Abstract
Purpose
The aim of this study was to examine the associations between the risk of cognitive impairment and the serum levels of folate, vitamin B12, and homocysteine (Hcy).
Methods
Subjects were persons aged 60–79 years who participated in the Yangpyeong Cohort study between 2011 and 2012. Cognitive impairment and normal subjects consisted of 100 pairs of old adults matched by age, sex, and education levels. Cognitive function was evaluated with the Korean version of the Mini-Mental State Examination for Dementia Screening (MMSE-DS). Pearson’s partial correlation coefficients and conditional multiple logistic regression analysis were applied to determine the associations between cognitive function and the serum levels of folate, vitamin B12, and Hcy.
Results
Compared with the matched normal group, the cognitive impairment group had higher proportions of folate deficiency (< 3 ng/mL) and hyperhomocysteinemia (≥ 15 µmol/L). Serum Hcy concentrations were inversely associated with serum folate (r = − 0.234, p = 0.001) and MMSE-DS score (r = − 0.150, p = 0.037) after adjusting for age, sex, and education. The high Hcy group showed a higher prevalence of cognitive impairment (4th vs. 1st quartile, OR 3.30, 95% CI 1.12–9.72, p for trend = 0.014) after adjusting for exercise.
Conclusions
The present findings suggest a putative protective role of high serum folate and normal Hcy against cognitive impairment among older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia not only destroys the quality of life for patients and their family, but also introduces a number of health and social problems [1]. In addition, patients with dementia incur higher medical expenditures per head than those with hypertension or diabetes [1]. South Korea has one of the most rapidly aging populations in the world [2]. According to a nationwide study in 2008, the prevalence of patients with dementia and mild cognitive impairment (MCI) aged ≥ 65 years was estimated to increase by 9.6 and 24.4% in 2012, and by 13.2 and 26.6% until 2050 [1]. Because the conversion rate of MCI to dementia is approximately 5–10% [3], early detection and therapy for MCI are important to reduce the population with dementia [4].
Low serum folate and vitamin B12 concentrations were reported to be connected with elevated homocysteine (Hcy) in many cross-sectional and longitudinal studies [2, 5, 6]. Several potential mechanisms, such as one-carbon metabolism playing a contributory role in methylation reactions, have been proposed to explain the connections among folate and vitamin B12 and Hcy [7]. Furthermore, hyperhomocyteinemia (HHcy) has been suggested as a risk factor for cognitive impairment [8]. More recently, it has been demonstrated that increased Hcy serves as a neurotoxin to promote neurodegeneration via apoptosis through DNA breakage [7]. Despite many studies, the relationships of cognitive function to serum levels of folate, vitamin B12, and Hcy are inconclusive.
Thus, the aim of the present study was to examine the associations between cognitive impairment and the levels of serum folate, vitamin B12, and Hcy concentrations in 100 pairs of adults aged 60–79 years matched by age, sex, and education levels.
Methods
Data source and study population
The Yangpyeong cohort was initiated in 2004 to determine the risk factors for cardiovascular disease and obtain genomic data in adults aged ≥ 40 years. A health interview and health examination survey obtained using a questionnaire, as well as anthropometry and blood samples of this cohort data between 2011 and 2012 from adults aged 60–79 years were used. Among 700 participants, we excluded 44 subjects who had not provided education information (n = 5) or MMSE-DS scores (n = 35) and those who had implausible energy intake (> 4000 or < 500 kcal, n = 4) [9]. 656 subjects were categorized into subjects with cognitive impairment (n = 169) or normal subjects (n = 487) using MMSE-DS score. Cognitive impairment and matched normal group consisted of 100 pairs from 656 subjects matched by age (year of birth, ± 2 years), sex (43 pairs of male, 57 pairs of female) and education level. The present study was approved by the Institutional Review Boards of Hanyang University and was performed in accordance with the Declaration of Helsinki. All subjects gave written informed consent.
General characteristics, anthropometric data, and health behavior data
Data from the health interview and health examination survey were used to determine general characteristics, anthropometric data, health behavior data, and disease prevalence. The height (cm) and weight (kg) of the subjects were measured, and the body mass index (BMI) was calculated as weight (kg)/height (m2). Regular exercise was defined as a weekly frequency of sweat-inducting activity. Medical history of diabetes mellitus, stroke, and hypertension was obtained from face to face interviews by a trained researcher. Supplement users were identified as persons who had taken vitamins (A, C, E, or D), folate, beta-carotene, multi-vitamins, minerals, or fatty acids supplements since the last checkup. Depressive symptoms were investigated using the Center for Epidemiologic Studies Depression (CES-D) scale. The CES-D consists of 20 total items with self-reporting of depression symptoms (0 point; “not at all”, 1 point; “1–2 days/week”, 2 points; “3–4 days/week”, 3 points; “have experience of depression symptoms over 5 days/week”). The highest score in this scale is 60 points, and high scores indicate a high risk of depressive symptoms.
Cognitive functional examination
The Mini-Mental Status Examination (MMSE) is commonly used as a simple tool for screening cognitive impairment [10]. In Korea, the Korean MMSE (K-MMSE) or MMSE in the Korean version (MMSE-KC) is commonly used. The diagnostic accuracy of the Korean version of the Mini-Mental State Examination for Dementia Screening (MMSE-DS) is better than that of the K-MMSE and MMSE-KC [11]. Therefore, the current study employed MMSE-DS to evaluate the cognitive function of the subjects. The diagnostic accuracy of the MMSE-DS was proved using the area under the receiver-operating characteristic curve [11]. The reliability was confirmed by an inter-rater (r = 0.968, p < 0.001) and test–retest (r = 0.825, p < 0.001) method, and the validity was identified by the Clinical Dementia Rating (r = − 0.698, p < 0.05) [12]. The MMSE-DS was administered by a trained researcher in a one-on-one manner. The MMSE-DS consists of a total of 19 questions including time orientation (5 questions, 5 points), place orientation (5 questions, 5 points), registration and recall (2 questions, 6 points), attention and calculation (1 question, 5 points), naming and repetition (2 questions, 3 points), three-stage command (1 question, 3 points), copying interlocking pentagons (1 question, 1 point), and judgment and abstract thinking (2 questions, 2 points). The highest score for this screening tool is 30 points, and lower scores show worse cognitive function. Subjects were divided into cognitive impairment and normal subjects according to sex, age, and education levels (‘0–3’, ‘4–6’, ‘7–12’, ≥ 13 years) using the normative table presented in the MMSE-DS. Cognitive impairment was classified using ≤ − 1.5 standard deviation (SD) of MMSE-DS mean score [13]. SD was calculated from the MMSE-DS mean of 1008 healthy volunteers aged ≥ 60 years that did not have dementia, MCI, physical health problems, or psychoneurotic disease [13].
Biochemical examination
Blood samples were collected from the participants, who did not eat for at least 12 h before the examination. The coagulated blood was transferred to Eppendorf tubes and serum was extracted using a centrifugal separator. Serum was stored in the refrigerator at − 70 °C before analysis. Serum concentrations of folate, vitamin B12, and Hcy were measured using ADVIA Centaur Folate, VB12, HCY reagent, and Centaur XP (Siemens Healthcare, Inc., Malvern, PA, USA). Coefficients of variation (CV) between and within experimenters were as follows (5.26–7.19% and 4.54–7.93% for folate; 2.7–9.2% and 2.4–5.0% for vitamin B12; 1.5–5.2% and 2.3–4.4% for Hcy). Serum folate (< 3 ng/mL) [14], and vitamin B12 (< 80 pg/mL) [14] were considered as deficient, respectively. Levels of Hcy (≥ 15 µmol/L) were considered as hyperhomocysteinemia [15].
Statistical analysis
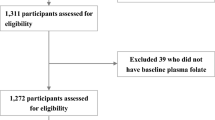
The general characteristics of the cognitive impairment and the matched normal group were analyzed using the paired t test for continuous variables and the McNemar’s Chi-square test for categorical variables. To compare the prevalence of the deficiency of folate (< 3 ng/mL), vitamin B12 (< 80 g/mL), and HyperHcy (≥ 15 µmol/L) between cognitive impairment and the matched normal group, Fisher’s exact test and McNemar’s Chi-square test were performed. The general linear model and the Cochran–Armitage trend test were applied to determine characteristics across serum quartile levels of folate, vitamin B12, and Hcy. The associations among serum folate, vitamin B12, Hcy, and MMSE-DS score were assessed using the partial correlation coefficients after adjusting for age, sex, and education levels. Conditional multiple logistic regression analysis was applied to obtain the odds ratios (OR) and p for the trend in models. The first model was a crude model and no variables were adjusted. In the second model (adjusted model), the regular exercise variable was additionally adjusted. In the analyses of serum vitamin B12, three pairs of subjects who had ≥ 10,000 pg/mL were excluded. A p value < 0.05 was considered significant. All statistical analyses were performed using SAS 9.3 statistical package (SAS Institute, Inc., Cary, NC, USA) (Fig. 1).
Results
The characteristics of the cognitive impairment and the matched normal group are described in Table 1. Both groups had equal proportions of age, sex, and education levels, because they were matched for these covariates. MMSE-DS scores (mean ± SD) were significantly lower among cognitive impairment (20.8 ± 3.6) as compared to matched normal group (26.0 ± 2.3). Compared with the matched normal subjects, the cognitive impairment had lower proportions of regular exercise (≥ 1 day/week).
Serum concentrations of folate (< 3 ng/mL), vitamin B12 (< 80 pg/mL), and Hcy (≥ 15 µmol/L) between the cognitive impairment and the matched normal group are compared in Table 2. The proportions of normal concentrations in folate and Hcy were lower in the cognitive impairment group than the matched normal group.
Table 3 shows the characteristics of matched normal subjects according to serum concentrations of folate, vitamin B12, and homocysteine. The proportions of supplement users increased across the quartiles of serum folate concentrations. Current drinker decreased across the quartiles of serum vitamin B12 concentrations. The mean age increased with the quartiles of serum Hcy concentrations, but supplement users decreased across the quartiles of serum Hcy concentrations.
The relationships among serum folate, vitamin B12, Hcy concentrations, and MMSE-DS scores are presented in Table 4. Serum Hcy concentration was inversely associated with serum folate (r = − 0.234, p = 0.001), and MMSE-DS score (r = − 0.150, p = 0.037) after adjusting for age, sex, and education levels.
Associations between the quartiles of serum folate, Hcy, and vitamin B12 and the prevalence of cognitive impairment are shown in Table 5. Serum folate concentrations were inversely associated with cognitive impairment in the crude model (4th vs. 1st quartile, OR 0.31, 95% CI 0.12–0.77, p for trend = 0.024). However, after additional adjustment for regular exercise, the inverse association between cognitive impairment and serum folate was no longer significant (4th vs. 1st quartile, OR 0.38, 95% CI 0.14–1.01, p for trend = 0.096). There was a decreasing trend between serum vitamin B12 concentrations and the risk of cognitive impairment, but the associations were not significant. Serum Hcy concentrations showed positive associations with the risk of cognitive impairment in two models.
Discussion
This study included 100 subjects with cognitive impairment and 100 matched normal subjects among older adults aged 60–79 years. The present study was conducted to examine the associations between the risk of cognitive impairment and the serum status of folate, vitamin B12, and Hcy.
Subjects with cognitive impairment had 2 and 16% higher prevalence of deficiency in folate (< 3 ng/mL) and HyperHcy (≥ 15 µmol/L) than the matched normal subjects in the present study. These results were the same as the previous studies [16, 17]. Wahlin et al. reported the association of low levels of serum vitamin B12 and folic acid with episodic memory performance in persons aged 75–96 years [16]. The cognitive impairment group with low levels of B vitamins had lower memory performance than the matched normal group with normal levels of B vitamins.
In the present study, decreased folate levels were associated with raised Hcy levels. Serum folate was inversely associated with cognitive impairment in the crude model, but this association was no longer significant after additionally adjusting for exercise. The association between folate and Hcy can be explained by one-carbon metabolism [7]. N5-methyl tetrahydrofolate (THF), which is converted to folate in the body, plays a role as a methyl donor [18]. Methionine receives a methyl group from THF, changing into Hcy [18]. Hence, if serum folate concentrations are deficient, Hcy levels are higher [19]. This is similar to the results in the majority of existing studies [5, 6]. Furthermore, a meta-analysis with 25 randomized controlled trials (RCTs) reported a dose-dependent reduction in plasma Hcy concentrations with incremental doses of folate supplementation up to 0.8 mg/d [20]. In an RCT by Durga et al., 3-year folic acid supplementation decreased Hcy concentrations and improved domains of cognitive function [21].
This study found that elevated Hcy concentrations were associated with decreased cognitive score. Moreover, the prevalence of cognitive impairment was elevated across increasing quartiles of Hcy concentrations. The connection between HHcy and cognitive decline such as MCI and AD can be explained by the following potential mechanism. Elevated Hcy enters intracellular sites through a membrane transporter [22]. In the brain, HHcy may promote hypomethylation apoptosis and DNA breakage caused by impaired DNA transmethylation [7, 8, 23]. As a result, HHcy may be connected with cognitive decline [8]. Several studies demonstrated that HHcy was related to cognitive decline or cognitive impairment prevalence [6, 24,25,26,27,28]. However, some studies are inconsistent with our results [26, 29]. The baseline data in other studies revealed associations similar to our results, but the follow-up results were inconclusive [30, 31]. In prospective studies, the findings suggest that high Hcy levels at the baseline may predict cognitive decline in 5-year follow-up [32]. On the other hand, other longitudinal study reported that red blood cell folate and plasma Hcy were related to better global cognition at the baseline, but they were not associated with the rate of decline over 5 years [31]. In an intervention study, supplement containing folate, vitamin B6, and vitamin B12 during 14 weeks significantly decreased serum total Hcy and improved cognitive function in middle-aged and elderly patients with HyperHcy [33]. However, in a meta-analysis with 11 trials, Hcy lowering using B vitamins did not slow the rate of cognitive aging [34].
However, serum vitamin B12 concentrations were not connected with serum Hcy in the present study. Vitamin B12 performs the role of a cofactor in one-carbon metabolism [18]. Thus, if serum vitamin B12 concentrations were deficient, serum Hcy concentrations may be increased [19]. However, unlike this mechanism, the results of many previous investigations like our study also suggested that there was no association between vitamin B12 and Hcy levels [35]. In addition, serum vitamin B12 concentration was not associated with cognitive score in our results. No definite associations were made with vitamin B12, but there are several speculations. The first reason is the small sample size in the present study. Second, our study did not use newer biomarkers such as holotranscobalamin (holoTC) and methylmalonic acid (MMA) [35]. Systematic review of O’Leary et al. revealed that future studies should use holoTC and/or MMA to establish a clear link between vitamin B12 and cognitive decline [35].
In this study, there was an interesting finding about exercise. Matched normal subjects had higher proportions of regular exercise than cognitive impairment subjects. Moreover, the relationship between cognitive impairment and levels of serum folate vanished in the model after adjusting for regular exercise. The existing studies suggested that exercise reduces the risk of cognitive impairment by attenuating neurodegenerative processes and loss of neuropil and synaptic connections [36,37,38]. These biologic mechanisms may be related to the rise of neurotropic factors such as brain-derived neurotrophic factor, vascular endothelial growth factor, and insulin-like growth factor 1 about exercise [39,40,41,42,43,44,45,46,47,48]. In meta-analyses studies of 29 RCTs over 1–12 months, exercise improved cognitive scores in healthy adults without dementia [49]. In addition, exercise in older adults was associated with increased brain volumes after 6-month RCTs compared with sedentary interventions [50].
Our study has the following limitations. First, it is difficult to apply the results to a general population of older adults, because the majority of subjects in the Yangpyeong cohort were farmers and housewives. Second, education level, which is widely known to be associated with cognitive function, differs between the Yangpyeong subjects and the general public aged 60–79 years [51]. Third, the number of subjects was small to detect small-to-medium effects. Fourth, since blood samples and MMSE-DS scores were collected at the same time, we cannot conclude causality of the serum folate, vitamin B12, and Hcy with cognitive function.
Nevertheless, this study had several strengths. First, the accuracy of the study was enhanced by matching the subjects according to age, sex, and education levels, which are reported to be associated with cognitive function [13]. Second, selection bias was reduced, because cognitive impairment subjects were selected with normal subjects from the same region.
Considering the findings above, lower concentrations of serum folate correlate with higher concentrations of serum Hcy, and higher serum Hcy increases the prevalence of cognitive impairment in those 60 years or older living in rural areas. Although this study is limited, because the Yangpyeong cohort resided in rural areas, the findings suggest a putative protective role of high serum folate and normal Hcy against cognitive impairment among older adults. Further studies are necessary to examine these findings in prospective and intervention studies.
References
Cho M, Kim K, Kim M, Kim M, Kim S, Kim J (2008) Nationwide study on the prevalence of dementia in Korean elders. Seoul: Seoul National University Hospital. Ministry of Health, Welfare, and Family Affairs 227
Kim G, Kim H, Kim KN, Son JI, Kim SY, Tamura T, Chang N (2013) Relationship of cognitive function with B vitamin status, homocysteine, and tissue factor pathway inhibitor in cognitively impaired elderly: a cross-sectional survey. J Alzheimer’s Dis 33:853–862
Mitchell AJ, Shiri-Feshki M (2009) Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119:252–265
Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56:1133–1142
Koike T, Kuzuya M, Kanda S, Okada K, Izawa S, Enoki H, Iguchi A (2008) Raised homocysteine and low folate and vitamin B-12 concentrations predict cognitive decline in community-dwelling older Japanese adults. Clin Nutr 27:865–871
Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ (2002) Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr 75:908–913
Sachdev PS (2005) Homocysteine and brain atrophy. Prog Neuro-Psychopharmacol Biol Psychiatry 29:1152–1161
Lehmann M, Gottfries C, Regland B (1999) Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord 10:12–20
Willett WC (1998) Nutritional epidemiology. Oxford University Press, New York
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Kim TH, Jhoo JH, Park JH, Kim JL, Ryu SH, Moon SW, Choo IH, Lee DW, Yoon JC, Do YJ (2010) Korean version of mini mental status examination for dementia screening and its’ short form. Psychiatry Invest 7:102–108
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Han JW, Kim TH, Jhoo JH, Park JH, Kim JL, Ryu SH, Moon SW, Choo IH, Lee DW, Yoon JC (2010) A normative study of the Mini-Mental State Examination for Dementia Screening (MMSE-DS) and its short form (SMMSE-DS) in the Korean elderly. J Korean Geriatr Psychiatry 14:27–37
World Health Organization (1968) Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967]
Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH (1993) Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem 39:1764–1779
Wahlin Å, Hill RD, Winblad B, Bäckman L (1996) Effects of serum vitamin B12 and folate status on episodic memory performance in very old age: A population-based study. Psychol Aging 11:487
Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW (2005) Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 82:1346–1352
Finkelstein J (1998) The metabolism of homocysteine: pathways and regulation. Eur J Pediatr 157:S40-S44
Vogel T, Dali-Youcef N, Kaltenbach G, Andres E (2009) Homocysteine, vitamin B12, folate and cognitive functions: a systematic and critical review of the literature. Int J Clin Pract 63:1061–1067
Homocysteine Lowering Trialists’ Collaboration (2005) Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 82:806–812
Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P (2007) Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369:208–216
Grieve A, Butcher S, Griffiths R (1992) Synaptosomal plasma membrane transport of excitatory sulphur amino acid transmitter candidates: kinetic characterisation and analysis of carrier specificity. J Neurosci Res 32:60–68
Mattson MP, Shea TB (2003) Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 26:137–146
Ravaglia G, Forti P, Maioli F, Vettori C, Grossi G, Bargossi AM, Caldarera M, Franceschi C, Facchini A, Mariani E (2001) Elevated plasma homocysteine levels in centenarians are not associated with cognitive impairment. Mech Ageing Dev 121:251–261
Morris MS (2003) Homocysteine and Alzheimer’s disease. Lancet Neurol 2:425–428
Stewart R, Asonganyi B, Sherwood R (2002) Plasma Homocysteine and Cognitive Impairment in an Older British African-Caribbean Population. J Am Geriatr Soc 50:1227–1232
De Luis D, Fernandez N, Arranz M, Aller R, Izaola O (2002) Total homocysteine and cognitive deterioration in people with type 2 diabetes. Diabetes Res Clin Pract 55:185–190
Sachdev PS, Valenzuela MJ, Brodaty H, Wang XL, Looi J, Lorentz L, Howard L, Jones M, Zagami AS, Gillies D, Wilcken DE (2003) Homocysteine as a risk factor for cognitive impairment in stroke patients. Dement Geriatr Cogn Disord 15:155–162
Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Jolles J, Clarke R, Breteler MM, Rotterdam Scan Study (2002) Homocysteine and cognitive function in the elderly: the Rotterdam Scan study. Neurology 59:1375–1380
Kado D, Selhub J, Seeman T (2001) Plasma total homocysteine levels and cognitive function in older high functioning adults: Macarthur Studies of Successful Aging. J Am Geriatr Soc 49:S13–S13
Mendonca N, Granic A, Mathers JC, Martin-Ruiz C, Wesnes KA, Seal CJ, Jagger C, Hill TR (2017) One-carbon metabolism biomarkers and cognitive decline in the very old: the Newcastle 85+ study. J Am Med Dir Assoc 17:1.e1–1.e9
McCaddon A, Hudson P, Davies G, Hughes A, Williams JH, Wilkinson C (2001) Homocysteine and cognitive decline in healthy elderly. Dement Geriatr Cogn Disord 12:309–313
Cheng D, Kong H, Pang W, Yang H, Lu H, Huang C, Jiang Y (2016) B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr Neurosci 19:461–466
Clarke R, Bennett D, Parish S et al (2014) Effects of homocysteine with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 100:657–666
O’Leary F, Allman-Farinelli M, Samman S (2012) Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr 108:1948–1961
Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC (2011) Physical exercise as a preventive or disease-modifying treatment of dementia brain aging. Mayo Clin Proc 86:876–884
Terry R, Katzman R (2001) Life span and synapses: will there be a primary senile dementia? Neurobiol Aging 22:347–348
Hof PR, Morrison JH (2004) The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci 27:607–613
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99:11946–11950
Zigova T, Pencea V, Wiegand SJ, Luskin MB (1998) Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci 11:234–245
Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS (2000) Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20:2896–2903
Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, Lee KH, Kim SY, Han SH, Woo JI (2002) Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B 57:P47–P53
Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F (2006) Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140:823–833
Trejo JL, Carro E, Torres-Aleman I (2001) Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21:1628–1634
Ding Y, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y (2006) Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res 3:15–23
Cotman CW, Berchtold NC, Christie L (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431
Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G (2006) Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging 27:1505–1513
Smith AD, Smith SM, De Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H (2010) Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE 5:e12244
Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A 61:1166–1170
Statistics Korea (2010) Population by sex, age and educational attainment (6 years old and over). http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parmTabId=M_01_01#SubCont. Accessed 19 May 2016
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A1041792).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The corresponding authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, S., Choi, B.Y., Nam, J.H. et al. Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur J Nutr 58, 399–408 (2019). https://doi.org/10.1007/s00394-017-1604-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1604-y