Abstract
Purpose
To assess if the associations found between three previously identified dietary patterns with breast, prostate and gastric cancer are also observed for colorectal cancer (CRC).
Methods
MCC-Spain is a multicase-control study that collected information of 1629 incident cases of CRC and 3509 population-based controls from 11 Spanish provinces. Western, Prudent and Mediterranean data-driven dietary patterns—derived in another Spanish case-control study—were reconstructed in MCC-Spain. Their association with CRC was assessed using mixed multivariable logistic regression models considering a possible interaction with sex. Risk by tumor site (proximal colon, distal colon, and rectum) was evaluated using multinomial regression models.
Results
While no effect of the Prudent pattern on CRC risk was observed, a high adherence to the Western dietary pattern was associated with increased CRC risk for both males [ORfourth(Q4) vs. first(Q1)quartile (95% CI): 1.45 (1.11;1.91)] and females [ORQ4 vs. Q1 (95% CI): 1.50 (1.07;2.09)] but seem to be confined to distal colon [ORfourth(Q4) vs. first(Q1)quartile (95% CI): 2.02 (1.44;2.84)] and rectal [ORQ4 vs. Q1 (95% CI): 1.46 (1.05;2.01)] tumors. The protective effect of the Mediterranean dietary pattern against CRC was observed for both sexes [males: ORQ4 vs. Q1 (95% CI): 0.71 (0.55;0.92); females: ORQ4 vs. Q1 (95% CI): 0.56 (0.40;0.77)] and for all cancer sites: proximal colon [ORQ4 vs. Q1 (95% CI): 0.70 (0.51;0.97)], distal colon [ORQ4 vs. Q1 (95% CI): 0.65 (0.48;0.89)], and rectum (ORQ4 vs. Q1 (95% CI): 0.60 (0.45;0.81)].
Conclusion
Our results are consistent with most of the associations previously found between these patterns and breast, prostate and gastric cancer risk and indicate that consuming whole fruits, vegetables, legumes, olive oil, nuts, and fish and avoiding red and processed meat, refined grains, sweets, caloric drinks, juices, convenience food, and sauces might reduce CRC risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of colorectal cancer (CRC) has increased in Europe in the last decades [1], being the second most diagnosed cancer in 2012 [2]. According to the scientific evidence, 40–50% of CRC cases are attributable to modifiable risk factors such as diet, physical activity and body weight [3, 4], providing major opportunities for prevention. The current evidence, points to a possible protective effect of foods containing dietary fiber and calcium against CRC [5, 6] and a detrimental effect of red and processed meat [6, 7] and alcohol consumption [6, 8, 9].
Despite the importance of these findings for individual foods, some authors suggest that the evaluation of the effects of full dietary patterns might be more appropriate, since it allows the exploration of the effect of food and nutrient interactions in disease [10,11,12]. Many indexes have been developed in the last years that evaluate dietary quality against predefined criteria [13, 14] and a recent metaanalysis found an inverse association between a high score for these indexes and cancer mortality and/or incidence [15]. However, these indexes are based on results in the area of cardiovascular disease and they refer to a theoretical diet that do not necessarily reflect the eating habits of a particular population. Moreover, moderate alcohol intake is positively considered in most of these indexes although alcohol consumption as low as one drink per day increases the risk of several tumors, including colorectal cancer [6]. In fact, some authors suggested that the lack of concordance of the results found for diet quality indexes and cancer might be due to their positive scoring for alcohol consumption [16]. As an alternative approach, dietary patterns that accurately represent the diet in a specific population can be identified with statistical methods like principal component analysis. These patterns also present the advantage of being extracted independently of disease associations, which allows exploration of the role of actual dietary habits in different health conditions. The scarce existing results for data-driven dietary patterns and CRC, indicate a possible protective effect of the so called Mediterranean/Healthy/Prudent dietary pattern [17,18,19,20,21,22] on CRC and a harmful effect of a pattern labelled as Western/Unhealthy diet [17,18,19,20,21,22,23], but the evidence is still insufficient.
A recent Spanish study on female breast cancer (BC)—EpiGEICAM—identified three data-driven dietary patterns [24] labelled as Western (associated with increased risk of BC), Prudent (not associated with BC) and Mediterranean (protective against BC). EpiGEICAM presents the novelty of being able to identify, over the same population, two different patterns (Prudent and Mediterranean) commonly interchanged in the literature [9, 18, 20, 21, 23, 25]. However, these patterns do not always represent the same dietary habits and the differences might be determinant in their association with disease risk, as it was the case for BC in the EpiGEICAM context [25]. Therefore, the replication of these patterns in different populations and the exploration of their association with tumors other than BC are of great scientific interest. In fact, the reproducibility of the patterns found has already been assessed in a different sample of Spanish women [26] and similar associations have been observed for other tumors and individuals. The detrimental effect of a high adherence to the Western dietary pattern has been corroborated for breast cancer [27] and also observed for gastric [28] cancer. These studies also show different results for the Prudent (null effect) and Mediterranean (protective) patterns in the case of breast [27], prostate [29] and gastric cancer [28].
The objective of the present study is to assess if the associations found between these dietary patterns—Western, Prudent and Mediterranean—with breast [24, 27], prostate [29] and gastric cancer [28] risk in our country are also observed for CRC risk, and to evaluate possible differences by sex and cancer site.
Patients and methods
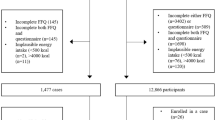
The multicase-control MCC-Spain study [30] recruited between 2008 and 2013 histologically confirmed incident cases of five tumors: breast, prostate, colorectal, stomach and chronic lymphocytic leukemia. Cases were recruited in 23 hospitals from 12 provinces and a single set of population controls, frequency matched by age and sex with the overall distribution of cases in each province, were randomly selected from the lists of residents assigned to primary health-care centers belonging to the same catchment area of each collaborating hospital. MCC-Spain recruited 2140 histologically confirmed CRC cases and 3950 population-based controls from 11 of the 12 contributing provinces. These numbers, represented the 64% of the CRC cases and the 53% of controls invited to participate (supplementary material, Figure S1). Potential participants had to be able to answer the questionnaire, had to live in the study area for at least 6 months before the diagnosis (cases) or at recruitment (controls) and had to be 20–85 years. Cases were identified as soon as possible after the diagnosis, and histologically confirmed incident cases of colon (ICD10 codes C18: malignant neoplasm of colon and D01.0: Carcinoma in situ of colon) or rectum (ICD10 codes C19: Malignant neoplasm of rectosigmoid junction; C20: Malignant neoplasm of rectum; D01.1: Carcinoma in situ of rectosigmoid junction and D01.2: Carcinoma in situ of rectum) cancer with no prior history of the disease and diagnosed within the recruitment period were included. They were classified according to the localization of tumor in proximal colon (including caecum, ascending and transverse colon and hepatic and splenic flexures), distal colon (including descending and sigmoid colon) and rectal cancer. When more than one tumor in different locations were diagnosed at the same time, the site in which the tumor was more invasive was assigned.
Information on socio-demographic factors, lifestyle and personal/family medical history was collected with a questionnaire administered by trained personnel in a face-to-face interview. Subsequent telephone contact was made to complete missing values on key variables. Height and weight at different ages were self-reported and diet was assessed with a 154-items semi-quantitative food frequency questionnaire (FFQ), which was based on an instrument validated in Spain [31]. Dietary information referred to the previous year before diagnosis (cases) or before interview (controls).
In the present study, three dietary patterns identified in a previous Spanish case-control study (EpiGEICAM) on female breast cancer (BC) [24] are examined: A western dietary pattern positively associated with BC risk that is characterized by high intakes of high-fat dairy products, processed meat, refined grains, sweets, caloric drinks, convenience food and sauces and by low intakes of low-fat dairy products and whole grains; A Prudent pattern not related to BC that represented high intakes of low-fat dairy products, vegetables, fruits, whole grains and juices; and a Mediterranean pattern that seemed to be protective and denoted a high intake of fish, vegetables, legumes, boiled potatoes, fruits, olives and vegetable oil—mainly olive oil (72%), and olives (22%) in our context-, and a low intake of juices. These three dietary patterns were identified in the EpiGEICAM sample in 2014 by grouping the dietary intake information collected with a 117 FFQ into 26 inter-correlated food groups and applying principal components analysis (PCA) without rotation of the variance–covariance matrix over these 26 food groups [32]. This method defines a set of weights (pattern loadings) associated with each food group that represents the correlation between food consumption and the component/pattern scores. Pattern loadings can be used to reproduce such patterns in other samples as explained in detail elsewhere [25, 26]. Briefly, we grouped 146 of the 154 items of the MCC-Spain FFQ (excluding non-caloric and alcoholic beverages) into 26 food groups defined in the EpiGEICAM study (see Table 1 for detailed information on the composition of food groups and their weight in the patterns). Afterwards, the scores of adherence to the Western, Prudent and Mediterranean dietary patterns of the MCC-Spain participants were calculated as a linear combination of the pattern loadings for each food group and dietary pattern extracted from the EpiGEICAM study [24] (Table 1) and the food group consumption reported by the MCC-Spain participants.
After describing the data, crude and adjusted associations between adherence to each dietary pattern and CRC risk were evaluated using logistic regression models with random province-specific intercepts. As fixed-effects terms, caloric and alcohol intake, self-reported body mass index (BMI) and physical activity [metabolic equivalents (METs)] during the 10 years before diagnosis (cases)/interview (controls), age, smoking status, education, family history of CRC and sex were considered as potential confounders. Scores of adherence were analyzed both, as categorical (grouping the scores of adherence into quartiles of their distribution among controls) and continuous variables (1-standard deviation increase taking into account the dispersion among controls). Heterogeneity of the effects by sex was tested including in the models an interaction term between the score of adherence and sex. Multinomial logistic regression models were used to evaluate the association of the adherence to the Western, Prudent and Mediterranean dietary patterns with proximal colon, distal colon and rectal cancer separately. These models were adjusted by the same set of variables described before but including province of residence as a fixed effect term.
Finally, assuming a causal relationship between the adherence to each of the patterns and CRC risk for all analyses, the population attributable fraction (PAF%) was calculated using Hanley’s J.A. formula [33] to estimate the proportion of total cancer in the population that hypothetically would not have occurred if all participants were in the optimal quartile of adherence to the dietary patterns (first quartile for Western and fourth quartile for Prudent and Mediterranean dietary patterns). Confidence intervals for PAF were computed using bootstrap with 500 iterations.
Analyses were performed using STATA/MP (version 14.1, 2015, StataCorp LP) and statistical significance was set at 2-sided p < 0.05.
Results
Among the initially recruited participants, 3509 (89%) controls and 1889 (88%) cases reported data on diet. Cases that provided dietary information later than 6 months after diagnosis were excluded (n = 260). Therefore, 1629 cases and 3509 controls aged 22–85 years were included in the study (supplementary Figure S1). The multivariable analyses were carried out over 1530 cases and 3240 controls, because data on either BMI (< 5%), physical activity (< 1%), smoking status (< 1%), total energy (< 2%) or alcohol intake (< 2%) was missing for 99 cases and 269 controls.
Compared to controls, CRC cases were more adherent to the Western (p < 0.001) and Mediterranean (p = 0.015) dietary patterns and reported higher energy (p < 0.001) and alcohol (p = 0.001) intake. CRC cases were also older (p < 0.001) and reported higher BMI (p < 0.001) and lower levels of physical activity (p < 0.001). The proportion of former smokers (p < 0.001), males (p < 0.001), participants with no formal education (p < 0.001) or with family history of CRC (p < 0.001) was also higher among cases (Table 2).
Results from Table 3 revealed a positive association between a high adherence to the Western dietary pattern and global CRC [ORfourth(Q4) vs. first(Q1)quartile (95% CI): 1.50 (1.20;1.87)] risk that was similar among males and females (p-interaction = 0.615). Once the difference in calorie intake was taken into account, a high adherence to the Mediterranean pattern appeared to be protective [ORQ4 vs. Q1 (95% CI): 0.65 (0.53;0.80)], with this effect slightly stronger among females [ORQ4 vs. Q1 (95% CI): 0.56 (0.40;0.77)] than among males [ORQ4 vs. Q1 (95% CI): 0.71 (0.55;0.92)], though the p value for the heterogeneity of the linear effects was not significant (p-interaction = 0.733). Assuming a causal relationship between diet and CRC risk, the estimations indicate that 1/4 and 1/5 of CRC cases could have been prevented if all the participants had been in the lowest category of adherence to the Western and in the highest category of adherence to the Mediterranean dietary patterns, respectively.
Stratified results by tumor subtype (Table 4) also indicate a detrimental effect of a high adherence to the Western dietary pattern over CRC risk that seems to be confined to distal colon [ORQ4 vs. Q1 (95% CI): 2.02 (1.44;2.84)] and rectal tumors [ORQ4 vs. Q1 (95% CI): 1.46 (1.05;2.01); p-heterogeneity = 0.087], while the protective effect of the Mediterranean dietary pattern was similar for all tumor sites [Proximal colon: ORQ4 vs. Q1 (95% CI): 0.70 (0.51;0.97); Distal Colon: ORQ4 vs. Q1 (95% CI): 0.65 (0.48;0.89); Rectum: ORQ4 vs. Q1 (95% CI): 0.60 (0.45;0.81); p-heterogeneity = 0.746]. In agreement with these results, it was estimated that more than 1/3 of distal colon and 1/4 of rectum tumors could have been prevented if all the study participants were in the lowest quartile of adherence to the Western dietary pattern and 1/5 for distal colon and 1/4 for rectum tumors could have been prevented with the highest adherences to Mediterranean dietary pattern.
A high adherence to the prudent pattern did not show an association with CRC risk.
Discussion
The detrimental effect of a high adherence to the Western dietary pattern for breast [24, 27] and gastric [28] cancer and the differential effect of a high adherence to the Prudent (null) and to the Mediterranean (protective) dietary patterns over breast [24, 27], prostate [29] and gastric cancer [28] identified in the previous studies was also found for CRC in the present work. Concretely, we found that a high adherence to the Western dietary pattern might increase CRC risk in both males and females and that such risk might be confined to distal colon and rectal cancer. Also, a high adherence to the Mediterranean dietary pattern showed a general protective effect against CRC that was very similar among males and females and for all cancer sites. On the contrary, the adherence to the Prudent dietary pattern was not associated to CRC.
Some recent reviews and metaanalysis [9, 19, 22], also report a positive association between a high adherence to the Western dietary pattern and CRC risk and a protective effect of a diet rich in fruits, vegetables, legumes and/or fish. The studies published after these reviews, also report a positive association of a high adherence to the Western dietary pattern with global CRC risk [18, 20, 21, 23] and a possible protective effect of a Healthy diet against this tumor [18, 20, 21]. In agreement with our results, some authors conclude that the effect of the Western and Healthy diet might be stronger for distal colon and rectal cancer [21, 22] or indicate stronger effects of the Western diet in distal colon tumors [9]. Only a few of these studies provide information of a possible interaction between diet and sex [18, 20, 21] and none of them report significant differences. Similarly, the current evidence for index based dietary patterns point to a detrimental effect of pro-inflammatory diets (similar to our Western pattern) for CRC risk [34] and a protective effect of diets that share common characteristics with our Mediterranean pattern against this type of tumor [34, 35]. One of the most important findings of the present study is the difference in the associations found for Prudent and Mediterranean dietary patterns. To understand these differences, we explored the associations of CRC risk with individual food groups (supplementary Table S1). We believe that the protective effect of the Mediterranean pattern against the null effect of the Prudent might be greatly explained by the protective effect of oily fish, nuts and olives and olive oil, only present in the Mediterranean pattern, but also by the detrimental effect of juices intake, only included in the Prudent pattern, that might counteract the positive effect of a high consumption of fruits, vegetables and whole grains characteristic of this pattern.
Some biological mechanisms support the associations found. On the one hand, the “Western”-like diet high in fat, refined grains, red and processed meats and sweets has been associated with higher levels of inflammatory markers [36] and with inflammation-related chronic diseases [37]. Moreover, the high content of iron in meat products present in this pattern generates free radicals that attack DNA and damage the tissue [38]. In addition, processing meat at high temperatures produces carcinogens such as N-nitroso and polycyclic aromatic hydrocarbons [39]. On the other hand, the antioxidants from fruits, vegetables and legumes present in the Mediterranean pattern may reduce risk by quenching free radicals and reducing oxidative damage to DNA [40]. Furthermore, fiber dilutes faecal content, decreases transit time and increases stool weight [41] contributing to a healthier gastrointestinal tract. Different carcinogenic pathways in proximal and distal tumors have been suggested, based on their molecular differences [42]. In this sense, the higher effect of the Western dietary pattern (characterized by a low dietary fiber intake) in distal colon and rectal tumors, might reflect a higher susceptibility to dietary carcinogens due to a less mature phenotype and lower immune activity of dendritic cells involved in immunologic surveillance at this location [43]. Olive oil intake has also been suggested to inhibit colon cancer development by inducing apoptosis and down-regulating the expression of cyclooxygenase2 and Bcl-2 proteins that have a crucial role in colorectal carcinogenesis [44]. Finally, the gut microbiome seems to play an important role in colorectal carcinogenesis [45], and dietary habits strongly influence it [46]. Turnbaugh et al. [46] recently demonstrated in an animal model that changing from low-fat, plant based diets to high-fat, high-sugar diets can shift the structure of the microbiota, modify the representation of metabolic pathways in the microbiome, and alter microbiome gene expression.
Our results should be interpreted in the context of the study’s limitations. Recall bias is always a concern in case-control studies. Anticipating the existence of this bias, some questions about general dietary habits were included in the questionnaire and used to adjust the responses to the FFQ [47]. In addition, only cases that responded to the questionnaire within the 6 months following their diagnosis were included. On the other hand, the participation rates (64% among CRC cases and 53% among controls) might give rise to some concerns about a possible selection bias. In this sense, participating controls might have healthier lifestyles than the general population, resulting in an overestimation of the effects. However, no effect was found for the prudent pattern that includes consumption of products widely known as “Healthy”. Therefore, we believe that this bias, if it exists, would be non-differential. Finally, the biological plausibility of the associations found, their strength, their consistency across sex and tumor site, their consistency with the results from other studies on CRC [9, 17,18,19,20,21,22,23] and the reproducibility of the results across different studies and tumors [24, 27,28,29], deem it unlikely that our findings are a result of recall or selection bias.
One of the main strengths of the current research is the recruitment of histologically confirmed incident cases of CRC and population controls. Furthermore, the geographical variability of the recruited participants, coming from 11 provinces from the North, South, Center, West and East of the country, ensured the representation of the different diets coexisting within Spain. Also, the sample size allowed the evaluation of potential interactions of diet and sex and the exploration of the associations by tumor localization. We also carried out a sensitivity analysis to explore all the associations excluding 42 in situ cases and obtained very similar results that led to the same exact conclusions (supplementary material Tables S2 and S3). In addition, as mentioned before, we explored the associations of CRC risk with individual food groups to ensure the associations found for patterns are not only due to the presence in the pattern of one or two foods associated with this tumor (supplementary material Table S1). High consumers of high-fat dairy products, meats, refined grains and sweets (products characteristic of the Western Pattern) showed higher risk of CRC, while high consumers of oily fish, vegetables, fruits, nuts and olive oil (foods present in the Mediterranean pattern) seemed to be protected against this tumor. Therefore, most of the components of the two patterns associated with CRC were also individually associated with this tumor, making it unlikely that the associations found for the whole dietary patterns are due only to the association of CRC with some individual foods. Finally, the reproducibility [26] and applicability [25] of the Western, Prudent and Mediterranean dietary patterns applied here was previously tested, demonstrating that the scores of adherence to these patterns can be calculated following the exact same rules over different populations, resulting in different levels of adherence but still being valid, which is supported by the similitude of the results found for breast [24, 27], prostate [29] and gastric cancer [28] and the present results found for CRC.
Our results provide evidence about very specific associations between diet and CRC that could be useful to clinical practitioners and public health professionals to offer nutritional recommendations based on avoiding the Western dietary pattern and promoting the Mediterranean diet. Even though other risk factors are involved in the genesis of these type of tumors, diet is a key risk factor for colorectal cancer. In this sense, if a country like Spain, with a high compliance with the Mediterranean diet and a moderate adherence to the Western diet, can benefit from abandoning the latter in favor of the former, the benefit might be greater in countries with unhealthier diets.
Conclusion
A high consumption of fruits, vegetables and whole grains combined with a low dietary fat intake might not be enough to prevent CRC. A fair percentage of colorectal cancer cases could be reduced in the general population by providing dietary recommendations based in a decrease of the consumption of high-fat dairy products, red and processed meat, refined grains, sweets, caloric drinks, juices, convenience food and sauces in favor of an increase in the intake of whole fruits, vegetables, legumes, olive oil, nuts and fish, especially for distal colon and rectal tumors.
References
Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D, Soerjomataram I (2015) Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur J Cancer 51(9):1164–1187. https://doi.org/10.1016/j.ejca.2013.09.002
Stewart B, Wild C (eds) (2014) World Cancer Report 2014. International Agency for Research on Cancer, Lyon
WCRF (2015) Cancer preventability estimates for diet, nutrition, body fatness, and physical activity. http://www.wcrf.org/int/cancer-facts-figures/preventability-estimates/cancer-preventability-estimates-diet-nutrition. Accessed 26 Oct 2016
Whiteman DC, Webb PM, Green AC, Neale RE, Fritschi L, Bain CJ, Parkin DM, Wilson LF, Olsen CM, Nagle CM, Pandeya N, Jordan SJ, Antonsson A, Kendall BJ, Hughes MC, Ibiebele TI, Miura K, Peters S, Carey RN (2015) Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 39(5):477–484. https://doi.org/10.1111/1753-6405.12471
Song M, Garrett WS, Chan AT (2015) Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148(6):1244–1260.e1216. https://doi.org/10.1053/j.gastro.2014.12.035
WCRF/AICR (2017) World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Diet, nutrition, physical activity and colorectal cancer. 2017. http://www.wcrf.org/sites/default/files/CUP%20Colorectal%20Report_2017_Digital.pdf. Accessed 10 Oct 2017
Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Kampman E, Norat T (2013) Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control CCC 24(4):611–627. https://doi.org/10.1007/s10552-012-0139-z
Cai S, Li Y, Ding Y, Chen K, Jin M (2014) Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev 23(6):532–539. https://doi.org/10.1097/cej.0000000000000076
Magalhaes B, Peleteiro B, Lunet N (2012) Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev 21(1):15–23. https://doi.org/10.1097/CEJ.0b013e3283472241
Barkoukis H (2007) Importance of understanding food consumption patterns. J Am Diet Assoc 107(2):234–236
Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13(1):3–9
Jacques PF, Tucker KL (2001) Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 73(1):1–2
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 82(1):163–173
Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, Schap TE, Reedy J (2015) The dietary patterns methods project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 145(3):393–402. https://doi.org/10.3945/jn.114.205336
Schwingshackl L, Bogensberger B, Hoffmann G (2018) Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 118(1):74–100.e111. https://doi.org/10.1016/j.jand.2017.08.024
Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, Morazzoni P, Negri E, Pelucchi C, Pezzotti M, Rondanelli M (2013) Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev 22(1):90–95. https://doi.org/10.1097/CEJ.0b013e328354d2d7
Safari A, Shariff ZM, Kandiah M, Rashidkhani B, Fereidooni F (2013) Dietary patterns and risk of colorectal cancer in Tehran Province: a case-control study. BMC Public Health 13:222. https://doi.org/10.1186/1471-2458-13-222
Park Y, Lee J, Oh JH, Shin A, Kim J (2016) Dietary patterns and colorectal cancer risk in a Korean population: a case-control study. Medicine 95(25):e3759. https://doi.org/10.1097/md.0000000000003759
Feng YL, Shu L, Zheng PF, Zhang XY, Si CJ, Yu XL, Gao W, Zhang L (2017) Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev 26(3):201–211. https://doi.org/10.1097/cej.0000000000000245
Haslam A, Wagner Robb S, Hebert JR, Huang H, Ebell MH (2017) Association between dietary pattern scores and the prevalence of colorectal adenoma considering population subgroups. Nutr Diet J Dietit Assoc Aust. https://doi.org/10.1111/1747-0080.12400
Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, Fung TT, Hamada T, Masugi Y, da Silva A, Shi Y, Li W, Gu M, Willett WC, Fuchs CS, Giovannucci EL, Ogino S, Chan AT (2017) Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 152(8):1944–1953.e1941. https://doi.org/10.1053/j.gastro.2017.02.015
Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A (2010) Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev 68(7):389–408. https://doi.org/10.1111/j.1753-4887.2010.00299.x
Tayyem RF, Bawadi HA, Shehadah I, Agraib LM, AbuMweis SS, Al-Jaberi T, Al-Nusairr M, Bani-Hani KE, Heath DD (2017) Dietary patterns and colorectal cancer. Clin Nutr (Edinburgh Scotland) 36(3):848–852. https://doi.org/10.1016/j.clnu.2016.04.029
Castello A, Pollan M, Buijsse B, Ruiz A, Casas AM, Baena-Canada JM, Lope V, Antolin S, Ramos M, Munoz M, Lluch A, de Juan-Ferre A, Jara C, Jimeno MA, Rosado P, Diaz E, Guillem V, Carrasco E, Perez-Gomez B, Vioque J, Boeing H, Martin M (2014) Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 111(7):9. https://doi.org/10.1038/bjc.2014.434
Castello A, Buijsse B, Martin M, Ruiz A, Casas AM, Baena-Canada JM, Pastor-Barriuso R, Antolin S, Ramos M, Munoz M, Lluch A, de Juan-Ferre A, Jara C, Lope V, Jimeno MA, Arriola-Arellano E, Diaz E, Guillem V, Carrasco E, Perez-Gomez B, Vioque J, Pollan M, Researchers G (2016) Evaluating the applicability of data-driven dietary patterns to independent samples with a focus on measurement tools for pattern similarity. J Acad Nutr Dietet 116(12):1914–1924 e1916. https://doi.org/10.1016/j.jand.2016.05.008
Castello A, Lope V, Vioque J, Santamarina C, Pedraz-Pingarron C, Abad S, Ederra M, Salas-Trejo D, Vidal C, Sanchez-Contador C, Aragones N, Perez-Gomez B, Pollan M (2016) Reproducibility of data-driven dietary patterns in two groups of adult Spanish women from different studies. Br J Nutr 116(4):734–742. https://doi.org/10.1017/S000711451600252X
Castello A, Boldo E, Perez-Gomez B, Lope V, Altzibar JM, Martin V, Castano-Vinyals G, Guevara M, Dierssen-Sotos T, Tardon A, Moreno V, Puig-Vives M, Llorens-Ivorra C, Alguacil J, Gomez-Acebo I, Castilla J, Gracia-Lavedan E, Davila-Batista V, Kogevinas M, Aragones N, Amiano P, Pollan M (2017) Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 103:8–15. https://doi.org/10.1016/j.maturitas.2017.06.020
Castelló A, Fernández de Larrea N, Martin M, Dávila-Batista V, Boldo E, Guevara M, Moreno V, Castaño-Vinyals G, Gómez-Acebo I, Fernandez-Tardon G, Peiro R, Olmedo-Requena R, Capelo R, Navarro C, Pacho-Valbuena S, Pérez-Gómez B, Kogevinas M, Pollán M, Aragonés N (2017) High adherence to the Western, Prudent and Mediterranean dietary patterns and risk of gastric adenocarcinoma. Gastric Cancer. https://doi.org/10.1007/s10120-017-0774-x
Castello A, Boldo E, Amiano P, Castano-Vinyals G, Aragones N, Gomez-Acebo I, Peiro R, Jimenez-Moleon JJ, Alguacil J, Tardon A, Cecchini L, Lope V, Dierssen-Sotos T, Mengual L, Kogevinas M, Pollan M, Perez-Gomez B, Researchers M-S (2017) Mediterranean dietary pattern is associated to low risk of aggressive prostate cancer: MCC-Spain study. J Urol. https://doi.org/10.1016/j.juro.2017.08.087
Castano-Vinyals G, Aragones N, Perez-Gomez B, Martin V, Llorca J, Moreno V, Altzibar JM, Ardanaz E, de Sanjose S, Jimenez-Moleon JJ, Tardon A, Alguacil J, Peiro R, Marcos-Gragera R, Navarro C, Pollan M, Kogevinas M, Group MC-SS (2015) Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gaceta Sanitaria SESPAS 29(4):308–315. https://doi.org/10.1016/j.gaceta.2014.12.003
Garcia-Closas R, Garcia-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, Tardon A, Carrato A, Castano-Vinyals G, Dosemeci M, Moore L, Rothman N, Sinha R (2007) Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer 43(11):1731–1740. https://doi.org/10.1016/j.ejca.2007.05.007
Burt C (1948) Factor analysis and canonical correlations. Br J Math Stat Psychol 1(2):95–106
Hanley JA (2001) A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health 55(7):508–514
Steck SE, Guinter M, Zheng J, Thomson CA (2015) Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr (Bethesda Md) 6(6):763–773. https://doi.org/10.3945/an.115.009746
Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu9101063
Barbaresko J, Koch M, Schulze MB, Nothlings U (2013) Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 71(8):511–527. https://doi.org/10.1111/nure.12035
Thorburn AN, Macia L, Mackay CR (2014) Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40(6):833–842. https://doi.org/10.1016/j.immuni.2014.05.014
Ashmore JH, Rogers CJ, Kelleher SL, Lesko SM, Hartman TJ (2016) Dietary iron and colorectal cancer risk: a review of human population studies. Crit Rev Food Sci Nutr 56(6):1012–1020. https://doi.org/10.1080/10408398.2012.749208
Santarelli RL, Pierre F, Corpet DE (2008) Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer 60(2):131–144. https://doi.org/10.1080/01635580701684872
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition (Burbank, Los Angeles County, Calif) 18(10):872–879
Giovannucci E, Willett WC (1994) Dietary factors and risk of colon cancer. Ann Med 26(6):443–452
Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S (2014) Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 25(10):1995–2001. https://doi.org/10.1093/annonc/mdu275
Bernardo D, Durant L, Mann ER, Bassity E, Montalvillo E, Man R, Vora R, Reddi D, Bayiroglu F, Fernandez-Salazar L, English NR, Peake ST, Landy J, Lee GH, Malietzis G, Siaw YH, Murugananthan AU, Hendy P, Sanchez-Recio E, Phillips RK, Garrote JA, Scott P, Parkhill J, Paulsen M, Hart AL, Al-Hassi HO, Arranz E, Walker AW, Carding SR, Knight SC (2016) Chemokine (C-C Motif) receptor 2 mediates dendritic cell recruitment to the human colon but is not responsible for differences observed in dendritic cell subsets, phenotype, and function between the proximal and distal colon. Cell Mol Gastroenterol Hepatol 2(1):22–39 e25. https://doi.org/10.1016/j.jcmgh.2015.08.006
Pelucchi C, Bosetti C, Negri E, Lipworth L, La Vecchia C (2011) Olive oil and cancer risk: an update of epidemiological findings through 2010. Curr Pharm Des 17(8):805–812
Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13(11):800–812. https://doi.org/10.1038/nrc3610
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1(6):6ra14. https://doi.org/10.1126/scitranslmed.3000322
Calvert C, Cade J, Barrett JH, Woodhouse A (1997) Using cross-check questions to address the problem of mis-reporting of specific food groups on Food Frequency Questionnaires. UKWCS Steering Group. United Kingdom Women’s Cohort Study Steering Group. Eur J Clin Nutr 51(10):708–712
Acknowledgements
This work was supported by Carlos III Institute of Health grants (PI12/00488, PI12/00265, PI12/00715, PI12/01270, PI11/01403, PI11/01889, PI11/00226, PI11/01810, PI11/02213, PI09/00773, PI09/01286, PI09/01903, PI09/02078, PI09/01662, PI08/1770, PI08/0533, PI08/1359), Spanish Ministry of Economy and Competitiveness (IJCI-2014-20900), Consejería de Salud de la Junta de Andalucía (PI-0306-2011; PI-0571-2009); Catalan Government DURSIgrant (2014SGR647);Instituto de Salud Carlos III, co-funded by FEDER funds—a way to build Europe—PI14-00613; Fundación Marqués de Valdecilla (API 10/09); Acción Transversal del Cancer, approved by the Spanish Ministry Council on October 11, 2007; Red Temática de Investigación del Cáncer del ISCIII (RD12/0036/0036); Junta de Castilla y León (LE22A10-2); Consejería de Salud de la Junta de Andalucía (2009-S0143); Conselleria de Sanitat de la Generalitat Valenciana (AP_061/10); Recercaixa (2010ACUP 00310); Regional Government of the Basque Country; Consejería de Sanidad de la Región de Murcia; European Commission grants (FOOD-CT-2006-036224-HIWATE); Spanish Association Against Cancer Scientific Foundation; Fundación Caja de Ahorros de Asturias; University of Oviedo. None of the sponsors intervened in any of the stages of the research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical standards
The MCC-Spain study protocol was approved by the Ethics Committee of each the participating institutions and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All participants were informed about the study objectives and signed an informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castelló, A., Amiano, P., Fernández de Larrea, N. et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr 58, 1495–1505 (2019). https://doi.org/10.1007/s00394-018-1674-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1674-5