Abstract
Purpose
To test whether the inflammatory potential of diet, as measured using the dietary inflammatory index (DII), is associated with risk of lung cancer or other respiratory conditions and to compare results obtained with those based on the aMED score, an established dietary index that measures adherence to the traditional Mediterranean diet.
Methods
In 4336 heavy smokers enrolled in a prospective, non-randomized lung cancer screening program, we measured participants’ diets at baseline using a self-administered food frequency questionnaire from which dietary scores were calculated. Cox proportional hazards and logistic regression models were used to assess association between the dietary indices and lung cancer diagnosed during annual screening, and other respiratory outcomes that were recorded at baseline, respectively.
Results
In multivariable analysis, adjusted for baseline lung cancer risk (estimated from age, sex, smoking history, and asbestos exposure) and total energy, both DII and aMED scores were associated with dyspnoea (p trend = 0.046 and 0.02, respectively) and radiological evidence of emphysema (p trend = 0.0002 and 0.02). After mutual adjustment of the two dietary scores, only the association between DII and radiological evidence of emphysema (Q4 vs. Q1, OR 1.30, 95 % CI 1.01–1.67, p trend = 0.012) remained statistically significant. At univariate analysis, both DII and aMED were associated with lung cancer risk, but in fully adjusted multivariate analysis, only the association with aMED remained statistically significant (p trend = 0.04).
Conclusions
Among heavy smokers, a pro-inflammatory diet, as indicated by increasing DII score, is associated with dyspnoea and radiological evidence of emphysema. A traditional Mediterranean diet, which is associated with a lower DII, may lower lung cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, lung cancer is among the most common type of cancer in men and women and the leading cause of cancer death [1]. In the context of the Italian COSMOS (Continuous Observation of Smoking Subjects) study, a screening program for the early diagnosis of lung cancer in high-risk individuals using annual low-dose computed tomography (LD-CT) [2], we previously examined the association between intake of selected nutrients and foods and the alternate Mediterranean diet (aMED) score and lung cancer risk [3, 4].
Research into the role of diet in inflammation and lung cancer suggests that diet represents a complicated set of exposures which often interact, and whose cumulative effect modifies both inflammatory responses and health outcomes [5–9]. In order to further address the effect of diet on inflammation, researchers at the University of South Carolina’s Cancer Prevention and Control Program developed the dietary inflammatory index (DII), which can be used in diverse populations to assess the inflammatory potential of diet assessed by various dietary assessment tools (i.e., 24-h dietary recalls, food frequency questionnaires (FFQs), and food records) [10–12]. To date, validation of the DII has shown its ability to predict serum CRP levels in a large longitudinal epidemiological study [13]. Previously, we observed that shift workers tend to have a pro-inflammatory diet (higher DII scores) compared to their day-working counterparts [14].
Despite that higher DII scores have been linked to known inflammation-related conditions, including colorectal cancer and asthma [11, 12], the DII has not yet been applied to a population with lung cancer and other lung diseases as outcomes. The purpose of this study was to examine the association between the DII and a series of lung conditions for which tobacco smoke-induced inflammation could be an important biological pathway [15]. These include chronic obstructive pulmonary disease (COPD), emphysema, or lung cancer. We also sought to compare the DII with the aMED score, an established dietary index that measures adherence to the traditional Mediterranean diet and has been associated with lower concentrations of inflammatory biomarkers [16].
Our working hypothesis is that a higher DII score (indicating a pro-inflammatory diet) increases risk of developing lung cancer and other lung disorders.
Materials and methods
Study population
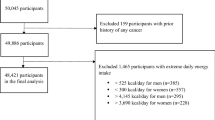
The study is based on participants in the COSMOS study, a non-randomized lung cancer screening trial for the early diagnosis of lung cancer in high-risk individuals. Study details have been published elsewhere [2]. In brief, a total of 5203 asymptomatic volunteers, free of cancer at baseline (except treated non-melanoma skin cancer), aged ≥50 years, who were current smokers or had quit smoking for <10 years and had smoked ≥20 pack-years, were enrolled in the study between 2004 and 2005. All volunteers provided written consent to receive annual LD-CT. The study was approved by the Ethical Committee of the European Institute of Oncology, Milan, Italy.
Dietary assessment
At baseline, the self-administered FFQ developed for the Italian cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Italy) [17] was given to and filled out directly by the participants. Frequency of consumption (per day, week, month, or year) of 188 different food items and beverages representative of the Italian diet was collected to assess average food intake over the preceding year. Data from the FFQs were checked, coded, and computerized using optical reading technology. The average daily quantities of foods and energy consumed by participants were calculated with the Nutrition Analysis of Food Frequency software [18].
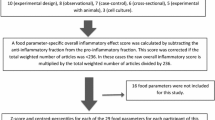
For each individual, FFQ-derived dietary information was used to calculate DII scores for all subjects, using procedures described in detail elsewhere [10, 19]. Briefly, the dietary data for each study participant were first linked to the regionally representative global database that we developed and which provides a robust estimate of a mean and standard deviation for each of the food parameters (i.e., foods, nutrients, and other food components, such as flavonoids) considered in order to derive a z-score, by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing” (a common occurrence with dietary data), this value was then converted to a centered percentile score which was then multiplied by the respective food parameter effect score (derived from a literature review and scoring of 1943 articles) in order to obtain each subject’s food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for every subject in the study. DII = b1 * n1 + b2 * n2…b(n) * n(n), where b refers to the literature-derived inflammatory effects score for each of the evaluable food parameters; n refers to the food parameter-specific centered percentiles, which were derived from the dietary data; and (n) refers to the total number of food parameters that will be available from this study. A positive score indicates a more pro-inflammatory diet, while a negative score reflects a diet that is more anti-inflammatory. Of a possible 45 food parameters, 24 retrieved from the FFQ were used for DII calculation (carbohydrate, protein, fat, alcohol, fiber, cholesterol, saturated fat, monounsaturated fat, polyunsaturated fat, omega-3, omega-6, niacin, thiamin, riboflavin, vitamin-B12, vitamin-B6, iron, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, beta carotene). The methodology is depicted in Fig. 1.
In addition, the aMED score was derived from the Mediterranean diet scale developed by Trichopoulou et al. [20, 21] and was calculated based on the intake of vegetables (excluding potatoes), fruits, nuts, cereals, legumes, fish, red and processed meats, and alcohol [3]. Intakes above the median value reported by all participants received 1 point for vegetables, fruits, nuts, cereals, legumes, and fish; otherwise, they received 0 points. Red and processed meat consumption below the median value received 1 point. Moderate alcohol intake received 1 point. The resulting aMED score values range from 0 (minimal adherence to the Mediterranean diet) to 9 (maximal adherence).
Outcomes
Past medical history, including information on history of pneumonitis, COPD/emphysema, or dyspnoea, was collected during a face-to-face interview with the participants at the time of baseline screening. At baseline, some participants also underwent a spirometry test, and their forced vital capacity (FVC %), forced expiratory flow (FEF %), forced expiratory volume in 1 s (FEV1 %), and FEV1/FVC ratio were recorded. The presence of emphysema on baseline LD-CT screen was visually assessed by the radiologist. Lung cancers were detected during annual LD-CT screening rounds following a detailed diagnostic protocol (at the time data were analyzed, participants were still on intervention and had entered their ninth screening round). Information on eventual interval cancers (i.e., incidental cancers diagnosed between two screening rounds) was obtained through the study follow-up program for those treated in other institution or who had quit attending the screening program. Participants not presenting at their last screening visit were individually contacted. If unreachable, their vital status was obtained from local health statistic office. All lung cancer cases were confirmed by either histology or cytology [2].
Statistical analysis
Pearson’s correlation coefficients were calculated between potential covariates at baseline, average daily intake of selected foods (including beverages), food groups, the aMED, and the DII. The cumulative incidence curves of lung cancer according to quartiles of the DII or categories of the aMED score were plotted using the Kaplan–Meier method. Differences between curves were assessed with the log-rank test. In addition, univariate and multivariable Cox proportional hazards regression and logistic regression models were fit to analyze the association between food intake and lung cancer risk or other baseline respiratory conditions. Multivariable models were adjusted for baseline lung cancer risk probability and total energy intake (both variables set as continuous). Lung cancer risk probability was calculated for each individual based on a recalibration of the model proposed by Bach et al. using information on age (years, continuous), sex, smoking duration (years, continuous), smoking intensity (cig/day, continuous), years of smoking cessation (years, continuous), and asbestos exposure (dichotomous), as reported on the baseline questionnaire [22]. We performed alternative models adjusted for all single-component variables instead of the composite risk score, plus additional variables such as education. Because the results were comparable across various models, we decided to present results from the simplest model. p values for trend were calculated using the quartile median values. Before presenting results from the log-rank tests or from the Cox models, we verified the proportional hazards assumption by introducing a constructed time-dependent variable and testing it for statistical significance. Analysis was performed with the SAS® software version 9.2 (Cary, NC). All p values are two-sided.
Results
A completed FFQ was returned by 4336 (84 %) of the 5203 participants to the COSMOS study, after exclusion of data from 27 participants who reported abnormal dietary values (total caloric intake ≥ |3| standard deviations). Main characteristics of the participants are shown in Table 1. Briefly, participants were mostly males (66.1 %), current smokers (79.8 %), had a median age of 57 years (range 50–84), and had smoked a median of 44 pack-years at enrollment. After a median follow-up of 8.5 years and based on 30,960 person-years of observation, lung cancer was diagnosed in 200 subjects (143 men and 57 women). The detection rate of lung cancer increased significantly with age, increasing pack-years, increasing baseline lung cancer risk probability, and in participants with a low education level. It was similar across body mass index categories and among current smokers and recent quitters (Table 1).
Correlations between potential confounding variables, including average daily intake of selected foods (including beverages), food groups, the aMED score, and the DII score, are shown in Table 2. Age, current smoking status, pack-years of tobacco use, and baseline lung cancer risk probability calculated using a recalibration of the Bach model [22] were directly correlated with the DII score, while total energy, consumption of fruits and vegetables, olive oil, fish, red meat, and the aMED score were inversely correlated. Figure 2 presents a series of box and whiskers plots to depict the correlation between the DII score and the aMED score.
Figure 3 presents the cumulative incidence of screening-detected lung cancer according to the aMED score and quartiles of the DII score. Univariate analysis revealed that both scores are associated with an increased risk of lung cancer. In particular, the DII score is associated with a 64 % increased risk (Q4 vs. Q1, OR 1.64, 95 % CI 1.10–2.44, p trend = 0.02) of lung cancer; however, the association lost statistical significance after adjustment for baseline lung cancer risk probability (data not shown) or after adjustment for baseline lung cancer risk probability and total energy. Conversely, the association with the aMED score remained statistically significant in multivariable analysis, even after adjusting for the DII score. Compared to participants with a low adherence to the Mediterranean diet (aMED scores 0–1), those with a strong adherence (aMED scores 8–9) had an 80 % lower risk of being diagnosed with lung cancer at screening HR 0.20 (95 % CI 0.04–0.90).
Cumulative incidence of lung cancers detected through repeated annual screening LD-CTs according to the alternate Mediterranean diet (aMED) score and the dietary inflammatory index (DII) score, COSMOS study, 2004–2005. Hazards ratios (HRs) and 95 % confidence intervals (CIs) obtained from multivariable Cox proportional hazards regression model adjusted for (1) baseline risk probability (based on age, sex, smoking duration, smoking intensity, years of smoking cessation, and asbestos exposure) and total energy, and (2) aMED and DII scores mutual adjusted. The number of participants at risk (on screening) at baseline, at 2nd, 3rd, 4th, 5th, 6th, 7th, 8th, and 9th year were, respectively, 4336, 4249, 4061, 3865, 3640, 3413, 3219, 3028, 2727, and 883
Table 3 provides information on the association between the DII score and past medical history, respiratory symptoms, respiratory function, and radiological findings at baseline screening LD-CT. Five hundred and fifty-five participants (12.8 %) reported a past history of pneumonitis at baseline visit, 661 (15.2 %) reported suffering from COPD or emphysema, 1387 (32.0 %) from dyspnoea, and 1721 (39.7 %) had radiological evidence of emphysema at baseline screening LD-CT. Five hundred and seventy-one (28.4 %) of the 2013 participants who had a lung function test done had a forced vital capacity <80 %.
Univariate analysis revealed that the DII score was inversely associated with past history of pneumonitis and positively associated with a history of COPD, dyspnoea, reduced respiratory function (FVC < 80 %), and radiological evidence of emphysema. In multivariable analysis, adjusted for baseline lung cancer risk (estimated from age, sex, smoking history, and asbestos exposure) and total energy, only the association with dyspnoea (Q4 vs. Q1, OR 1.30, 95 % CI 1.03–1.64, p trend = 0.046) and with radiological evidence of emphysema (Q4 vs. Q1, OR 1.41, 95 % CI 1.13–1.75, p trend = 0.0002) remained statistically significant (Table 4). After further adjustment for the aMED score, only the association with radiological evidence of emphysema (Q4 vs. Q1, OR 1.30, 95 % CI 1.01–1.67, p trend = 0.01) remained statistically significant. In contrast, in multivariable-adjusted analyses accounting for baseline lung cancer risk and total energy, aMED score was significantly associated with dyspnoea (p trend = 0.02) and with radiological evidence of emphysema (p trend = 0.02). However, both associations disappeared after further adjustment for DII score (Table 4).
Discussion
In the current study, we observed a positive association between a pro-inflammatory diet, as evidenced by an increasing DII score, and dyspnoea and radiological evidence of emphysema. Compared to subjects in quartile 1, those in quartile 4 were 30 % more likely to have dyspnoea and 41 % more likely to have radiological emphysema. We also observed an association with lung cancer (64 % increase risk) and COPD (27 % increased risk) after univariate analyses. Although we did not observe a significant association with lung cancer and COPD after multivariable analyses, the results were suggestive of a positive association. With longer follow-up or a larger sample size, the results might have reached statistical significance. We also observed an inverse association between DII and pneumonitis, in univariate analysis only. This could be due to chance, as there are multiple causes for pneumonitis, including infections; thus, diet may not play a major role in determining this particular outcome. Finally, we showed that the DII score and the aMED score were strongly correlated, but in multivariable analysis, DII was stronger than aMED to predict radiological evidence of emphysema, while aMED more strongly predicted lung cancer.
This is the first study to explore the association between DII and lung cancer and other lung disorders in a cohort of heavy smokers enrolled in a lung cancer screening trial. Previous findings from this study showed a protective effect of a “vitamins and fiber” pattern score for lung cancer [4], whereas red meat consumption was associated with increased risk [3]. In this study, the DII was inversely correlated with red meat (ρ = −0.26), this could be due to the fact that DII takes into account diet as a whole; so, people with high red meat consumption might be consuming large amounts of other anti-inflammatory dietary components such as spices, olive oil, and vegetables. In fact, we did observe a strong inverse correlation between DII and olive oil (ρ = −0.67), and fruits and vegetables (ρ = −0.77). Red meat consumption was not associated with lung cancer risk, while vegetable consumption was found to be protective against lung cancer in a cohort study conducted in Europe [23, 24]. In contrast, results from a recent meta-analysis suggest an increased risk of lung cancer with high intake of red meat [25, 26], whereas white meat consumption showed an inverse association with lung cancer among non-smokers in a large case–control study conducted in Singapore [27].
We also observed strong inverse correlation between DII and aMED scores, which is along expected lines because a higher DII score indicates a pro-inflammatory or an unhealthier eating pattern, whereas for aMED score a higher score indicates a healthier diet [28]. The fact that the aMED score better predicted lung cancer than the DII score and that the DII score performed better than the aMED score to identify those with radiological evidence of emphysema highlights potential distinct features of these two “healthy” dietary indices and possibly suggests different mechanisms of action. The DII score has previously been shown to be associated with CRP and interleukin-6 levels [9, 13], which are recognized systemic inflammatory biomarkers of chronic obstructive pulmonary disease [29]. The aMED score may define a broader “healthy” diet characterized by other properties such as increased antioxidant potential [30].
Previous studies have shown poor diet to be associated with increased risk of dyspnoea, emphysema, and other lung disorders [31]. Of interest, di Giuseppe et al. [32] examined associations between dietary total antioxidant capacity (TAC) and pulmonary function in an Italian healthy population. They found a positive association of TAC with FEV1 and FVC among women (Q5 vs. Q1), which was more pronounced in premenopausal never smokers. TAC has an effect opposite to that of the DII and probably protects the lungs from oxidative stress due to the higher concentrations of bioactive compounds with antioxidant properties. Previously, the DII has been shown to be associated with asthma and lung function tests [11]. A possible mechanism of diet’s action may be through its effect on the production of inflammatory cytokines, including bronchoconstrictive leukotrienes in lung tissue. Previously, omega 3 and omega 6 fatty acids consumption has been shown to reduce the production of these cytokines [33]. The inflammatory components that have been shown to be involved in the development of the lung cancer include a variety of cytokines, chemokines, and cytotoxic mediators such as reactive oxygen species (ROS), metalloproteinases, interleukins, and interferons [34]. Most of these inflammatory markers are increased by tobacco consumption [35], and diet has previously been shown to have an effect on these inflammatory components [9, 36].
A major strength of our study is its reliance on a well-defined population composed of asymptomatic heavy smokers participating to a lung cancer screening program, for whom individualized lung cancer risk probability has been calculated [22]. Our noninvasive screening protocol based on annual LD-CT, PET and evaluation of nodule doubling time, and the close follow-up of participants also ensured steady early-stage lung cancers detection over the study period [2]. A study limitation is the use of a single baseline FFQ to assess diet over the entire study period. Because participants were repeatedly advised to stop smoking, they also may have modified their diets following general health recommendations. FFQs also are subject to measurement errors that may have affected the assessment of the DII score [37–41]. It must be noted, however, that measurement biases are very culturally specific; so, factors such as social desirability that have been observed in North American populations may not exert much of an effect in this Italian population. Another limitation relates to the potential for recall bias for the outcomes that were ascertained at baseline interview, e.g., history of pneumonitis, COPD, or dyspnoea. Finally, despite the large size of the screened cohort, the number of lung cancers diagnosed during the study period was relatively small, conferring limited statistical power to the study. We also tested the association between DII and aMED scores with multiple potential outcomes, and some of the positive associations found might have been due to chance. In contrast, multivariable models may have suffered from overadjustment due to the high correlation between the two dietary scores. The disappearance of a significant association between the DII and lung cancer may be due simply to the fact that much of what drives the DII toward lower values in this population reflects adherence to the Mediterranean dietary prescription.
In conclusion, our study suggests that, among heavy smokers, a pro-inflammatory diet, as shown by increasing DII score, is associated with reduced dyspnoea, radiological evidence of emphysema, while a traditional Mediterranean diet, which is associated with a lower DII, lowers the risk of developing lung cancer. Hence, targeting an improvement in DII may be added to the list of general health recommendations for heavy smokers.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Veronesi G, Bellomi M, Mulshine JL, Pelosi G, Scanagatta P, Paganelli G, Maisonneuve P, Preda L, Leo F, Bertolotti R, Solli P, Spaggiari L (2008) Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer 61(3):340–349
Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G (2013) Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol 24(10):2606–2611
Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G (2013) Nutrient intake and nutrient patterns and risk of lung cancer among heavy smokers: results from the COSMOS screening study with annual low-dose CT. Eur J Epidemiol 28(6):503–511
Ziegler RG, Mayne ST, Swanson CA (1996) Nutrition and lung cancer. Cancer Causes Control 7(1):157–177
Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA (2004) Environmental and chemical carcinogenesis. Semin Cancer Biol 14(6):473–486
Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK (2013) Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105(24):1871–1880
Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC (2011) Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 103(14):1112–1122
Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I (2015) Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr 2:1–7
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17(8):1689–1696
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR (2015) Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy 45(1):177–183
Shivappa N, Prizment AE, Blair CK, Jacobs DR Jr, Steck SE, Hebert JR (2014) Dietary inflammatory index (DII) and risk of colorectal cancer in Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 23(11):2383–2392
Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hébert JR (2014) A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 17(8):1825–1833
Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hébert JR (2014) Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. J Occup Environ Med 56(2):145–148
Milara J, Cortijo J (2012) Tobacco, inflammation, and respiratory tract cancer. Curr Pharm Des 18:3901–3938
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82:163–173
Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F (1997) Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 26(Suppl 1):S152–S160
Pala V, Sieri S, Palli D, Salvini S, Berrino F, Bellegotti M, Frasca G, Tumino R, Sacerdote C, Fiorini L, Celentano E, Galasso R, Krogh V (2003) Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori 89(6):594–607
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR (2009) A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr 139(12):2365–2372
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348(26):2599–2608
Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB (2009) Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 119(8):1093–1100
Maisonneuve P, Bagnardi V, Bellomi M, Spaggiari L, Pelosi G, Rampinelli C, Bertolotti R, Rotmensz N, Field JK, Decensi A, Veronesi G (2011) Lung cancer risk prediction to select smokers for screening CT—a model based on the Italian COSMOS trial. Cancer Prev Res (Phila) 4(11):1778–1789
Büchner FL, Bueno-de-Mesquita HB, Ros MM, Overvad K, Dahm CC, Hansen L, Tjønneland A, Clavel-Chapelon F, Boutron-Ruault MC, Touillaud M, Kaaks R, Rohrmann S, Boeing H, Nöthlings U, Trichopoulou A, Zylis D, Dilis V, Palli D, Sieri S, Vineis P, Tumino R, Panico S, Peeters PH, van Gils CH, Lund E, Gram IT, Braaten T, Sánchez MJ, Agudo A, Larrañaga N, Ardanaz E, Navarro C, Argüelles MV, Manjer J, Wirfält E, Hallmans G, Rasmuson T, Key TJ, Khaw KT, Wareham N, Slimani N, Vergnaud AC, Xun WW, Kiemeney LA, Riboli E (2010) Variety in fruit and vegetable consumption and the risk of lung cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 19(9):2278–2286
Linseisen J, Rohrmann S, Bueno-de-Mesquita B, Büchner FL, Boshuizen HC, Agudo A, Gram IT, Dahm CC, Overvad K, Egeberg R, Tjønneland A, Boeing H, Steffen A, Kaaks R, Lukanova A, Berrino F, Palli D, Panico S, Tumino R, Ardanaz E, Dorronsoro M, Huerta JM, Rodríguez L, Sánchez MJ, Rasmuson T, Hallmans G, Manjer J, Wirfält E, Engeset D, Skeie G, Katsoulis M, Oikonomou E, Trichopoulou A, Peeters PH, Khaw KT, Wareham N, Allen N, Key T, Brennan P, Romieu I, Slimani N, Vergnaud AC, Xun WW, Vineis P, Riboli E (2011) Consumption of meat and fish and risk of lung cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control 22(6):909–918
Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, Yang YS, Wu QJ, Zhang W, Xiang YB (2012) Meat consumption and risk of lung cancer: evidence from observational studies. Ann Oncol 23(12):3163–3170
Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J (2014) Red and processed meat consumption and the risk of lung cancer: a dose-response meta-analysis of 33 published studies. Int J Clin Exp Med 7(6):1542–1553
Lim WY, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, Ng A, Poh WT, Tee A, Teh M, Salim A, Seow A (2011) Meat consumption and risk of lung cancer among never-smoking women. Nutr Cancer 63(6):850–859
Sofi F, Macchi C, Abbate R, Gensini GF, Casini A (2013) Mediterranean diet and health. Biofactors 39(4):335–342
MacNee W (2013) Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med 45(3):291–300
Visioli F, Galli C (2001) The role of antioxidants in the Mediterranean diet. Lipids 36(Suppl):S49–S52
Fonseca Wald EL, van den Borst B, Gosker HR, Schols AM (2014) Dietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic review. Respirology 19(2):176–184
di Giuseppe R, Arcari A, Serafini M, Di Castelnuovo A, Zito F, De Curtis A, Sieri S, Krogh V, Pellegrini N, Schünemann HJ, Donati MB, de Gaetano G, Iacoviello L, Moli-sani Project Investigators (2012) Total dietary antioxidant capacity and lung function in an Italian population: a favorable role in premenopausal/never smoker women. Eur J Clin Nutr 66(1):61–68
Schwartz J (2000) Role of polyunsaturated fatty acids in lung disease. Am J Clin Nutr 71(1 Suppl):393S–396S
Gomes M, Teixeira AL, Coelho A, Araujo A, Medeiros R (2014) The role of inflammation in lung cancer. Adv Exp Med Biol 816:1–23
Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, Kitahara CM, Furr M, Li Y, Kemp TJ, Goedert JJ, Chang CM, Engels EA, Caporaso NE, Pinto LA, Hildesheim A, Chaturvedi AK (2014) Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. doi:10.1093/jnci/dju294
Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, Mavroeidi A, Simpson WG, Duthie GG, Macdonald HM (2014) Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr 112(8):1341–1352
Egan MB, Fragodt A, Raats MM, Hodgkins C, Lumbers M (2007) The importance of harmonizing food composition data across Europe. Eur J Clin Nutr 61(7):813–821
Kelemen LE (2006) Food frequency questionnaires: not irrelevant yet. Cancer Epidemiol Biomarkers Prev 15(5):1054
Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK (1995) Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol 24(2):389–398
Hebert JR, Ma Y, Clemow L, Ockene IS, Saperia G, Stanek EJ 3rd, Merriam PA, Ockene JK (1997) Gender differences in social desirability and social approval bias in dietary self report. Am J Epidemiol 146(12):1046–1055
Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L (2002) Systematic errors in middle-aged women’s estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol 12(8):577–586
Acknowledgments
This work was in part supported by the Italian Association for Cancer Research (AIRC), the Italian Foundation for Cancer Research (FIRC), and the European Institute of Oncology (IEO). Dr. Hébert was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975).
Conflict of interest
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is a paid employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project. On behalf of all other co-authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Patrick Maisonneuve and Nitin Shivappa have contributed equally to this article and therefore share first-authorship.
Rights and permissions
About this article
Cite this article
Maisonneuve, P., Shivappa, N., Hébert, J.R. et al. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr 55, 1069–1079 (2016). https://doi.org/10.1007/s00394-015-0920-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0920-3