Abstract
Purpose
These days, obesity threatens the health for which one of the main interventions is calorie restriction (CR). Due to the difficulty of compliance with this treatment, CR mimetics such as resveratrol (RSV) have been considered. The present study compared the effects of RSV and CR on hypothalamic remodeling in a diet-switching experiment.
Methods
C57BL/6 male mice received high-fat diet (HFD) for 4 weeks, subsequently their diet switched to chow diet, HFD + RSV, chow diet + RSV or CR diet for a further 6 weeks. Body weight, fat accumulation, hypothalamic apoptosis and expression of trophic factors as well as generation and fate specification of newborn cells in arcuate nucleus (ARC) were evaluated.
Results
Switching diet to RSV-containing foods leading to weight and fat loss after 6 weeks. In addition, not only a significant reduction in apoptosis but also a considerable increase in production of newborn cells in ARC occurred following consumption of RSV-enriched diets. These were in line with augmentation of hypothalamic ciliary neurotrophic factor and leukemia inhibitory factor expression. Interestingly, RSV-containing diets changed the fate of newborn neurons toward generation of more proopiomelanocortin than neuropeptide Y neurons. The CR had effects similar to those of RSV-containing diets in the all-evaluated aspects besides neurogenesis in ARC.
Conclusions
Although both RSV-containing and CR diets changed the fate of newborn neurons to create an anorexigenic architecture for ARC, newborn neurons were more available after switching to RSV-enriched diets. It can be consider as a promising mechanism for future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is one of the serious medical conditions worldwide. It has been found to reduce life expectancy because of being associated with several diseases, including diabetes mellitus, cancers, cardiovascular diseases, asthma and obstructive sleep apnea [1]. Obesity is created when the balance between dietary energy intake and energy consumption is destroyed [2]. To date, the main non-pharmacological intervention proposed to solve this problem is calorie restriction (CR) in which calorie intake is reduced under ad libitum levels [3]. In this sense, sirtuins are the main molecular target for CR [4]. Although dietary restriction has beneficial effects in control of metabolic rate [5], reduction of eating for the aim of maintaining a desirable metabolic profile is improbable to reach widespread compliance. Hence, scientists search CR mimetics with some anti-obesity effects like CR without need for eating less. Since one of the most potent activators of sirtuins is resveratrol (RSV), it is considered as an alternative agent for correction of energy imbalance observed in obesity [6]. This naturally occurring polyphenolic compound, which is found in various plant species, has shown a wide range of pharmacological and therapeutical activities [7]. Previous investigations indicated the effects of resveratrol on lipid metabolism in adipose tissue, liver and skeletal muscles [8]. The suggested anti-obesity mechanisms for RSV are inhibition of adipogenesis and lipogenesis, stimulation of lipolysis, induction of apoptosis in adipocytes and enhancement of fatty acid oxidation and thermogenesis [9]. Despite these peripheral mechanisms, the central effects of resveratrol on hypothalamic areas related to energy homeostasis have not clearly described.
In recent years, there is an exponential increase in research on adult hypothalamic neurogenesis as an important modulator of energy homeostasis [10]. Since the hypothalamus includes diverse nucleuses locating around an active neurogenic niche, even the modest activity of the stem cells can have considerable effect on the architecture and finally function of hypothalamus. On the other hand, previous studies revealed a change in hypothalamic neurogenesis following consuming high-fat diet (HFD) [11], intraventricular administration of ciliary neurotrophic factor (CNTF) [12] and using CR [11, 13]. It should be noted that, studies in this field are still in early exploratory stages and conclusive judgment in this case is extremely difficult. However, the results of the all studies so far point out two things: first, there is a relationship between diet and intensity of adult neurogenesis in sub-regions of hypothalamus. Second, alteration in hypothalamic proliferative remodeling and in turn in hypothalamic neuroarchitecture can lead to changes in body energy homeostasis and finally changes in body weight [14, 15].
However, so far no study has examined the effects of RSV on hypothalamic neurogenesis. Here, we created a HFD-induced obesity model of mice and investigated the effects of resveratrol on neurogenesis and apoptosis in the arcuate nucleus (ARC) of the hypothalamus. Moreover, the fate of newborn neurons in this area has been determined. Finally, these central effects of RSV were compared with those of CR.
Materials and methods
Animals
Sixty C57BL/6 male mice were obtained from the Pastor institute (Karaj, Iran) and maintained in a temperature-controlled room with a 7:00 A.M.–7:00 P.M. light/dark cycle. Mice were offered ad libitum access to water and subjected to different dietary regimens as described below. All experiments were carried out in accordance with the guiding principles for the care and use of animals and were approved by the ethic committee of Tehran University of Medical Sciences.
Experimental design
A summary of the study design is shown in Fig. 1. After 1 week of acclimatization, 6-week-old mice were provided with ad libitum access to either a laboratory chow diet (Chow, n = 10) for 10 weeks or an in-house prepared HFD containing 60% kcal fat for 4 weeks (n = 50). Then, animals received HFD were either maintained on HFD or they were switched to chow diet (HFD/Chow), HFD + RSV (HFD/HFD + RSV), chow diet + RSV (HFD/Chow + RSV) or CR diet (HFD/Chow CR) for a further 6 weeks. The chow diet was purchased from Behparvar industry in which 9% of total kcal was as fat. In HFD, the chow in powder form was mixed by adding soy oil and butter (1:9 v/w) up to 60% per total kilocalorie (Table 1). After homogenization, a dough-like texture was shaped and the obtained blocks were dried and utilized for feeding. RSV (Tokyo Chemical Industry, TCI) with purity of more than 99% was mixed with either powdered chow or HFD to provide approximately a 400 mg/kg dose, and pellets were then reconstituted. All processes of food preparing were done in dark. Diet-containing RSV was stored at − 20 °C and food was provided in cages for no more than 2 days. Animals in the HFD/Chow CR group were fed 60% of the daily intake of age-matched chow-fed controls during last 6 weeks of the study.
Food intake and body weight monitoring
Food intake throughout the experiment was evaluated by substracting the amount of food left from the amount served in 24 h. Cages were monitored for evidence of food spillage. Body weight of the animals was measured weekly.
Bromodeoxyuridine (BrdU) labeling
Four weeks after beginning the study, for labeling proliferating cells, animals of all groups (n = 5 per each group) received BrdU (Sigma, USA) administrated in the morning and evening by intraperitoneal injection at 50 mg/kg of body weight for 9 days. To prepare a BrdU solution for injection, BrdU was dissolved in saline and adjusted pH to 7.35.
Tissue preparation
At the end of the experiment, 16-week-old animals were deeply anesthetized with intraperitoneal injection of ketamine and xylazine (80 and 10 mg/kg, respectively) and transcardially perfused with 0.9% cold normal saline followed by 4% paraformaldehyde in 0.1 M PBS with pH of 7.4. Epididymal white adipose tissues (WAT) were rapidly dissected out from mice according to defined anatomical landmarks. Following brain and WAT dissection, the tissues were postfixed in 4% paraformaldehyde for 24 h at 4 °C. Subsequently, tissue processing was performed. Then, the tissues were embedded in paraffin, and sectioned at 5 µm thickness using cryostat and placed on glass slides. Brain sections were collected in the coronal plane throughout the caudal hypothalamus (− 1.22 to − 2.12 from bregma). These slides were then used for immunohistochemistry, apoptosis assay as well as hematoxylin and eosin (H&E) staining.
H&E staining and analysis of adipocyte size
Multiple sections obtained from WAT depots (three section per animal) were analyzed systematically with respect to adipocyte cell morphology and size. For this reason, sections were hydrated with decreasing concentrations of ethanol. Then, slides stained with H&E and finally, dehydrated with increasing concentration of ethanol. Images captured by a light microscope (Olympus, Japan). The mean area of adipocytes was calculated using the infinity software.
Evaluation of apoptosis
The Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) method was performed using a TUNEL assay kit (DNA Fragmentation/Fluorescence Staining, Millipore, USA) to detect apoptosis in ARC of the hypothalamus as manufacture’s instruction. In brief, tissue sections were deparaffinized and covered with diluted proteinase K (20 mg/ml) and incubated for 15–30 min at 37 °C. Next, sections were equilibrated in terminal deoxynucleotidyl transferase (TdT) buffer for 10 min. Sections were then incubated in TdT end-labeling cocktail (a mixture of TdT Buffer, Biotin-dUTP and TdT) in a humid chamber for 60 min at 37 °C. The reaction was stopped by immersing the sections in termination buffer (TB) for 5 min at room temperature (RT). For minimizing non-specific staining, sections were incubated 20 min at RT with blocking buffer. Sections were then incubated with Avidin-FITC solution for 30 min at RT. Finally, the tissues were counterstained with 4′,6-diamidino-2-phenylindole to reveal normal and apoptotic nuclear morphology. In positive controls, proteinase K-treated section were incubated with low concentration of DNase (1 µg/ml during 1 h). TUNEL staining was analyzed bilaterally in ARC using a fluorescent microscope (Olympus, Japan); apoptotic cells were quantified by counting the percentage of TUNEL-positive cells against the total number of nucleated cells per section.
Immunofluorescence assay
To visualize cells expressing BrdU or CNTF, the immunohistochemistry was done. Briefly, sections (at least three sections per animal) were washed in phosphate-buffered saline (PBS), and then treated with 2 M HCl for 15 min followed by 12 min incubation with 0.1 M sodium tetraborate (Sigma, USA) (these steps were done only for BrdU detection). Then, the tissues were blocked with 5% normal goat serum (NGS) and 1% bovine serum albumin (BSA) in PBS for 60 min. After that, tissues were incubated with mouse anti-BrdU (1:50; Abcam, USA) or rabbit anti-CNTF (1:20; Santa Cruz, Germany) at 4 °C overnight. Next, goat anti-mouse IgG (Alexa flour 647, 1:600; Invitrogen, USA) or goat anti-rabbit IgG (FITC; 1:700; Abcam, USA) were used for 60 min at RT and sections were counterstained with DAPI (1 g/ml, Santa Cruz, Germany) or propidium iodide (PI, Molecular Probes, USA) and evaluated bilaterally by a fluorescent microscope (Olympus, Japan). The primary antibodies were omitted in control samples in which no immunoreactivity was detected. The percentage of immunopositive cells per area was calculated by the following formula: (the number of positive cells × 100)/the total number of nuclei.
Total RNA isolation and cDNA synthesis
For molecular analysis, animals were deeply anesthetized and decapitated (five mice per group). Then, the brain was rapidly removed from the skull and hypothalamic tissue from the preoptic area to the mammillary body was dissected out. Samples were snap frozen in liquid nitrogen and stored at − 80 °C. Hypothalamic total RNA was extracted using Ribo Ex (Hybrid-RTM miRNA, Gene all, Korea) according to the manufacturer’s recommendation. The purity and concentration of the RNA extracted from all samples was verified and quantified using a RNA NanoDrop 2000c Spectrophotometer. RNA samples were rendered genomic DNA free using DNase I kit (Qiagen, Germany).
For synthesis of complementary DNA (cDNA), 500 ng total RNA from each sample in a total reaction volume of 10 µl was reverse transcribed using the cDNA Synthesis Kit (Thermo Scientific, EU) according to the manufacturer’s instruction. Reactions were incubated initially at 37 °C for 15 min and subsequently at 75 °C for 10 min. All the cDNA samples were stored at − 20 °C until reverse transcription quantitative polymerase chain reaction (RT-qPCR) analyses.
Quantification of mRNA
Brain-derived neurotrophic factor (BDNF) and leukemia inhibitory factor (LIF) were quantified with RT-q PCR with CFX 96 Real-Time System (Bio-Rad, Germany). β-Actin mRNA levels were similarly measured and served as the internal control. The PCR reagent mixture were prepared in a total volume of 20 µl containing: 5 µl template, 0.5 µl of each 10 µM primer, 4 µl of 5× HOT FIREPol® EvaGreen® qPCR Mix Plus (Solis BioDyne, Estonia) and 10 µl RNase/DNase-free sterile water (Sigma, USA). The primers for the BDNF gene were:5′-TGCCGCAAACATGTCTATGA-3′ (forward) and 5′-CCGGGACTTTCTCTAGGACT-3′ (reverse). The primers for the LIF gene were: 5′-CAGGGATTGTGCCCTTACTG-3′ (forward) and 5′-CCCCTTGAGCTGTGTAATAGGAA-3′ (reverse). The sequences of the primers for the β-actin gene were: 5′-AAGTCCCTCACCCTCCCAAAAG-3′ (forward) and 5′-AAGCAATGCTGTCACCTTCCC-3′ (reverse). Amplification was performed as followed: an initial denaturation for 15 min at 95 °C followed by 45 cycles of denaturation at 95 °C for 30 s, primer annealing at defined annealing temperature for 30 s and elongation at 72 °C for 30 s. The procedure was followed by a melting curve analysis to determine product specificity. Meanwhile, wells of no template control (NTC) and no reverse transcriptase control (NRT) were prepared as the negative controls to evaluate DNA contamination. All sample mRNA levels were normalized to the values of β-actin and the 2−ΔΔCt method was used to calculate relative BDNF and LIF mRNA, and fold changes in mRNA levels.
Double immunofluorescence assay
To evaluate the fate of newborn cells in ARC, double immunohistochemistry technique was utilized. Briefly, the brain sections were washed with PBS with 1% Triton X-100 and then bathed with 2 M HCl for 15 min at 37 °C. The sections were then neutralized with 0.1 M sodium tetraborate (Sigma, USA) for 12 min at RT. Next, 5% NGS and 1% BSA in PBS were used for 60 min. After that, slides were incubated with mouse anti-BrdU (1:50; Abcam, USA) in combination with rabbit anti-proopiomelanocortin (POMC; 1:200; Abcam, USA) or rabbit anti-neuropeptid Y (NPY; 1:1000; Abcam, USA) at 4 °C overnight. Subsequently, goat anti-mouse IgG (FITC; 1:700; Abcam, USA) and goat anti-rabbit IgG (Alexa flour 647, 1:600; Invitrogen, USA) were used for 60 min at RT. The nuclei were stained with DAPI (1 g/ml, Santa Cruz, Germany). Using a fluorescent microscope (Olympus, Tokyo, Japan), immunopositive cells were analyzed. The percentage of BrdU-positive cells, which expressed a defined marker (POMC or NPY) per area, was calculated by the following formula: (the number of BrdU-defined marker-positive cells × 100)/the total number of BrdU-positive cells.
Statistical analyses
Results are presented as mean ± standard error of the mean. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software (version 23, Chicago, USA). For the analyses in which change over groups was assessed one-way ANOVA test was used. Pairwise comparison of means was accomplished by LSD post hoc test. In all analyses, P < 0.05 was regarded as statistically significant.
Results
Body weight and food intake following diet switching
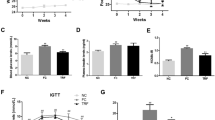
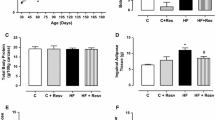
As represented in Fig. 2a, evaluation of body weight of mice in the different groups indicated that this index had an increasing trend during the first four weeks of study so that animals that received HFD had significantly higher body weight than mice that consumed chow diet in the 3rd and 4th week of study (P < 0.01). Indeed, weight gaining in HFD group occurred from the third week (arrow head in Fig. 2a). After 4 weeks, diet switching was performed and some clear changes in body weight were seen. The animals in the HFD/Chow and HFD/Chow CR groups showed dramatically loss of weight compared to the HFD group from 6th week of the study to the end (P < 0.05). The body weight in the HFD/Chow + RSV indicated a decreasing trend during 6th and 7th weeks of the study. At week 9, the animals in this group revealed a considerable decrease in body weight gain compared to the HFD group (P < 0.05). In addition, animals switched to HFD + RSV had significantly lower weight gaining compare to that of the HFD group at 10th week of the study (P < 0.05). Indeed, reduced weight gaining was seen in HFD/Chow + RSV and HFD/HFD + RSV groups at 9th and 10th week of study, respectively (arrows in Fig. 2a). More analysis of the results indicated that there was no statistical differences in the body weight between HFD/Chow + RSV and HFD/HFD + RSV groups. In addition, the body weight in the HFD/Chow and HFD/Chow + RSV groups was not significantly different. Moreover, the body weight in the CR group was significantly lower than that of the groups received RSV in 6th and 7th weeks of the study (P < 0.05). In summary, foods containing RSV reduced weight-gaining process at the last weeks of the study, but this effect was not as effective as the effects of CR and Chow diets. Assessment of daily food intake (gr/day) of animals showed that this index was not significantly different among the groups except the HFD/Chow CR group in which food intake/mice/day was considerably lower than that of others (P < 0.05, Fig. 2b). In conclusion, adding RSV to either chow or HFD in diet-switching protocol needed a lag phase to reduce the weight-gaining process.
Evaluation of body weight, food intake and fat accumulation. The animals in the different groups were evaluated in terms of body weight (a), food intake (b) and adipocyte size (c) during 10 weeks. The epididymal WAT was collected and stained by H&E (d, ×100). *P < 0.01 vs other groups. #P < 0.05 vs other groups except the HFD/Chow CR. $P < 0.05 vs other groups except the HFD/Chow CR and HFD/Chow. &P < 0.05 vs HFD/Chow, HFD/Chow CR and Chow. @P < 0.05 vs other groups except the HFD/HFD + RSV. +P < 0.05 vs other groups. ¶P < 0.001 vs other groups
Fat storage following diet switching
The results of H&E staining indicated that consuming HFD resulted in significant fat accumulation in adipose tissue, determined by the size of adipocytes in epididymal WAT (P < 0.001, Fig. 2c). In contrast, diet switching to chow or chow CR decreased considerably the mean adipocyte area in WAT (P < 0.001). In addition, in the groups received RSV either with HFD or chow diet the size of adipocytes was lower than that of the HFD group (P < 0.001). In summary, adding RSV to diet could slow the speed of fat accumulation process following HFD consuming.
Apoptosis rate in ARC following diet switching
As indicated in Fig. 3, analyzing the results of TUNNEL assay test revealed a considerable enhancement in the percentage of apoptotic cells in ARC of animals consumed HFD compared to that of other groups received chow or switched diets (P < 0.001). In other words, diet switching significantly decreased apoptosis rate in ARC. Although, in ARC of animals switched to chow + RSV or chow CR diet, the percentage of TUNNEL-positive cells was remarkably lower than that of mice switched to HFD + RSV diet (P < 0.05). We can conclude that, the decreasing effect of switching diet to chow + RSV on apoptosis in ARC was similar to that of switching diet to chow CR diet.
Distribution of TUNNEL-positive cells. a–c Using TUNNEL assay test, the apoptotic cells (green) in ARC of animals in the studied groups was determined. d–f Magnified pictures indicate TUNNEL + cells. g The percentage of apoptotic cells in different groups. *P < 0.001 vs other groups. #P < 0.05 vs the HFD and HFD/HFD + RSV groups. 3V third ventricle
Generation of new cells in ARC following diet switching
Six weeks after BrdU injection, the newborn cells were determined by immunostaining of BrdU-positive cells in ARC of the animals (Fig. 4). The results represented that consuming HFD tended to increase the percentage of BrdU+ cells in ARC but this effect was not statistically significant (P = 0.9). There was no difference among the chow, HFD/chow and HFD/chow CR groups in the percentage of BrdU+ cells in ARC. However, this index dramatically increased in HFD/chow + RSV and HFD/HFD + RSV groups compare to that of the chow and HFD/chow CR groups (P < 0.05). These results showed that switching diet to foods containing RSV, enhanced the percentage of newborn cells in ARC.
Tracing the newborn cells. a–c The distribution of newborn cells in ARC of the studied animals was assessed by immunostaining of BrdU-positive cells (red) 6 weeks following intraperitoneal injection of BrdU. d–f Magnified pictures show BrdU + cells. g Quantification of newborn cells in ARC of mice in different groups. *P < 0.05 vs the chow and HFD/chow CR groups. 3V third ventricle
Distribution of CNTF+ cells in ARC following diet switching
The results of immunostaining for CNTF in ARC showed that HFD did not change the percentage of CNTF+ cells in ARC (Fig. 5). While the percentage of CNTF-expressing cells in ARC significantly increased in other groups in which diet switching was performed (P < 0.05 vs the chow and HFD groups). Overly, switching from HFD to RSV-containing diet as well as chow diet enhanced CNTF expression in ARC similar to chow CR diet.
Distribution of CNTF-positive cells. a–c Using immunostaining technique, the cells expressed CNTF (green) were determined in ARC. d A magnified picture indicates CNTF+ cells in ARC. Note the presence of these cells in the subependymal layer (arrow) as well as ARC. The data of different groups were quantified in e. *P < 0.05 vs the chow and HFD groups. 3V third ventricle, ME medial eminence
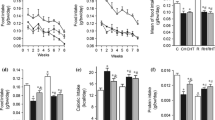
Expression of BDNF and LIF in hypothalamus following diet switching
To evaluate the effect of diet switching in mRNA expression of two main growth factors in the hypothalamus, RT-qPCR was performed (Fig. 6). The results indicated that consuming HFD for 10 weeks caused a significant enhancement in BDNF gene expression (P < 0.05 vs other groups except the HFD/chow + RSV group). In addition, switching HFD to HFD + RSV increased considerably LIF mRNA expression in hypothalamus compared to that of the chow group (P < 0.05). According to the results, a tendency to increase of LIF mRNA expression could be seen in all other groups compared to the chow group but these changes were not statistically significant. Together, diet switching had a decreasing effect on hypothalamic BDNF expression. In contrast, a cumulative increasing effect of HFD and RSV was seen on levels of LIF in the hypothalamus.
Production of new NPY neurons in ARC following diet switching
To identify what percentage of newborn cells (BrdU-positive cells) became NPY-expressing cells, a double immunostaining was performed (Fig. 7). Comparing the results of the chow and HFD groups revealed that the percentage of new NPY cells in ARC did not change following HFD consumptions for 10 weeks. In contrast, switching diet to HFD + RSV, chow + RSV or chow CR diet significantly decreases the percentage of newborn NPY cells in ARC compare to that of the chow and HFD groups (P < 0.01). In summary, adding RSV to diet following HFD consumption changes the fate of new cells toward production of less NPY cells. This effect was similar to that of CR diet.
New NPY-positive cells in ARC. To determine newborn cells that expressed NPY marker, double immunostaining was performed. The pictures of Brdu (a, green), NPY (b, red) and nuclei (c, blue) were merged (d). e A magnified picture in which arrows indicate samples of BrdU + NPY + cells and the arrowhead shows a NPY + cell. The percentage of BrdU + NPY + cells was quantified in F. *P < 0.01 vs the chow and HFD groups. 3V third ventricle, ME medial eminence
Production of new POMC neurons in ARC following diet switching
The results of double immunostaining for BrdU and POMC revealed the percentage of new POMC-positive neurons in ARC (Fig. 8). Although there was no significant difference in the percentage of POMC-positive cells in ARC between the chow and HFD groups, this index increased dramatically in the HFD + RSV, chow + RSV and chow CR groups compared to that of the chow and HFD groups (P < 0.05). It can be concluded that consuming CR diet after HFD enhanced the production of new POMC neurons in ARC. Interestingly, RSV-containing diets mimicked this increasing effect.
New POMC-positive cells in ARC. Using double immunostaining for BrdU (a, green) and POMC (b, red), the new POMC-positive cells were determined in ARC. The nuclei were indicated in blue color (c). e A magnified picture in which arrow indicates a sample of BrdU + POMC + cells and the arrowhead shows a POMC + cell. The percentage of BrdU + POMC + cells was quantified in f. *P < 0.05 vs the chow and HFD groups. 3V third ventricle, ME medial eminence
Discussion
The present study evaluated a novel aspect of anti-obesity effects of RSV in a diet-switching model. Adding RSV to chow or HFD decreased weight gaining after 5 or 6 weeks of study, respectively. This phenomenon was linked to reduction in fat accumulation in WAT. In addition, not only a significant anti-apoptotic effect of RSV was seen in ARC, but also RSV enhanced production of newborn cells in this area of hypothalamus. Among the studied growth factors, CNTF, and to some extent, LIF seems to have a relation with increasing effect of RSV on neurogenesis in ARC. Amazingly, RSV-containing diets changed the fate of newborn neurons toward generation of more anorexigenic (POMC) neurons than orexigenic (NPY) ones. Interestingly, CR diet mimicked all mentioned effects of RSV except stimulation of neurogenesis in ARC.
Since previous epidemiological investigations have indicated a positive relationship between obesity and dietary fat intake, inducing obesity via dietary fat in animals is an appropriate model for studying different aspects of obesity. In the present study, common sources of fatty acids, butter and soy oil were added to diet of C57 BL/6 mice to create a reliable animal model of obesity [16]. The results revealed that in mice that received HFD, significant weight gaining started at the third week of the study and continued to end. In addition, similar to the results of previous studies, fat accumulation in WAT was observed in obese animals [17, 18]. On the other hand, switching diet to CR led to weight loss at 6th week of the study. Moreover, chow + RSV or HFD + RSV diet prevented more weight gaining in 9th or 10th week of study, respectively. The dose of RSV used in the animal studies ranged from 1 mg up to more than 1 g/kg bw/day [19]. Based on the previous investigations, RSV in high doses induced anti-obesity effects. Thus, the dose of 400 mg/kg bw/day was utilized in the present study similar to Lagouge et al. [20] and Kim et al. [21] studies. Using body surface area normalization method, a human equivalent dose is 32.4 mg/kg, which equates to a 2.3 g dose of resveratrol for a 70 kg person [22]. Administration of RSV at this level of dose for human was reported in the previous studies. Dvid et al. showed that single doses of 0.5, 1, 2.5, or 5 g of RSV in healthy volunteers did not cause serious adverse events [23]. In another study, healthy persons ingested RSV at 0.5, 1.0, 2.5 or 5.0 g daily for 29 days. The researchers reported that RSV was safe, but the doses of 2.5 and 5 g showed mild to moderate gastrointestinal symptoms [24]. In the present study, the amount of dietary fiber in chow diet was more than that of HFD. It has been suggested that dietary fibers reduce food intake and fat accumulation by decreasing calorie, increasing intestinal satiety and reducing food ingestion rate [25]. In addition, dietary fibers may have impact on nutritional behaviors via gut–brain axis. Indeed, gut microorganisms digest and ferment fibers into short-chain fatty acids like acetate, propionate, and n-butyrate, which are known to exert neuroactive functions [26]. Although, in our study, the difference for fibers between the foods did not have any effect on food intake. However, what is certain is that adding RSV to HFD reduced weight gaining (by comparing the HFD and HFD/HFD + RSV groups). In addition, histological assessments indicated a decreasing effect of RSV-containing diets and CR diet on fat accumulation. Based on the results of the previous studies, fat mobilization is affected by RSV [27]. This natural phenol decreases the number of adipocytes as well as reduces lipid accumulation. RSV induces apoptosis in adipocytes via modulation of Akt pathway [28], decreases adipogenesis by activation of sirtuin1 (SIRT1) [29], reduces lipid synthesis via phosphorylation of 5′ AMP-activated protein kinase [7] and increases lipolysis by enhancing levels of cAMP [7]. What is clear, on the other hand, is that CR plays some of its beneficial effects via inducing SIRT1 expression too. For instance, CR reduced adipose tissue inflammation created by HFD [30, 31]. Although all mechanisms by which CR and RSV exert their effects are not similar [32]. This may explain why the onset of weight losing observed in the present study following diet switching was not the same.
Based on our results, adding RSV to foods produced new hunger neurons in ARC without any significant changes in food intake during 6 weeks. There are some explanation for this phenomenon. First, in the present study, we just showed generation of new neurons by immunofluorescence assay. After birth, a new neuron should migrate and integrate with other cells to exert its electrochemical function and finally change behavior [33]. Maybe, time of 6 weeks was a short period for changing food intake behavior. Second, POMC neurons act via two main mechanisms; decrease in food intake and increase in energy expenditure [34]. Thus, one of the probable effects for RSV via generation of POMC neurons can be regulation of energy and metabolic homeostasis. Lagouge et al. reported that treatment of mice with RSV improves mitochondrial function and energy homeostasis without any effect on food intake [20]. Further investigations are needed for determining the exact effect of RSV on food intake via remodeling of hypothalamus.
The present study indicated that apoptosis in ARC was enhanced by HFD consumption. This is in line with other studies that considered induction of hypothalamic neuronal apoptosis by HFD was due to inflammatory signal transduction [35, 36]. On the other side, both CR and RSV revealed anti-apoptotic effects in ARC. Previous investigations have been reported tissue-specific pro- and anti-apoptotic effects of RSV. Although RSV induced program cell death in cancer cells [37, 38] and adipocytes [28, 39], it decreased apoptosis in hippocampus and cortex via regulation of Bcl-2, Bax and caspase-3 expression and suppression of the mitochondrial death pathway [40]. Based on the previous reports, CR inhibits Bax-mediated apoptosis via increasing the expression of CIRT1 [30]. Together, the architecture of ARC and consequently the function of this area can be influenced by anti-apoptotic effects of RSV and CR.
After introducing an active neurogenesis in adult hypothalamus [10], researchers tried to study the probable effects of different conditions on hypothalamic neurogenesis and vice versa. Our study showed that the percentage of newborn cells in ARC did not significantly change following HFD consumption for 10 weeks. In this regard, the results of previous studies are inconsistent. Bless et al. [41] reported that receiving HFD for 6 weeks had enhancing effect on the number of BrdU-positive cells in the hypothalamus of adult female mice. In contrast, Li et al. [42] found that eating HFD for 16 weeks reduced hypothalamic neurogenesis. Similarly, McNay et al. [11] reported a decreasing effect of HFD consumption for 8 weeks on generation of hypothalamic new cells. Considering these results and the report of Gouazé et al. [43], we can conclude that the effect of HFD on hypothalamic neurogenesis is biphasic so that this effect is increasing at early phases and is reducing by chronic exposure to HFD. It seems that enhancement of neurogenesis in early phases of HFD consumption is a compensatory mechanism against neurodegeneration [44], but this process eventually fails.
What is remarkable in the present study is that switching diet to RSV-containing diets considerably increased the percentage of BrdU-positive cells in ARC. Although no pervious study has been reported about this issue, some investigations evaluated the effect of RSV on neurogenesis in other regions of the brain especially the hippocampus. Kumar et al. [45] indicated that RSV increased the number of newly generated cells in the hippocampus which was associated with upregulation of SIRT1 protein. In addition, RSV increased neurogenesis in the hippocampus of the prenatally stressed animals [46]. Moreover, similar effects have been reported by other groups [47, 48]. On the other hand, our results revealed that CR did not have any significant effect on the percentage of newborn cells in ARC. In this sense, McNay et al. [11] showed that the number of neural stem-like cells derived from hypothalamic tissue of obese animals switched to CR diet for 4 weeks was not significantly changed. Although the results of their in-vivo investigation showed that the loss of hypothalamic progenitor cells caused by HFD can be rescued by CR, the other report violated this statement [49]. Overall, our results indicated that RSV and CR had different effects on generation of new cells in ARC.
In the present study, although the percentage of CNTF-positive cells in ARC of animals used HFD did not change, this index considerably increased in ARC of mice for which diet switching was performed. What can be concluded from analyzing the data of weight and CNTF in our study is weight loss was in line with increase in the percentage of CNTF-positive cells. In other words, any diet switching from HFD enhanced endogenous CNTF in ARC. Although exogenous CNTF induces weight loss via leptin-like pathways [50, 51], determining the exact effects of endogenous CNTF on energy homeostasis needs to be elucidated. Some investigations demonstrated that endogenous CNTF was influenced by 6 weeks of consumption of high-sucrose diet but not by HFD [51]. In contrast, Severi et al. [52] revealed that CNTF expression in tuberal and mammillary regions of mouse hypothalamus increased after using HFD for 12 weeks. In addition, they reported that CR diet showed an opposite effect on hypothalamic CNTF expression. None of the previous studies have been designed as diet-switching model. More investigations are needed to determine the mechanisms involved in hypothalamic CNTF expression changes following diet switching. In the present study, an elevated levels of LIF in hypothalamus was seen following switching diet to HFD + RSV. Previous studies showed that production of LIF, as transcription 3 activator, increased following treatment of medulloblastoma cells by RSV [53]. Moreover, expression of LIF receptor enhanced in intestinal mucosa after RSV treatment [54]. On the other hand, central LIF reduced body weight, food intake and adiposity [55]. Meanwhile, previous studies indicated that LIF promoted neurogenesis and proliferation of neural stem cells [56]. We, therefore, considered that some anti-obesity and neurogenic effects of RSV might be created via enhancement of LIF expression in the hypothalamus. In our study, an increase in hypothalamic BDNF expression was seen following HFD consumption which could be a compensatory mechanism. It should be noted that the satiety effects of this neurotrophin was reported previously [57]. What is clear in the present study is that the switched diets did not exert their anti-obesity effects via modulating hypothalamic BDNF expression.
Attempting fate determination of newborn cells in our study indicated that 10 weeks of consumption of HFD did not create considerable change in the architecture of ARC in terms of generation of new POMC and NPY neurons. Although switching diet to RSV-containing diets and CR diet leading to production of more POMC-positive cells than NPY-positive ones. In other words, these diets changed the architecture of ARC to generate more anorexigenic neurons that prevent more weight gaining. Based on the results of McNay et al. [11], the number of both BrdU + NPY-positive and BrdU + POMC-positive neurons in ARC of mice increased after HFD consumption. It is worth to point out that, in their study, the labeling of proliferating cells was performed in embryogenic stage then the animals exposed to HFD from 6th to 16th week of age. Indeed, the study evaluated the retention of embryonic neural stem cells not the fate of adult proliferating cells [11]. Another investigation reported that the number of POMC + cells enhanced after exposure to HFD for 3 weeks [43]. Considering the duration of the mentioned study (3 weeks) compared to that of our investigation (10 weeks), it seems that an unsuccessful compensatory phase could be created after HFD consumption.
In conclusion, resveratrol shifted remodeling patterns of ARC in mice on HFD toward those of animals on CR diet. Considering the increasing effect of RSV on adult neurogenesis in ARC, we propose that the anti-obesity effect of RSV can be more lasting than that of CR diet. We need to bear in mind that neurogenesis process consists of various steps including cell proliferation, migration, differentiation, maturation and integration during which many environmental signals affect neural stem cells [58,59,60]. In addition, RSV exerts its effects in dose- and duration-dependent manner [61, 62]. Therefore, more investigations are required to get new insight into the hypothalamic remodeling as well as rebounding weight gain following switching diet of obese individuals to RSV-enriched diets.
References
Haslam DW, James WP (2005) Obesity. Lancet 366(9492):1197–1209. https://doi.org/10.1016/S0140-6736(05)67483-1
Ravussin E, Swinburn BA (1992) Pathophysiology of obesity. Lancet 340(8816):404–408
Fontana L, Klein S (2007) Aging, adiposity, and calorie restriction. JAMA 297(9):986–994
Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6(4):298–305
Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM (2006) Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295(13):1539–1548
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14(5):612–622
Szkudelska K, Szkudelski T (2010) Resveratrol, obesity and diabetes. Eur J Pharmacol 635(1):1–8
Aguirre L, Fernández-Quintela A, Arias N, Portillo MP (2014) Resveratrol: anti-obesity mechanisms of action. Molecules 19(11):18632–18655
Wang S, Zhu M-J, Du M (2015) Prevention of obesity by dietary resveratrol: how strong is the evidence? Taylor & Francis, London
Rojczyk-Gołębiewska E, Pałasz A, Wiaderkiewicz R (2014) Hypothalamic subependymal niche: a novel site of the adult neurogenesis. Cell Mol Neurobiol 34(5):631–642
McNay DE, Briançon N, Kokoeva MV, Maratos-Flier E, Flier JS (2012) Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Investig 122(1):142–152
Kokoeva MV, Yin H, Flier JS (2005) Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310(5748):679–683
Lee DA, Yoo S, Pak T, Salvatierra J, Velarde E, Aja S, Blackshaw S (2014) Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front Neurosci 8:157
Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H (2012) Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 15(5):700–702
Lee DA, Blackshaw S (2012) Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int J Dev Neurosci 30(8):615–621
Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23(02):270–299
Gao M, Ma Y, Liu D (2015) High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10(3):e0119784
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T (1999) PPARγ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Mol Cell 4(4):597–609
Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomas-Barberan A, Teresa Garcia-Conesa F, Carlos Espin M J (2013) Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 19(34):6064–6093
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127(6):1109–1122
Kim S, Jin Y, Choi Y, Park T (2011) Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81(11):1343–1351
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661
Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ (2007) Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Prev Biomark 16(6):1246–1252
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G (2010) Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 70(22):9003–9110
Van Itallie TB (1978) Dietary fiber and obesity. Am J Clin Nutr 31(10):S43-S52
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701
Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, Della-Fera MA (2011) Effect of resveratrol on fat mobilization. Ann N Y Acad Sci 1215(1):40–47
Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA (2008) Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res 22(10):1367–1371
Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR. Nature 429(6993):771–776
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392
Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S (2011) SirT1 regulates adipose tissue inflammation. Diabetes 60(12):3235–3245
Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C (2008) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3(6):e2264
Belvindrah R, Lazarini F, Lledo P-M (2009) Postnatal neurogenesis: from neuroblast migration to integration into mature circuits. Rev Neurosci 20(5–6):331–346
Millington GW (2007) The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab 4(1):18
Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ (2009) High-fat diet induces apoptosis of hypothalamic neurons. PLoS One 4(4):e5045
Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL (2014) Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immunity 35:33–42
Gusman J, Malonne H, Atassi G (2001) A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 22(8):1111–1117
Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ (2008) Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66(8):445–454
Yang J-Y, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA (2008) Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci 82(19):1032–1039
Bastianetto S, Ménard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1852 (6):1195–1201
Bless E, Reddy T, Acharya KD, Beltz B, Tetel M (2014) Oestradiol and diet modulate energy homeostasis and hypothalamic neurogenesis in the adult female mouse. J Neuroendocrinol 26(11):805–816
Li J, Tang Y, Cai D (2012) IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 14(10):999–1012
Gouazé A, Brenachot X, Rigault C, Krezymon A, Rauch C, Nédélec E, Lemoine A, Gascuel J, Bauer S, Pénicaud L (2013) Cerebral cell renewal in adult mice controls the onset of obesity. PLoS One 8(8):e72029
Pierce AA, Xu AW (2010) De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci 30(2):723–730
Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS, Yadav S, Khanna VK (2016) Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6:28142
Madhyastha S, Sekhar S, Rao G (2013) Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int J Dev Neurosci 31(7):580–585
Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K (2011) Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem 22(12):1150–1159
Moriya J, Chen R, Yamakawa J-i, Sasaki K, Ishigaki Y, Takahashi T (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 34(3):354–359
McNay DE, Speakman JR (2013) High fat diet causes rebound weight gain. Mol Metab 2(2):103–108
Lambert P, Anderson K, Sleeman M, Wong V, Tan J, Hijarunguru A, Corcoran T, Murray J, Thabet K, Yancopoulos G (2001) Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci 98(8):4652–4657
Vacher C-M, Crepin D, Aubourg A, Couvreur O, Bailleux V, Nicolas V, Férézou J, Gripois D, Gertler A, Taouis M (2008) A putative physiological role of hypothalamic CNTF in the control of energy homeostasis. FEBS Lett 582(27):3832–3838
Severi I, Perugini J, Mondini E, Smorlesi A, Frontini A, Cinti S, Giordano A (2013) Opposite effects of a high-fat diet and calorie restriction on ciliary neurotrophic factor signaling in the mouse hypothalamus. Front Neurosci 7:263
Yu L-J, Wu M-L, Li H, Chen X-Y, Wang Q, Sun Y, Kong Q-Y, Liu J (2008) Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia 10(7):736–744
Schneider Y, Duranton B, Goss F, Schleiffer R, Seiler N, Raul F (2001) Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutrition cancer 39(1):102–107
Beretta E, Dhillon H, Kalra PS, Kalra SP (2002) Central LIF gene therapy suppresses food intake, body weight, serum leptin and insulin for extended periods. Peptides 23(5):975–984
Oshima K, Teo DTW, Senn P, Starlinger V, Heller S (2007) LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol 7(1):112
Rios M (2013) BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci 36(2):83–90
Gage FH (2000) Mammalian neural stem cells. Science 287(5457):1433–1438
Faigle R, Song H (2013) Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochimica et Biophysica Acta (BBA) Gen Subjects 1830(2):2435–2448
Attari F, Zahmatkesh M, Aligholi H, Mehr SE, Sharifzadeh M, Gorji A, Mokhtari T, Khaksarian M, Hassanzadeh G (2015) Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to curcumin. DARU J Pharm Sci 23(1):33
Scapagnini G, Davinelli S, Kaneko T, Koverech G, Koverech A, Calabrese EJ, Calabrese V (2014) Dose response biology of resveratrol in obesity. J Cell Commun Signal 8(4):385–391
Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, Bach T, Zhao Y (2012) Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One 7(5):e37162
Acknowledgements
This investigation was supported by Tehran University of Medical Sciences and Health Services (Grant No. 93-01-161-25558), we wish to thank the Shefa Neuroscience Research Center scientists and staff.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Safahani, M., Aligholi, H., Noorbakhsh, F. et al. Switching from high-fat diet to foods containing resveratrol as a calorie restriction mimetic changes the architecture of arcuate nucleus to produce more newborn anorexigenic neurons. Eur J Nutr 58, 1687–1701 (2019). https://doi.org/10.1007/s00394-018-1715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1715-0