Abstract
Background
Current evidence indicates that red and processed meat intake increases the risk of colorectal cancer; however, the association with colorectal adenomas is unclear.
Objective
To conduct a systematic review and meta-analysis of epidemiological studies of red and processed meat intake and risk of colorectal adenomas as part of the Continuous Update Project of the World Cancer Research Fund.
Design
PubMed and several other databases were searched for relevant studies from their inception up to 31 December 2011. Summary relative risks (RRs) were estimated using a random effects model.
Results
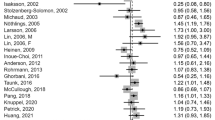
Nineteen case–control studies and seven prospective studies were included in the analyses. The summary RR per 100 g/day of red meat was 1.27 (95 % CI 1.16–1.40, I 2 = 5 %, n = 16) for all studies combined, 1.20 (95 % CI 1.06–1.36, I 2 = 0 %, n = 6) for prospective studies, and 1.34 (95 % CI 1.12–1.59, I 2 = 31 %, n = 10) for case–control studies. The summary RR per 50 g/day of processed meat intake was 1.29 (95 % CI 1.10–1.53, I 2 = 27 %, n = 10) for all studies combined, 1.45 (95 % CI 1.10–1.90, I 2 = 0 %, n = 2) for prospective studies, and 1.23 (95 % CI 0.99–1.52, I 2 = 37 %, n = 8) for case–control studies. There was evidence of a nonlinear association between red meat (p nonlinearity < 0.001) and processed meat (p nonlinearity = 0.01) intake and colorectal adenoma risk.
Conclusion
These results indicate an elevated risk of colorectal adenomas with intake of red and processed meat, but further prospective studies are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most common cancer worldwide with 1.2 million new cases diagnosed in 2008 [1]. Colorectal cancer is thought to develop through the adenoma-carcinoma sequence, with a stepwise progression leading to dysplastic changes in the epithelium of the colon and rectum [2]. The histologic type, size, and number of adenomas determine the risk of developing colorectal cancer [3]. Screening for colorectal adenomas and removal of such adenomas by colonoscopy is an important strategy to reduce colorectal cancer risk [4]. Although lifestyle factors are considered to be of major importance in colorectal cancer etiology [5–9], less is known about how such factors are related to risk of colorectal adenomas. Studying risk factors for colorectal adenomas could enhance our understanding of the early stages of colorectal carcinogenesis.
Red and processed meat intake was judged to be convincing risk factors for colorectal cancer in the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) report “Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective” from 2007, and we recently confirmed a positive association between red and processed meat intake and colorectal cancer in an updated meta-analysis of the evidence from prospective studies up to 2011 [9]. However, the WCRF/AICR report did not find a significant association between red or processed meat intake and colorectal adenomas, but the number of studies assessed was modest (a total of 5 prospective studies, 4 case–control studies) [5]. A number of additional case–control [10–16] and prospective studies [17–22] have since been published on the subject. We update the evidence as accumulated up to December 2011 and explore whether the associations reported differed by study design and other study characteristics. We further investigated whether the association between red and processed meat intake differs for small and large adenomas.
Methods
Search strategy
We updated the systematic literature review published in 2007 [5] by searching the PubMed database from its inception up to December 2011 for studies of red and processed meat intake and colorectal adenoma risk. Several reviewers at Wageningen University carried out the literature search and extracted data up to end of December 2005 during the systematic literature review for the WCRF/AICR report (http://www.dietandcancerreport.org/cancer_resource_center/downloads/SLR/Colorectal_polyps_SLR.pdf). Initially, several databases were used for the searches, including PubMed, Embase, CAB Abstracts, ISI Web of Science, BIOSIS, Latin American and Caribbean Center on Health Sciences Information, Cochrane library, Cumulative Index to Nursing and Allied Health Literature, the Allied and Complementary Medicine Database, National Research Register, and In Process Medline. However, as all the relevant studies were identified through PubMed, a change was made to the protocol and only PubMed was used for the updated searches. A predefined protocol was used for the review (http://www.dietandcancerreport.org/cup/report_overview/index.php) and includes details of the search terms used. The search from January 2006 and up to end of December 2011 was conducted by one of the authors (DSMC). Data were extracted by three authors (DSMC, DANR, and ARV). We also reviewed the reference lists of the relevant articles and previously published systematic reviews for additional studies [23, 24]. We followed standard criteria for conducting and reporting meta-analyses [25].
Study selection
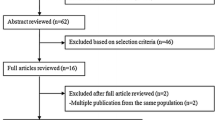
Studies were eligible for inclusion if they were prospective or case–control studies and presented estimates of the relative risk (such as hazard ratio, risk ratio, or odds ratio) with the 95 % confidence intervals or the information to calculate the confidence intervals. Prospective studies were defined as studies where the diet of the participants was assessed at baseline before diagnosis of colorectal adenoma and the population was followed up over time for development of colorectal adenomas. Case–control studies were defined as studies where individuals with colorectal adenomas and controls were asked to recall their past diet (most of the studies asked about diet in the year before colorectal adenoma diagnosis or colonoscopy/interview for controls). In these studies, there was no follow-up period. Studies that asked for current diet at the time of colorectal adenoma diagnosis were considered to be cross-sectional studies and were not included in our analysis. For the dose–response analysis, a quantitative measure of intake had to be provided. When we identified duplicate publications, we selected the publication with the largest number of cases. In a few cases, several papers were published from the same study, but reported on different meat items or subgroups in the different papers, and in this case, several papers from the same study were included, but each publication was only included once in each analysis. Fifty-seven potentially relevant full text publications [10–22, 26–69] were identified. We excluded eight duplicate publications [21, 31, 46, 49–53]. Additional publications that did not report on red or processed meat intake [54, 55, 57–65], or reported only on serrated polyps [66], or a combined adenoma and cancer outcome (neoplasia) [48] or adenoma recurrence [67–69] were also excluded. For the dose–response analysis, we further excluded three publications because there were only two categories of exposure [14, 37] or the intake was not quantified [32]. We used data from a previous publication from the Nurses’ Health study [34] in the dose–response analysis because the most recent publication only provided a high versus low comparison [18]. For the subgroup analysis by adenoma size, we used data from the publication by Gunter et al. [30] in the analysis of red meat because such results were not available in the original publication [26]. Authors of 7 papers [10, 12, 14, 17, 26, 29, 33] were contacted for clarification of the definition of red meat and sufficient detail was provided by 4 of these [10, 17, 29, 33].
Data extraction
The following data were extracted from each study: The first author’s last name, publication year, country where the study was conducted, study design, adenoma size when available, follow-up period, sample size, gender, age, number of cases, dietary assessment method (type, number of food items and whether it had been validated), meat exposure, quantity of intake, relative risks (RRs) and 95 % CIs and variables adjusted for in the analysis.
Statistical methods
We used random effects models to calculate summary RRs and 95 % CIs associated with red and processed meat intake [70]. The natural logarithm of the RR from each study was weighted by the inverse of its variance and pooled across studies. A two-tailed p < 0.05 was considered statistically significant. For studies that reported results stratified by gender [32, 33], adenoma size [38], or other subgroups [10, 28, 56], we calculated a combined estimate of the association by using a fixed effects model before including the study in the overall analysis.
We used the method described by Greenland and Longnecker [71] to compute study-specific slopes (linear trends) and 95 % CIs from the natural logs of the RRs and CIs across categories of red and processed meat intake. The method requires that the distribution of cases and person-years or non-cases and the RRs with the variance estimates for at least three quantitative exposure categories are known. We estimated the distribution of cases or person-years in studies that did not report these. The reported median or mean level of red and processed meat intake in each category of intake was assigned to the corresponding relative risk for each study. For studies that reported intake by ranges, we estimated the midpoint in each category by calculating the average of the lower and upper bound. When the highest or lowest category was open-ended, it was assumed that the open-ended interval length had the same length as the adjacent interval. When studies reported the intake in servings and times per day or week, we converted the intakes to grams of intake per day using standard units of 120 g for red meat and 50 g for processed meat [72]. Results are presented per 100 g per day for red meat and 50 g per day for processed meat for comparison with our previous results for colorectal cancer [9]. A potential nonlinear dose–response relationship was examined using fractional polynomial models [73]. We determined the best fitting second-order fractional polynomial regression model, defined as the one with the lowest deviance. A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity [73].
Statistical heterogeneity among studies was assessed by I 2 which is the amount of total variation that is explained by between-study variation and the Q test [74]. We conducted subgroup and meta-regression analyses by study characteristics to investigate potential sources of heterogeneity. Small study bias, such as publication bias, was assessed with funnel plots, Egger’s test [75] and with Begg’ test [76], and the results were considered to indicate potential small study bias when p < 0.10. We conducted sensitivity analyses excluding one study at a time to explore whether the results were robust to the influence of single studies. Results from these sensitivity analyses are presented excluding the two studies with the most positive and negative influence on the summary estimate.
Stata version 10.1 software (StataCorp, College Station, TX, USA) was used for the statistical analyses.
Results
Nineteen case–control studies (24 publications) [10–16, 26–30, 35–45, 56] and seven cohort studies (9 publications) [17–20, 22, 32–34, 47] were included in the analyses of red and processed meat intake and colorectal adenomas (Tables 1, 2). Ten studies were from Europe, twelve from the US, three from Asia, and one from Australia. A summary of the study characteristics of the included studies is provided in Tables 1, 2.
Red meat
Eleven case–control studies [10–16, 26–28, 56] and seven cohort studies [17–20, 22, 32, 33] investigated red meat intake and colorectal adenomas and included 21,493 cases among 234,451 participants. Some studies included processed red meat in the red meat variable (Tables 1, 2). The summary RR for high versus low intake was 1.22 (95 % CI: 1.11–1.34), with moderate heterogeneity, I 2 = 46 % and p heterogeneity = 0.02, and it was 1.16 (95 % CI: 1.03–1.30, I 2 = 48 %, p heterogeneity = 0.07) for prospective studies and 1.29 (95 % CI: 1.13–1.48, I 2 = 23 %, p heterogeneity = 0.23) for case-control studies (Supplementary Figure 1a). In the dose-response analysis the summary RR was 1.27 (95 % Cl: 1.16–0.40, I 2 = 5 %, p heterogeneity = 0.40) per 100 g/d (Fig. 1a). The summary RR for prospective studies was 1.20 (95 % CI 1.06–1.36, I 2 = 0 %, p heterogeneity = 0.97), and it was 1.34 (95 % CI 1.12–1.59, I 2 = 31 %, p heterogeneity = 0.16) for case–control studies (Fig. 1a), but there was no evidence of heterogeneity by study design, p heterogeneity = 0.27 (Table 3). In sensitivity analyses excluding the studies with the most influence on the summary estimate the summary RR ranged from 1.21 (95 % CI 1.10–1.34) when the study by Fu et al. [16] was excluded to 1.29 (95 % CI: 1.17–1.43) when the study by Wu et al. [49] was excluded. There was no indication of small study effects with Egger’s test, p = 0.80, or with Begg’s test, p = 0.50. The association between red meat intake and colorectal adenoma risk appeared to be nonlinear, p nonlinearity < 0.001, with the steepest increase in risk at the lower levels of intake (Fig. 1b, Supplementary Table 3). Further restricting the analysis to the studies that reported on fresh red meat and colorectal adenoma risk [10, 13, 15–17, 20, 22, 28, 33, 45, 56] did not materially alter the results, the summary RR for high versus low intake was 1.25 (95 % CI: 1.15–1.36, I 2 = 0 %, p heterogeneity = 0.51) for all studies combined, 1.17 (95 % CI: 1.02–1.34, I 2 = 0 %, p heterogeneity = 0.92) for prospective studies, and 1.28 (95 % CI: 1.12–1.47, I 2 = 15 %, p heterogeneity = 0.31) for case-control studies.
Processed meat
Nine case–control studies [11, 12, 16, 26, 35–39] and two cohort studies [17, 22] were included in the analysis of processed meat and colorectal adenoma risk and included 6,098 cases among 41,538 participants. The summary RR for high versus low intake was 1.29 (95 % CI 1.15–1.45), with low heterogeneity, I 2 = 18 %, p heterogeneity = 0.27 and it was 1.33 (95 % CI: 1.09–1.62, I 2 = 16 %, p heterogeneity = 0.28) for prospective studies and 1.27 (95 % CI: 1.09–1.48, I 2 = 27 %, p heterogeneity = 0.21) for case-control studies (Supplementary Figure 1b). The summary RR per 50 g/day increase in the intake was 1.29 (95 % CI 1.10–1.53), with low heterogeneity, I 2 = 27 %, p heterogeneity = 0.19 (Fig. 2a). The summary RR was 1.45 (95 % CI 1.10–1.90, I 2 = 0 %, p heterogeneity = 0.41) for prospective studies and 1.23 (95 % CI 0.99–1.53, I 2 = 37 %, p heterogeneity = 0.13) for case–control studies, with no evidence of heterogeneity by study design, p heterogeneity = 0.46. In sensitivity analyses excluding the studies with the most influence on the summary estimate, the summary RR ranged from 1.24 (95 % CI 1.02–1.50) when the study by Fu et al. [16] was excluded to 1.38 (95 % CI 1.20–1.58) when the study by Benito et al. [35] was excluded. There was no indication of small study effects with Egger’s test, p = 0.30, or with Begg’s test, p = 0.37. The association between processed meat intake and colorectal adenoma risk appeared to be nonlinear, p nonlinearity = 0.01, with a slight flattening of the curve at higher levels of intake (Fig. 2b, Supplementary Table 4).
Subgroup, sensitivity, and meta-regression analyses
In subgroup analyses of red meat intake and colorectal adenoma, there were positive associations across all strata, and heterogeneity between subgroups was not observed for red meat (Table 3). When we further restricted the subgroup analyses to prospective studies, the results for red meat persisted in all strata of subgroups with adjustment for different confounding factors (Supplementary Table 1 and 2). In the analyses of processed meat and colorectal adenomas, there was significant or borderline significant heterogeneity in subgroups defined by geographic location, p heterogeneity = 0.04, number of cases, p heterogeneity = 0.06, and adjustment for energy intake, p heterogeneity = 0.03 (Table 3). The association was restricted to American studies and was more pronounced in studies with a large number of cases and in studies that adjusted for energy intake. Exclusion of one study [37] that reported unadjusted results from the high versus low analysis of processed meat intake and colorectal adenoma did not change the conclusions, summary RR = 1.27 (95 % CI 1.14–1.41, I 2 = 11 %, p heterogeneity = 0.34) (the study was not included in the dose–response analysis). We also conducted nonlinear dose–response analyses stratified by study design (Supplementary Figure 2a and 2b), but the conclusions were similar, with a weaker effect for red meat in prospective studies and a stronger effect of processed meat in prospective studies compared with case–control studies.
Because adenomas often develop without symptoms, it is possible that some of the studies in the analysis may have included prevalent adenoma cases if no colonoscopy was conducted at baseline. For this reason, we conducted an additional sensitivity analysis among the four prospective studies of red meat with both a baseline and a follow-up colonoscopy which included only incident adenoma cases [17, 20, 22, 34]. The summary per 100 g/day RR was 1.18 (95 % CI: 1.01–1.37, I 2 = 0 %, p heterogeneity = 0.95), similar to the overall analysis.
For the case–control studies, we restricted the analysis to the two studies that reported that diet was assessed before colonoscopy (before the participants knew their case–control status) [11, 16], and the summary RR was 1.69 (95 % CI 1.34–2.12), while it was 1.26 (95 % CI 1.07–1.48) for the remaining case–control studies.
High versus low intake of beef (summary RR = 1.40, 95 % CI 1.18–1.67, I 2 = 19 %, p heterogeneity = 0.28) [16, 26, 29, 40–44], hamburgers (summary RR = 1.23, 95 % CI 1.06–1.43, I 2 = 0 %, p heterogeneity = 0.67) [16, 17, 44, 45], and pork (summary RR = 1.55, 95 % CI 1.05–2.30, I 2 = 37 %, p heterogeneity = 0.20) [16, 40, 44], but not bacon (summary RR = 1.12, 95 % CI 0.99–1.27, I 2 = 0 %, p heterogeneity = 0.58) [16, 39, 45], was also associated with significantly increased risk of colorectal adenomas (Table 3).
High versus low red meat intake was associated with an increased risk of large adenomas (≥1 cm diameter), summary RR = 1.57 (95 % CI 1.12–2.19, I 2 = 7 %) [17, 19, 30, 31], but not with small-sized adenomas (<1 cm), summary RR = 0.97 (95 % CI 0.66–1.42, I 2 = 0 %) [17, 19], although there was no heterogeneity between subgroups, p heterogeneity = 0.13. The association was similar for advanced, summary RR = 1.38 (95 % CI 1.04–1.84, I 2 = 0.31) and non-advanced adenomas, summary RR = 1.31 (95 % CI 1.10–1.57, I 2 = 0.31) [10, 16]. Because one of the criteria for advanced adenomas is a large adenoma size and because of the limited number of studies in the analyses by adenoma size and stage, we conducted an additional analysis where we combined studies that reported results for large and advanced adenomas and studies that reported on small and non-advanced adenomas. The summary RRs were 1.47 (95 % CI 1.18–1.81) for advanced or large adenomas [10, 16, 17, 19, 30, 31] and 1.24 (95 % CI 1.05–1.46) for non-advanced or small adenomas [10, 16, 17, 19], but there was no heterogeneity between subgroups, p heterogeneity = 0.26. Similar analyses were not possible for processed meat because of lack of studies.
Discussion
In this meta-analysis, we found an increased risk of colorectal adenomas with higher intake of red and processed meat intake, and the positive associations appeared to be consistent across strata in subgroup analyses. Although there was no heterogeneity by study design, the results for red meat appeared to be stronger in case–control studies than in cohort studies, while for processed meat, the opposite was observed.
The findings of this meta-analysis are consistent with the previously reported increased risks of colorectal cancer associated with red and processed meat intake [5, 9] and provide further support for an association between red and processed meat intake and colorectal carcinogenesis. Two previous meta-analyses did not find a significant association between intake of red and processed meat and colorectal adenomas, but were limited by a low number of studies included in the analyses [5, 23]. However, with a total of 26 studies accumulated up to 2011, we found significant associations between both red and processed meat and subtypes of meat, such as beef, pork, and hamburgers and increased risk of colorectal adenomas. A few additional studies did not find an association between meat intake and colorectal adenoma recurrence [67, 69, 77], but it is possible that risk factors differ for incidence and recurrence of adenomas.
Our meta-analysis may have several limitations that deserve comment. High intake of red and processed meat is oftentimes associated with other risk factors such as low intake of fiber, lower physical activity, higher prevalence of obesity, smoking, and high alcohol intake [22]. Many of the studies adjusted for these confounders, and in several subgroup analyses, we found that the results persisted across subgroups with adjustment for these and other potential confounders. In addition, there was little evidence that the results differed whether or not confounding factors had been adjusted for or not. However, we cannot exclude the possibility that residual confounding partly could explain the results. Small study effects, such as publication bias can be a problem in meta-analyses of published literature, but we found no evidence of small study effects in this analysis. Since we included case–control studies, there is a possibility that recall bias and selection bias partly could explain the results in such studies. However, when we restricted the results to the two studies that assessed diet before colonoscopy was conducted (before the subjects knew their case–control status) the results persisted. When we restricted the analysis to prospective studies, the results also persisted, although the results were somewhat weaker for red meat. Because adenomas often develop without symptoms, a potential limitation is that some of the studies may have included prevalent adenoma cases if a colonoscopy had not been conducted at baseline (in cohort studies) or previously (in case–control studies). None of the case–control studies conducted analyses restricted to subjects with a previous colonoscopy. In addition, although most of the case–control studies asked about past diet, it is still possible that the adenomas may already have existed at the time point they were asked to recall their diet for. However, when we restricted the analysis to the four cohort studies with both a baseline and a follow-up colonoscopy, which included only incident adenoma cases, the results were similar to the overall results for cohort studies for red meat.
Due to the limited number of studies reporting results for subsites within the colorectum, we did not have adequate power to clarify whether the risk differed between colon or rectum or proximal and distal colon. Although we found that the results for red meat did not differ by geographic location or study size, there was heterogeneity between these subgroups in the analysis of processed meat. The association between processed meat and colorectal adenomas was observed only in the American studies and not in the European studies, but it is not clear what the reason for this is. It might be due to differences in the consumption patterns or additives used for processing or it could be a chance finding because there were only three European studies in the analysis. The association between processed meat and adenomas was stronger in the larger studies than in the smaller studies. In addition, we cannot exclude the possibility that low numbers of observations at the low or high ends of the range of intakes partly could contribute to the nonlinear observations that we observed.
Measurement error in the dietary assessment is another limitation of our results. None of the studies included in our analysis made any corrections for measurement error.
Several mechanisms might explain an increased risk of colorectal adenoma with high red and processed meat intakes. Heterocyclic amines and polycyclic aromatic hydrocarbons, meat mutagens that are formed during frying and barbecuing of meats, have been shown to be gastrointestinal carcinogens in experimental animal studies [78]. These compounds can form DNA adducts and induce genetic alterations characteristic of colorectal tumors [79]. The heme–iron content of meats may contribute to colorectal neoplasia by inducing oxidative DNA damage [80] and by increasing endogenous formation of N-nitroso compounds [81] which are known to be powerful multisite carcinogens [82]. Red meat intake was positively associated with risk of large adenomas, but not small adenomas, although there were few studies in these analyses. However, when we grouped large and advanced adenomas and small and non-advanced adenomas together, the association was significant for both types, but was somewhat stronger for the large and advanced adenomas. Large or advanced adenomas convey a greater colorectal cancer risk than small or non-advanced adenomas [3], suggesting that red meat intake might play a role in the progression to malignancy. However, we cannot exclude the possibility that persons with a high intake of red and processed meat are less likely to undergo screening and that this could have contributed to this finding. The summary estimate per 100 g/day for red meat and colorectal adenomas among cohort studies, RR = 1.20 (95 % CI 1.06–1.36, n = 6) is similar to that of a recent meta-analysis [24] and is also similar to the summary estimate that we previously reported for colorectal cancer, RR = 1.17 (95 % CI 1.05–1.31), although for processed meat the results for adenomas are stronger, summary RR = 1.45 (1.10–1.90, n = 2) for colorectal adenomas versus 1.18 (95 % CI 1.10–1.28, n = 9) for colorectal cancer; however, there were only 2 cohort studies in the analysis of colorectal adenomas, and thus, this difference might have been due to chance [9].
Strengths of this meta-analysis include the comprehensive search strategy, dose–response, subgroup, and sensitivity analyses. With the large number of studies and study participants, we had adequate statistical power to detect significant associations in the main analyses.
In conclusion, we found a positive association between red and processed meat intake and risk of colorectal adenomas. Our results provide further support that red and processed meat intake is implicated in colorectal carcinogenesis; however, further prospective studies are warranted.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Leslie A, Carey FA, Pratt NR, Steele RJ (2002) The colorectal adenoma-carcinoma sequence. Br J Surg 89:845–860
Atkin WS, Morson BC, Cuzick J (1992) Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 326:658–662
Atkin WS, Edwards R, Kralj-Hans I et al (2010) Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375:1624–1633
World Cancer Research Fund/American Insitute for Cancer Research Food (2007) Nutrition, physical activity and the prevention of cancer: a global perspective. AICR, Washington, DC
Aune D, Chan DS, Lau R et al (2011) Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343:d6617
Aune D, Lau R, Chan DS et al (2011) Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 141:106–118
Aune D, Lau R, Chan DS et al (2012) Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol 23:37–45
Chan DS, Lau R, Aune D et al (2011) Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS ONE 6:e20456
Saebø M, Skjelbred CF, Brekke LK et al (2008) CYP1A2 164 A→C polymorphism, cigarette smoking, consumption of well-done red meat and risk of developing colorectal adenomas and carcinomas. Anticancer Res 28:2289–2295
Ferrucci LM, Sinha R, Graubard BI et al (2009) Dietary meat intake in relation to colorectal adenoma in asymptomatic women. Am J Gastroenterol 104:1231–1240
Wang H, Yamamoto JF, Caberto C et al (2011) Genetic variation in the bioactivation pathway for polycyclic hydrocarbons and heterocyclic amines in relation to risk of colorectal neoplasia. Carcinogenesis 32:203–209
Northwood EL, Elliott F, Forman D et al (2010) Polymorphisms in xenobiotic metabolizing enzymes and diet influence colorectal adenoma risk. Pharmacogenet Genomics 20:315–326
Ramadas A, Kandiah M (2009) Food intake and colorectal adenomas: a case-control study in Malaysia. Asian Pac J Cancer Prev 10:925–932
Burnett-Hartman AN, Newcomb PA, Mandelson MT et al (2011) Colorectal polyp type and the association with charred meat consumption, smoking, and microsomal epoxide hydrolase polymorphisms. Nutr Cancer 63:583–592
Fu Z, Shrubsole MJ, Smalley WE et al (2011) Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev Res (Phila) 4:1686–1697
Wu K, Giovannucci E, Byrne C et al (2006) Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol Biomarkers Prev 15:1120–1125
Cho E, Willett WC, Colditz GA et al (2007) Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst 99:1224–1231
Rohrmann S, Hermann S, Linseisen J (2009) Heterocyclic aromatic amine intake increases colorectal adenoma risk: findings from a prospective European cohort study. Am J Clin Nutr 89:1418–1424
Tantamango YM, Knutsen SF, Beeson WL, Fraser G, Sabate J (2011) Foods and food groups associated with the incidence of colorectal polyps: the adventist health study. Nutr Cancer 63:565–572
Cross AJ, Sinha R, Wood RJ et al (2011) Iron homeostasis and distal colorectal adenoma risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Prev Res (Phila) 4:1465–1475
Ferrucci LM, Sinha R, Huang WY et al (2012) Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer 106:608–616
Yoon H, Benamouzig R, Little J, Francois-Collange M, Tome D (2000) Systematic review of epidemiological studies on meat, dairy products and egg consumption and risk of colorectal adenomas. Eur J Cancer Prev 9:151–164
Xu X, Yu E, Gao X et al (2012) Red and processed meat intake and risk of colorectal adenomas: a meta-analysis of observational studies. Int J Cancer 132:437–448
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012
Haile RW, Witte JS, Longnecker MP et al (1997) A sigmoidoscopy-based case-control study of polyps: macronutrients, fiber and meat consumption. Int J Cancer 73:497–502
Sinha R, Chow WH, Kulldorff M et al (1999) Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res 59:4320–4324
Voskuil DW, Kampman E, Grubben MJ et al (2002) Meat consumption and meat preparation in relation to colorectal adenomas among sporadic and HNPCC family patients in The Netherlands. Eur J Cancer 38:2300–2308
Tiemersma EW, Voskuil DW, Bunschoten A et al (2004) Risk of colorectal adenomas in relation to meat consumption, meat preparation, and genetic susceptibility in a Dutch population. Cancer Causes Control 15:225–236
Gunter MJ, Probst-Hensch NM, Cortessis VK, Kulldorff M, Haile RW, Sinha R (2005) Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis 26:637–642
Shin A, Shrubsole MJ, Ness RM et al (2007) Meat and meat-mutagen intake, doneness preference and the risk of colorectal polyps: the Tennessee Colorectal Polyp Study. Int J Cancer 121:136–142
Kahn HS, Tatham LM, Thun MJ, Heath CW Jr (1998) Risk factors for self-reported colon polyps. J Gen Intern Med 13:303–310
Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S (2001) Diet and colorectal adenoma in Japanese males and females. Dis Colon Rectum 44:105–111
Chan AT, Ma J, Tranah GJ et al (2005) Hemochromatosis gene mutations, body iron stores, dietary iron, and risk of colorectal adenoma in women. J Natl Cancer Inst 97:917–926
Benito E, Cabeza E, Moreno V, Obrador A, Bosch FX (1993) Diet and colorectal adenomas: a case-control study in Majorca. Int J Cancer 55:213–219
Macquart-Moulin G, Riboli E, Cornee J, Kaaks R, Berthezene P (1987) Colorectal polyps and diet: a case-control study in Marseilles. Int J Cancer 40:179–188
Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C (2002) Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps—a case control study. Eur J Nutr 41:35–43
Senesse P, Boutron-Ruault MC, Faivre J, Chatelain N, Belghiti C, Meance S (2002) Foods as risk factors for colorectal adenomas: a case-control study in Burgundy (France). Nutr Cancer 44:7–15
Ward MH, Cross AJ, Divan H et al (2007) Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis 28:1210–1216
Kune GA, Kune S, Read A, MacGowan K, Penfold C, Watson LF (1991) Colorectal polyps, diet, alcohol, and family history of colorectal cancer: a case-control study. Nutr Cancer 16:25–30
Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL (1993) Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst 85:884–891
Lubin F, Rozen P, Arieli B et al (1997) Nutritional and lifestyle habits and water-fiber interaction in colorectal adenoma etiology. Cancer Epidemiol Biomarkers Prev 6:79–85
Breuer-Katschinski B, Nemes K, Marr A et al (2001) Colorectal adenomas and diet: a case-control study. Colorectal Adenoma Study Group. Dig Dis Sci 46:86–95
Chiu BC, Gapstur SM (2004) Changes in diet during adult life and risk of colorectal adenomas. Nutr Cancer 49:49–58
Probst-Hensch NM, Sinha R, Longnecker MP et al (1997) Meat preparation and colorectal adenomas in a large sigmoidoscopy-based case-control study in California (United States). Cancer Causes Control 8:175–183
Shin A, Shrubsole MJ, Rice JM et al (2008) Meat intake, heterocyclic amine exposure, and metabolizing enzyme polymorphisms in relation to colorectal polyp risk. Cancer Epidemiol Biomarkers Prev 17:320–329
Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC (1992) Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst 84:91–98
Lieberman DA, Prindiville S, Weiss DG, Willett W (2003) Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 290:2959–2967
Wu K, Hu FB, Fuchs C, Rimm EB, Willett WC, Giovannucci E (2004) Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Causes Control 15:853–862
Sinha R, Peters U, Cross AJ et al (2005) Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res 65:8034–8041
Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N (2005) Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev 14:2030–2034
Sinha R, Kulldorff M, Chow WH, Denobile J, Rothman N (2001) Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 10:559–562
Diergaarde B, Tiemersma EW, Braam H et al (2005) Dietary factors and truncating APC mutations in sporadic colorectal adenomas. Int J Cancer 113:126–132
Almendingen K, Hofstad B, Trygg K, Hoff G, Hussain A, Vatn M (2001) Current diet and colorectal adenomas: a case-control study including different sets of traditionally chosen control groups. Eur J Cancer Prev 10:395–406
Almendingen K, Hofstad B, Vatn MH (2004) Dietary habits and growth and recurrence of colorectal adenomas: results from a three-year endoscopic follow-up study. Nutr Cancer 49:131–138
Wark PA, Van der KW, Ploemacher J et al (2006) Diet, lifestyle and risk of K-ras mutation-positive and -negative colorectal adenomas. Int J Cancer 119:398–405
Ferrucci LM, Cross AJ, Gunter MJ et al (2010) Xenobiotic metabolizing genes, meat-related exposures, and risk of advanced colorectal adenoma. World Rev Nutr Diet 101:34–45
Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S (1990) A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res 81:1101–1108
Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K (1991) Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: a study of self-defense officials in Japan. J Clin Epidemiol 44:1255–1261
Kono S, Imanishi K, Shinchi K, Yanai F (1993) Relationship of diet to small and large adenomas of the sigmoid colon. Jpn J Cancer Res 84:13–19
Todoroki I, Kono S, Shinchi K et al (1995) Relationship of cigarette smoking, alcohol use, and dietary habits with sigmoid colon adenomas. Ann Epidemiol 5:478–483
Neugut AI, Garbowski GC, Lee WC et al (1993) Dietary risk factors for the incidence and recurrence of colorectal adenomatous polyps. A case-control study. Ann Intern Med 118:91–95
Faivre J, Boutron MC, Senesse P, Couillault C, Belighiti C, Meny B (1997) Environmental and familial risk factors in relation to the colorectal adenoma–carcinoma sequence: results of a case-control study in Burgundy (France). Eur J Cancer Prev 6:127–131
Hoshiyama Y, Kono S, Sasaba T, Shigematsu T, Kawaguchi T (2000) Relation of cigarette smoking, alcohol use, and dietary habits to colon adenomas: a case-control study in Saitama, Japan. Asian Pac J Cancer Prev 1:139–146
Skjelbred CF, Saebø M, Hjartaker A et al (2007) Meat, vegetables and genetic polymorphisms and the risk of colorectal carcinomas and adenomas. BMC Cancer 7:228
Wallace K, Grau MV, Ahnen D et al (2009) The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 18:2310–2317
Robertson DJ, Sandler RS, Haile R et al (2005) Fat, fiber, meat and the risk of colorectal adenomas. Am J Gastroenterol 100:2789–2795
Tseng M, Sandler RS, Greenberg ER, Mandel JS, Haile RW, Baron JA (1997) Dietary iron and recurrence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 6:1029–1032
Mathew A, Sinha R, Burt R et al (2004) Meat intake and the recurrence of colorectal adenomas. Eur J Cancer Prev 13:159–164
DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28:105–114
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Norat T, Lukanova A, Ferrari P, Riboli E (2002) Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer 98:241–256
Royston P (2000) A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 19:1831–1847
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Martinez ME, Jacobs ET, Ashbeck EL et al (2007) Meat intake, preparation methods, mutagens and colorectal adenoma recurrence. Carcinogenesis 28:2019–2027
Ohgaki H, Kusama K, Matsukura N et al (1984) Carcinogenicity in mice of a mutagenic compound, 2-amino-3-methylimidazo[4,5-f]quinoline, from broiled sardine, cooked beef and beef extract. Carcinogenesis 5:921–924
Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M (1998) High frequency of beta-catenin (ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res 58:1127–1129
Tappel A (2007) Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses 68:562–564
Cross AJ, Pollock JR, Bingham SA (2003) Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res 63:2358–2360
Lijinsky W (1987) Carcinogenicity and mutagenicity of N-nitroso compounds. Mol Toxicol 1:107–119
Acknowledgments
We thank the systematic literature review team at Wageningen University for their contributions to the colorectal adenoma database. We thank Dr. Camilla Furu Skjelbred, Professor Elin H. Kure, Dr. Chisato Nagata and Dr. Kana Wu for clarifying definitions of red meat in their studies. The views expressed in this review are the opinions of the authors. They may not represent the views of WCRF International/AICR and may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer risk. The systematic literature review team at the Wageningen University conducted the search, data selection, and data extraction up to December 2005. DSMC did the updated literature search and study selection. DSMC, DANR, and ARV did the updated data extraction. DA conducted the statistical analyses and wrote the first draft of the original manuscript. DCG was expert statistical advisor and contributed toward the statistical analyses. All authors contributed to the revision of the manuscript. DA had primary responsibility for final content. EK was PI of the SLR at Wageningen University and TN is the PI of the Continuous Update Project. All authors had full access to all of the data in the study. The authors declare that there are no conflicts of interest. This work was funded by the World Cancer Research Fund (grant number 2007/SP01) as part of the Continuous Update Project (www.dietandcancerreport.org).
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aune, D., Chan, D.S.M., Vieira, A.R. et al. Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control 24, 611–627 (2013). https://doi.org/10.1007/s10552-012-0139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-012-0139-z