(4):967–974. https://doi.org/10.3945/ajcn.2010.29345
Sun Y, Li Z, Li J, Li Z, Han J (2016) A healthy dietary pattern reduces lung cancer risk: a systematic review and meta-analysis. Nutrients 8(3):134. https://doi.org/10.3390/nu8030134
Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D, Stevens C, Greenwood D, Norat T (2016) Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 27(1):81–96. https://doi.org/10.1093/annonc/mdv381
Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J (2014) Red and processed meat consumption and the risk of lung cancer: a dose–response meta-analysis of 33 published studies. Int J Clin Exp Med 7(6):1542–1553
Kasala ER, Bodduluru LN, Barua CC, Gogoi R (2016) Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother 82:568–577. https://doi.org/10.1016/j.biopha.2016.05.042
Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, Kromhout D, Jacobs DR Jr (2012) Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr 95(3):580–586
Wirth MD, Hebert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, McMahon D, Shook RP, Blair SN (2016) Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res 36(3):214–219. https://doi.org/10.1016/j.nutres.2015.11.009
Shivappa N, Hebert JR, Kivimaki M, Akbaraly T (2017) Alternate Healthy Eating Index 2010, Dietary Inflammatory Index and risk of mortality: results from the Whitehall II cohort study and meta-analysis of previous Dietary Inflammatory Index and mortality studies. Br J Nutr 118(3):210–221. https://doi.org/10.1017/S0007114517001908
Hodge AM, Bassett JK, Dugue PA, Shivappa N, Hebert JR, Milne RL, English DR, Giles GG (2018) Dietary inflammatory index or Mediterranean diet score as risk factors for total and cardiovascular mortality. Nutr Metab Cardiovasc Dis 28(5):461–469
Dugué P-A, Hodge AM, Brinkman MT, Bassett JK, Shivappa N, Hebert JR, Hopper JL, English DR, Milne RL, Giles GG (2016) Association between selected dietary scores and the risk of urothelial cell carcinoma: A prospective cohort study. Int J Cancer 139(6):1251–1260
Acknowledgements
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study. The Singapore Cancer Registry assisted with the identification of cancer outcomes via database linkages. Finally, we acknowledge the founding, long-standing Principal Investigator of the Singapore Chinese Health Study—Mimi C. Yu.
Funding
The Singapore Chinese Health Study was supported by Grants R01 CA144034 and UM1 CA182876 from the United States National Institute of Cancer. This imputation of dietary inflammatory index score was supported by the Italian Foundation for Cancer Research (FIRC). Drs. Shivappa and Hébert were supported by Grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases. W-P Koh is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina to develop computer and smartphone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shivappa, N., Wang, R., Hébert, J.R. et al. Association between inflammatory potential of diet and risk of lung cancer among smokers in a prospective study in Singapore. Eur J Nutr 58, 2755–2766 (2019). https://doi.org/10.1007/s00394-018-1825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1825-8