Abstract
Purpose
While reports of inadequate vitamin D intakes among young children are widespread, data on the prevalence of vitamin D deficiency are inconsistent. We aimed to quantify vitamin D intake and serum 25-hydroxyvitamin D [25(OH)D] concentrations in children aged 2 years in the prospective Cork BASELINE Birth Cohort Study.
Methods
Serum 25(OH)D was analysed using UPLC-MS/MS in 741 children living in Cork, Ireland (51°N). Two-day weighed food diaries were collected in 467 children, and 294 provided both a blood sample and a food diary.
Results
Mean (SD) 25(OH)D concentrations were 63.4 (20.4) nmol/L [winter: 54.5 (19.9), summer: 71.2 (17.5)]. The prevalence of vitamin D deficiency (<30 nmol/L) was 4.6, and 26.7% were <50 nmol/L [45.2% during winter (November–April) and 10.4% in summer (May–October)]. With a mean (SD) vitamin D intake of 3.5 (3.1) µg/day, 96% had intakes below 10 µg/day, the current IOM estimated average requirement and the SACN safe intake value for this age group. After adjustment for season, vitamin D intake (µg/day) was associated with higher 25(OH)D concentrations [adjusted estimate (95% CI) 2.5 (1.9, 3.1) nmol/L]. Children who did not consume vitamin D-fortified foods or supplements had very low vitamin D intakes (1.2 (0.9) µg/day), and during winter, 12 and 77% were <30 and <50 nmol/L, respectively, compared with 6 and 44% of fortified food consumers.
Conclusion
There was a high prevalence of low vitamin D status during winter, especially among children who did not consume fortified foods or nutritional supplements. Our data indicate the need for dietary strategies to increase vitamin D intakes in this age group. This report provides further evidence that DRVs for vitamin D should be based on experimental data in specific population groups and indicates the need for dose–response RCTs in young children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary recommendations have a key role in protecting the population against vitamin D deficiency, and over the last 6 years, several authoritative agencies have redefined their vitamin D recommendations in the light of increasing evidence of adverse health effects at low vitamin D status, indicated by serum concentrations of 25-hydroxyvitamin D [25(OH)D]. Most agencies selected musculoskeletal outcomes as the health indicators with the most developed evidence base, particularly from randomised controlled trials (RCTs), on which to nominate serum 25(OH)D thresholds designating deficient/sufficient vitamin D status [1]. Following analysis of dose–response vitamin D intake—serum 25(OH)D data, collected under conditions of minimal UVB availability, these 25(OH)D thresholds provided the basis for estimating population and/or individual vitamin D intake recommendations [2–6]. The published dietary reference values (DRVs) are somewhat diverse; individual recommendations vary from 10 to 20 μg/day [400–800 IU], depending on the 25(OH)D target, ranging from 25 to 50 nmol/L.
In the UK, the Scientific Advisory Committee on Nutrition (SACN) proposed a ‘population protective’ serum 25(OH)D concentration of 25 nmol/L, defined as the minimum threshold that should be met or exceeded by almost everyone, and recommended a corresponding intake of 10 μg/day for all persons >1 year of age [3]. Four other agencies based their recommendations on achieving a serum 25(OH)D threshold ≥50 nmol/L [2, 4–6], with some important distinctions. The Institute of Medicine (IOM) [2] and NORDEN [5] recommended intakes of 15 and 10 μg/day, respectively, with increased allowances for older adults. These two agencies also proposed an average requirement (AR) intake of 10 and 7.5 μg/day, respectively, to meet the needs of 50% of the population—this value is not an individual recommendation but used by nutritional epidemiologists to evaluate the adequacy of vitamin D intakes in the population [7]. The IOM committee proposed the serum 25(OH)D concentration of 40 nmol/L as the adequacy threshold and this value was used to specify the EAR [2], while the Nordic AR is based on a threshold of 50 nmol/L [5]. The German Nutrition Society [6] and more recently the European Food Safety Authority (EFSA) [4] did not make firm intake recommendations, but opted for adequate intake (AI) values of 20 and 15 μg/day, respectively, with the 50 nmol/L threshold in mind. The AI is reserved for use only in cases of much uncertainty in the data, where it is not possible to make firmer recommendations. As such it has limited utility in public health or clinical practice.
Notwithstanding the disparity in DRVs from different authoritative agencies, the lack of both observational and experimental data in young children has meant that uncertainty around the dietary requirements for vitamin D for infants and children persists. DRVs for young children (aged 1–4 years) from a number of agencies, presented in Table 1, are the same as those for adults. Rather than setting a recommended nutrient intake (RNI) for infants and children up to 4 years of age, SACN recommended a safe intake of 8.5–10 µg/day for infants aged 0–11 months and 10 µg/day for children aged 1–3 years on the basis of insufficient evidence relating 25(OH)D concentrations to health outcomes for this age group [3]. While no age effect was found in the simulated dose–response relationship used by the IOM in establishing current DRVs, randomised controlled trials in children and adolescents were limited (n = 3) and no study included in the meta-regression included children younger than 6 years [2]. The recently derived EFSA AI of 15 µg/day for children aged 1–17 years was based mostly on adult data, while an analysis based on four RCTs in children (aged 2–17 years) showed that children tended to achieve the same mean 25(OH)D concentration as adults at lower intakes [4].
National nutrition surveys have reported that vitamin D intakes among young children are generally below 10 µg/day [8–11], including countries with mandatory or regular vitamin D fortification of staple foods [12, 13]. While nutritional supplements can be important contributors to vitamin D intake, the prevalence of supplement use varies between countries and between age groups and current rates of supplement use are too low to bridge the gap between actual vitamin D intakes and current recommendations [14]. In Ireland, mean intakes among children aged 1–4 years in the National Preschool Nutrition Survey (NPNS) ranged from 2.9 to 4.9 µg/day, and 93 and 78% had intakes <10 and <5 µg/day, respectively [11]. In the UK, the mean vitamin D intake in children aged 1.5–3 years participating in the National Diet and Nutrition Survey (NDNS) was 2.2 µg/day, while the prevalence of year-round plasma 25(OH)D concentrations <25 nmol/L was 8% [9].
Although intake data are few, the availability of large-scale or nationally representative data on vitamin D status in children is very limited and cross-comparisons by age are restricted. However, among studies with 25(OH)D concentrations across different age categories, the prevalence of deficiency and insufficiency was lowest in the youngest age groups [8, 9, 15, 16]. In a recent study in Canadian preschool children (n = 508), there was a large discrepancy between the prevalence of vitamin D intakes below the IOM EAR (95%) and the prevalence with 25(OH)D concentrations <40 nmol/L (4.5%), the proposed serum 25(OH)D to cover the average requirement [17]. Results from a recent dose–response RCT conducted during wintertime in 4- to 8-year-old white children living in Denmark estimated an EAR to achieve a serum 25(OH)D concentration of 40 nmol/L at 4 µg/day. In this study, an intake of ~20 µg/day was required to maintain 25(OH)D concentrations >50 nmol/L in 97.5% of children in winter [18]. This study demonstrates the need for experimentally derived estimates of vitamin D requirements for all population subgroups. The aims of the present study were to quantify vitamin D intake and describe serum 25(OH)D concentrations in children aged 2 years participating in a large prospective birth cohort study in Ireland and to identify determinants (including dietary determinants) of 25(OH)D all year round at this northerly latitude (51°N).
Materials and methods
Study participants
The Cork BASELINE (Babies after SCOPE: Evaluating the Longitudinal Impact using Neurological and Nutritional Endpoints) Birth Cohort Study was initiated in 2008 and is an ongoing prospective mother–infant birth cohort study based in Cork, Ireland. Participants were recruited from the SCOPE (Screening for Pregnancy Endpoints) study, a major multi-centre prospective pregnancy study involving nulliparous low-risk women with singleton pregnancies [19]. During their 20-week gestation visit, 1537 SCOPE Ireland participants consented to their infants participating in the birth cohort. A second stream of recruitment also took place where a further 600 mothers provided consent after delivery and the last infant was recruited in November 2011. After birth, the child’s early-life environment, diet and lifestyle was recorded, along with detailed monitoring of their health, growth and development. Paediatric follow-up with in-person appointments was repeated from the time of birth through to 2, 6 and 12 months and at 2 years, and assessments at 5 years are ongoing. Full details of the cohort and paediatric assessments have been provided by O’ Donovan et al. [20].
Research objectives and measurements in the Cork BASELINE Birth Cohort Study were conducted according to the guidelines laid down by the Declaration of Helsinki, and ethical approval was granted by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, ref ECM 5 (9) 01/07/2008. The study is registered with the United States National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov), ID: NCT01498965.
Dietary assessment
Dietary intake data at 2 years were collected using a 2-day weighed food diary collected on non-consecutive days. Caregivers were asked to record the amount and types of all foods, beverages and nutritional supplements consumed by the child over the two days, including brand information and cooking methods, where applicable. Food intake data were converted to nutrient intake data using the nutritional analysis software WISP© (Weighed Intake Software Package, Tinuviel Software, Anglesey, UK), which uses food composition data from the UK Composition of Foods [21] and the Irish Food Composition Database [22]. Our group continuously updates the vitamin D composition database to incorporate recipes for composite dishes, nutritional supplements, fortified foods and new food products (based on manufacturers’ data). In this study, food items specific to young children and composition data collected during the National Preschool Nutrition Survey (NPNS) [23] were also incorporated into the database.
Vitamin D intake (µg) was calculated for the total group and for specific subgroups including vitamin D-containing supplement users, consumers of vitamin D-fortified foods and non-consumers of vitamin D-containing supplements or vitamin D-fortified foods. Individual mean daily intakes (MDIs) were obtained by averaging a participant’s vitamin D intake over the two days. As intake data were only collected over 2 days, usual/habitual intakes, corrected for within-person variation, were also estimated using the NCI method for calculating habitual intakes in Crème Global Nutrition© Software. Important food sources of vitamin D were identified using the population proportion method described by Krebs-Smith et al. [24]. The method addresses the nutrient intake of the population rather than the average per person and is defined as the sum of the nutrient from a specific food group or food source for all participants, divided by the sum of the nutrient from all food sources for all participants and multiplied by 100. Actual contributions in µg/day of vitamin D intake from specific food groups were estimated by averaging the sum of vitamin D intake from that food group and dividing by the number of participants.
Additional data on supplement use over the previous month and current use of young child formula were collected for all participants, including those who did not complete a food diary. Formula intended for children aged 1–3 years is fortified with vitamin D at levels ranging from 1.5 to 1.7 µg/100 g.
Additional data collected
For the current analysis, complete data on maternal demographics and lifestyle factors were available from SCOPE. Questions relating to sun care at 2 years included whether parents applied sunscreen to their child’s skin during the summer, the sunscreen factor used, and whether they deliberately exposed their child’s skin to some sun without sunscreen or usually made a conscious effort to keep their child out of the sun.
At 2 years, naked weight was measured using digital scales (seca 384, seca, Birmingham, UK) correct to the nearest 0.1 kg. Standing height was measured using a wall-mounted stadiometer (seca 206). Age- and sex-specific body mass index (BMI) standard deviation scores (SDS) were generated using the UK World Health Organisation (WHO) 0- to 4-year growth reference data [25]. Overweight was defined as a BMI >91st and ≤ 98th percentile (SDS > 1.341 − ≤2.054) and obesity as a BMI >98th percentile (SDS > 2.054) [26].
Biological sample collection and analytical methods
A venous blood sample was collected at 2 years by a paediatric research nurse. Blood was processed to serum within three hours before storage in 200 µL aliquots at −80 °C until analysis. Circulating 25(OH)D concentrations in serum were measured at the Cork Centre for Vitamin D and Nutrition Research laboratory using a liquid chromatography–tandem mass spectrometry (LC–MS/MS) method that has been previously described in detail [27]. Briefly, the instrument used was a Waters Acquity UPLC system coupled to an Acquity Triple Quadrupole (TQD)® mass spectrometer detector (Waters, Santry, Dublin 9, Ireland). Concentrations of 25(OH)D3 and 25(OH)D2 were quantified individually, and their values summed to generate total 25(OH)D. Four levels of serum-based National Institute of Standards and Technology (NIST)-certified quality assurance material (SRM 972) were used for method validation, while quality control materials assayed in parallel to all samples were purchased from Chromsystems (Munich, Germany). NIST calibrators (SRM 2972) were used throughout the analysis. The intra- and inter-assay coefficients of variation (CV) were not greater than 6 and 5%, respectively, for each metabolite. The limit of detection (LoD) for 25(OH)D3 was 0.31, and the limit of quantitation (LoQ) was 1.03 nmol/L.
The quality and accuracy of vitamin D metabolite analysis in our laboratory using this LC–MS/MS method are assessed on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (DEQAS) (Charing Cross Hospital, London UK). We have been certified by the CDC Vitamin D Standardization Certification programme, which reports accuracy and bias for total 25(OH)D, 25(OH)D3, 3-epi-25(OH)D3 and 25(OH)D2, since 2013.
Vitamin D deficiency was defined as 25(OH)D <30 nmol/L and vitamin D sufficiency as 25(OH)D ≥50 nmol/L in line with IOM definitions [2].
Statistical analysis
Data were analysed using the statistical software package IBM SPSS® for Windows™ version 21.0 (IBM Corp. released 2012 Armonk, NY, USA). Spearman correlation coefficients were calculated to examine associations between total 25(OH)D and vitamin D intake. Multiple linear regression was used to explore the determinants of total 25(OH)D concentrations, and associations were expressed as the mean adjusted difference and 95% confidence intervals (95% CI). Season of sampling was dichotomised into winter (November–April) and summer (May–October). Subject characteristics including sex, BMI SDS, weight status (overweight or obese: yes or no), vitamin D intake (µg/day), supplement use in past months (yes or no), present consumption of vitamin D-fortified formula (yes or no) and sunscreen use during the summer (yes or no) were explored in unadjusted models. Maternal characteristics including ethnicity, tertiary education status and present smoking status were also explored in unadjusted models, and variables significant at the 10% level (P < 0.1) were included in the final multivariate models.
Results
Participants
Of the 2137 infants recruited to the Cork BASELINE Birth Cohort Study, 1537 children attended the 2-year assessment. Of those, 468 (30%) and 742 (48%) completed a food diary and gave a blood sample, respectively. A total of 295 children had both a food diary and blood collected at 2 years. One of these children was excluded from the analysis due to an inability to quantify their vitamin D intake from supplements. Characteristics of those who either completed a food diary, gave a blood sample or both at 2 years are presented in Table 2. There were no significant differences in subject characteristics between those with both blood and a food diary and those with only a blood sample (data not shown). There were also no significant differences in total 25(OH)D concentrations between those with and without a food diary [median (IQR) 63.7 (50.1, 75.6) vs. 62.8 (48.0, 78.3) nmol/L, P = 0.910] or in vitamin D intake between those with both a food diary and blood sample and those with only a food diary [median (IQR) 2.3 (1.4, 4.8) vs. 2.1 (1.2, 5.3) µg/day, P = 0.534].
Dietary intakes
The mean percentage contribution and actual contribution in µg/day of food groups to total vitamin D intake (n = 467) are presented in Table 3. With a mean (SD) daily vitamin D intake of 3.5 (3.1) µg, 96% of children had intakes below 10 µg/day, specified as an EAR by the IOM [2] and safe intake by SACN [3] and 90% had intakes below the Nordic AR of 7.5 µg/day [5]. The prevalence of intakes <4 µg/day is also presented in Table 3, on the basis of data from a recent dose–response vitamin D RCT in 4- to 8-year-old white children, where an intake of ~4 µg/day was required to maintain 25(OH)D >40 nmol/L in 50% of children during winter [18]. Individual MDIs ranged from 0.2 to 19.4 µg, with a median of 2.2 µg/day. Mean (SD) usual/habitual intake [3.5 (2.7) µg/day], 5th and 95th percentile intakes (0.8 and 8.6 µg/day) and the prevalence below 10 µg/day (96%) were similar to those calculated over 2 days. The main contributors to vitamin D intake were vitamin D-fortified foods, including breakfast cereals, vitamin D-fortified milks, yogurts and formula. Vitamin D intakes from the base diet (no fortification) were low with contributions from meat, fish, eggs and non-fortified milk and yogurts of 0.2–0.3 µg/day.
Most children (82%) consumed at least one vitamin D-fortified food (primarily fortified milk and yogurts and ready-to-eat breakfast cereals) over the recording period. However, the range of intakes from fortified foods varied greatly (0.03–17.21 µg/day). Ninety-six children (21%) consumed young child formula at least once over the recording period with a median (IQR) daily intake of 242 (138, 386) mL/day. There were no significant differences in intakes of specific foods or food groups between consumers and non-consumers of formula, except in intakes of cow’s milk [125 (68, 213) vs. 303 (175, 485) mL/day, P < 0.001]. Formula contributed 61% of the MDI of vitamin D (7.2 µg/day) among consumers. The prevalence of vitamin D-containing supplement use was low, with 10.5% (n = 49) using a supplement on at least one of the recording days (MDI 7.3 µg/day).
Mean (SD) and median (IQR) vitamin D intakes per kg body weight were 0.27 (0.25) and 0.18 (0.11, 0.36) µg/kg/d, respectively. No child exceeded the tolerable upper intake levels (UL) proposed for young children by the European Food Safety Authority [EFSA] (50 µg/day) [28].
Vitamin D status
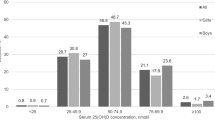
The mean (SD) total 25(OH)D concentration was 63.4 (20.4) nmol/L [median (IQR) 63.1 (48.8, 77.0), range 17.8–130.7 nmol/L], and vitamin D status as expected was better in summer [mean (SD) 71.2 (17.5) nmol/L] than in winter [54.5 (19.9) nmol/L], P < 0.001. The prevalence of total 25(OH)D at various cut-offs in the total group and stratified by season of sampling, supplement use and fortified food consumption is presented in Table 4. The overall prevalence of deficiency (<30 nmol/L) was 4.6%. Three children (0.4%) had a total 25(OH)D >125 nmol/L (range 125.6–130.7 nmol/L), the threshold at which the IOM recommended caution on the basis of some evidence for reverse J-shaped relationships for 25(OH)D and adverse outcomes [2]. Looking specifically at those with a completed food diary who were sampled in winter (n = 139), 94.2% had a vitamin D intake below 10 μg/day, specified as the IOM EAR and SACN safe intake [2, 3], and the prevalence with 25(OH)D concentrations <40 nmol/L (the IOM EAR-type value [2]) and <25 nmol/L (the SACN deficiency threshold [3]) was 24.5 and 3.6%.
Supplement use protected against vitamin D deficiency during winter, with 1 versus 10% of users and non-users, respectively, having wintertime 25(OH)D <30 nmol/L. Similarly, more than half (56%) of non-users had 25(OH)D <50 nmol/L versus a quarter (24%) of users. Almost all formula consumers (94%) had 25(OH)D >50 nmol/L, and none were vitamin D deficient. The highest prevalence of vitamin D deficiency was among those who neither used a vitamin D-containing supplement nor consumed a vitamin D-fortified food. Among these individuals, who represented ~17% of the sample with both intake and status data, the mean intake of vitamin D was just over 1 µg per day. During winter, the prevalence of vitamin D deficiency among these children was 12%, more than half (53%) were <40 nmol/L and more than three quarters (77%) were <50 nmol/L. Among those who consumed a vitamin D-containing fortified food but did not use a vitamin D-containing supplement, the prevalence of 25(OH)D <30, 40 and 50 nmol/L during winter was 6, 24 and 44%, respectively.
Determinants of 25(OH)D concentrations at 2 years
As expected, season was a major determinant of 25(OH)D concentrations (see Table 5). There was a strong association between vitamin D intake (µg/day) and status in winter (see Fig. 1) [Spearman’s r = 0.509, P < 0.001], and even during summer, vitamin D intake was associated with 25(OH)D [r = 0.224, P < 0.01]. The effect of intake (µg/day) on 25(OH)D concentrations was stronger in winter [β (95% CI) 3.1 (2.3, 3.9)] than in summer [1.7 (0.7, 2.6)]. This translated to the main dietary sources of vitamin D, as supplement use and consumption of formula were also associated with higher 25(OH)D. We found no associations with maternal characteristics, including ethnicity, tertiary education and smoking or with child sex, BMI SDS and weight status. Sunscreen use and deliberate exposure to the sun without sunscreen were also not significantly associated with 25(OH)D concentrations at 2 years (data not shown).
Discussion
This study reports vitamin D intakes and determinants of 25(OH)D concentrations in a large sample of well-characterised young children living in Ireland. We have reported that despite low vitamin D intakes, northerly latitude and self-reported adherence to sun-safe recommendations, there was a relatively low prevalence of vitamin D deficiency, and a much lower prevalence of inadequate status than inadequate intakes in this cohort of 2-year-old children. We also reported a high prevalence of very low vitamin D intakes and a higher prevalence of vitamin D deficiency in children not obtaining additional vitamin D from dietary or supplemental sources over and above the relatively small amounts contributed by the base diet.
As expected, 25(OH)D concentrations were lower in winter (November–April), reflecting an increase in cutaneous synthesis during the summer months. We found no significant association between sunscreen use and 25(OH)D concentrations; however, the high prevalence of use among participants may have masked any potential effect. Emphasis on skincare in the sun in Ireland, especially for infants and young children, may have induced a response bias, and our single question on sunscreen use is unlikely to have captured habitual sun care practices. While sunscreen has been shown to completely block dermal synthesis of vitamin D in controlled situations, it is unlikely to do so according to actual habitual use as many parents admitted to not applying sunscreen for limited or casual exposure, such as a brief walk. Additional factors such as incorrect application, where 100% coverage is not achieved, are also likely to be widespread in this and many populations [29].
Besides season of sampling, vitamin D intake (µg/day) and the consumption of important dietary sources of vitamin D, including fortified young child formula and supplements, were the only other significant determinants of vitamin D status. Each 1 µg/day of vitamin D was associated with an increase in 25(OH)D of 2.5 nmol/L (95% CI 1.9–3.1), which is slightly higher than the estimate of 1.8 nmol/L (95% CI 1.3, 2.2) per µg from a meta-analysis of data from RCTs in adults and children during wintertime at latitudes >49.5°N [30]. Associations between intake and status were stronger in winter, and a lower prevalence of supplement users was deficient in wintertime. Other studies in children have also reported higher 25(OH)D concentrations [17, 31–33] and less seasonal variation among supplement users [16, 34]. Maguire et al. [35] reported that among 1898 Canadian children aged 1–5 years, the use of vitamin D supplements and the consumption of vitamin D-fortified cow’s milk accounted for more variation in 25(OH)D concentrations than season or skin pigmentation.
Consistent with our reports in Irish adults and older children [10, 36], vitamin D intake from naturally occurring sources was low. Those who did not use a vitamin D-containing supplement or consume a vitamin D-fortified food had the lowest vitamin D intakes and a higher prevalence of low 25(OH)D concentrations, especially during winter. While the use of supplements was associated with higher vitamin D intakes and status, the prevalence of supplement use was relatively low; 23% reported using a supplement over the previous month, and 11% of diary participants used a supplement over the recording period. In addition, the frequency of supplement use and amount of vitamin D taken as a supplement varied in these children. Recommending daily vitamin D supplementation as a means of maintaining adequate year-round circulating 25(OH)D concentrations may not guarantee adherence and be effective across the population. The use of supplements across Europe is highly variable, and vitamin D supplementation policies for infants and young children, in addition to compliance with such policies, vary across countries [14, 37, 38]. It is worth noting that in Ireland, a vitamin D supplementation policy (5 µg/day) is currently only in place for infants up to 12 months of age [39].
Since 2006, legislation for the addition of vitamins and minerals to food and supplements has been harmonised across Europe [40]; however, fortification practices vary between countries. In Ireland, a number of foods are voluntarily fortified with vitamin D, and while most children consumed at least one vitamin D-fortified food over the diary recording period, vitamin D intakes from these foods varied greatly. No child had an intake that exceeded or even approached the EFSA UL of 50 µg/day. While the European Community (EC) legislation has provided for the setting of maximum amounts of vitamins and minerals to food supplements and fortified foods, these have yet to be set [40]. Strategic fortification of staple foods with vitamin D has been demonstrated to reduce the prevalence of vitamin D deficiency [41]. Milk is a widely consumed food among young children in Ireland and all participants who provided dietary data consumed at least one type of milk over the 2-day diary period [median (IQR) intake 332 (202, 491) mL/day]. Milk is not fortified with vitamin D on a mandatory basis in Ireland, and very few children (n = 32, ~7%) consumed a vitamin D-fortified milk other than formula. Other than cow’s milk, there were no significant differences in the food and food group intakes between consumers and non-consumers of formula, suggesting that young child formula is being used as a milk substitute rather than a food substitute. Formula was a major contributor to vitamin D intake, and even among those sampled in winter, few formula consumers (n = 3) were <50 nmol/L, compared to more than half (54.3%) of non-consumers. In Finland, a national recommendation for food manufacturers to fortify fluid milks and margarine was implemented in 2003 and while not mandatory was adopted by most companies. This fortification policy was successful in improving vitamin D intakes and status in 4-year-old Finnish children [42]. Studies in Canada (43–45°N), where milk is fortified with vitamin D on a mandatory basis, have reported a positive association between fortified milk consumption and 25(OH)D concentrations among preschool children [17, 43]. In New Zealand, where there is minimal fortification of food with vitamin D, the prevalence of concentrations <50 nmol/L decreased from 32% at baseline to 11% after 20 weeks in preschool children randomised to a vitamin D-fortified milk [0.8 µg/100 ml; mean (95% CI) intake from milk 3.6 (3.2, 4.2) µg/day] [44]. In another study in Germany, Hower et al. [45] reported that children aged 2–6 years randomised to a vitamin D-fortified formula (2.85 µg/100 ml, MDI from milk 7.1 µg) did not display the usual decrease in 25(OH)D concentrations during winter.
The prevalence of vitamin D deficiency (4.6% < 30 nmol/L) and insufficiency (26.7% < 50 nmol/L) was lower in this study than recently reported for a nationally representative sample of Irish adults aged 18–84 years participating in the National Adult Nutrition Survey (NANS) [11.2, 27.2 and 45.0% < 30, 40 and 50 nmol/L, respectively] [46] and in a large sample of pregnant women (n = 1768) in the SCOPE Ireland study [17, 31 and 44% < 30, 40 and 50 nmol/L, respectively] [47]. A number of nationally representative studies have reported a higher prevalence of low 25(OH)D concentrations in older children and adults compared with younger children [9, 15, 16]. Infants and children may require less sun exposure to produce sufficient quantities of vitamin D due to greater surface area for body size and because of the age-associated reduction in the capacity for cutaneous synthesis [48, 49]. Their smaller body size and volume may also have implications for vitamin D requirements [50]. The mean (SD) vitamin D intake per kg body weight among children in the current study [0.27 (0.25) µg/kg/d] was four times higher than that for Irish adults participating in NANS [0.07 (0.05) µg/kg/d)]. El Hayek et al. [17] reported that among children aged 2–5 years, the higher 25(OH)D concentrations observed in children in the first age tertile compared with the second and third tertile became non-significant after adjustment for body weight.
Despite a relatively low mean vitamin D intake (3.5 µg/day) in the current cohort, the prevalence of deficiency (<30 nmol/L) was low (3.4%), even during winter (5.7%). Furthermore, while 96% had intakes below 10 μg/day, 24.5 and 3.6% were <40 and 25 nmol/L, during winter. El Hayek et al. reported even greater discrepancies between the prevalence below the IOM EAR and the prevalence <40 nmol/L in Canadian preschool children. Agencies charged with setting DRVs for vitamin D have acknowledged that there are uncertainties around the EAR for children and adolescents, largely due to the lack of appropriate dose–response RCTs in younger age groups [2–5]. Two recently published dose–response RCTs conducted in winter in white 4- to 8-year-old children [18] and 14- to 18-year-old adolescents [51] reported that 4.4 and 6.6 µg/day, respectively, were required to maintain serum 25(OH)D concentrations >40 nmol/L in 50% of children and adolescents. Moreover, they reported that an intake of 19.5 and ~30 µg/day would be required to maintain serum 25(OH)D >50 nmol/L in 97.5% of children and adolescents, supporting suggestions that body size may influence dietary requirements for vitamin D. A further RCT in younger children (<4 years) may be required to establish vitamin D requirements in this age group.
The main strengths of this paper are the large sample size, the use of prospectively collected data from a well-characterised longitudinal birth cohort, allowing for the assessment of a range of potential determinants of 25(OH)D concentrations and a quality dietary assessment protocol compatible with the national dietary surveys of Ireland [11] and the UK [9]. The analytical method for 25(OH)D in our laboratory is traceable to a higher-order reference measurement procedure and provides a high degree of confidence in the measured 25(OH)D concentrations [27]. Our sample was mainly Caucasian, so our results are not generalisable to other ethnic groups.
In conclusion, we show a lower prevalence of vitamin D deficiency in this large cohort of young children than previously reported in adults. Stronger associations between vitamin D intake and status during winter than summer reflect both the impact of cutaneous synthesis on status during the summer and the important contribution of dietary intake to status during the winter at high latitudes. There was a high prevalence of low vitamin D intakes and status during winter, especially among children who did not consume fortified foods or nutritional supplements. Our data indicate the need for targeted dietary strategies to increase vitamin D intakes in this age group. Finally, this report provides further evidence that DRVs for vitamin D should be based on experimental data in specific population groups and indicates the need for dose–response RCTs in young children.
Authors’ contributions
C. ní C. carried out data collection, database construction and data analysis. M.K. designed the study, and C. ní C. and M.K. drafted the manuscript. M.K. had responsibility for the final content. E.K.M. carried out data collection. The Cork BASELINE Birth Cohort Study is a multidisciplinary collaboration led by D.M.M. (Principal Investigator), M.K., J.O’B.H., A.D.I. and L.C.K. All authors reviewed and approved the final submission.
References
Cashman KD, Kiely M (2014) Recommended dietary intakes for vitamin D: where do they come from, what do they achieve and how can we meet them? J Hum Nutr Diet 27(5):434–442. doi:10.1111/jhn.12226
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. The National Academies Press, Washington
Scientific Advisory Committee on Nutrition. Report on Vitamin D and Health (2016) Published online at http://www.sacn.gov.uk/pdf. Accessed 1 July 2016
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) (2016) Scientific opinion on Dietary Reference Values for vitamin D. EFSA Journal 2016; volume (issue):NNNN, 179 pp. doi:10.2903/j.efsa.2016
NORDEN Nordic Nutrition Recommendations, 5th Edition (NNR5)–Vitamin D (2013). http://www.slv.se/en-gb/Startpage-NNR/Public-consultation11/. Accessed Aug 2013
German Nutrition Society (2012) New reference values for vitamin D. Ann Nutr Metab 60(4):241–246. doi:10.1159/000337547
Carriquiry AL (1999) Assessing the prevalence of nutrient inadequacy. Public Health Nutr 2(1):23–33
Lennox A, Sommerville J, Ong K, Henderson H, Allen R (2011) Diet and Nutrition Survey of Infants and Young Children (DNSIYC). Public Health, England
Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, Swan G (2014) National Diet and Nutrition Survey. Results from years 1–4 (combined) of the rolling programme (2008/2009–2011/2012)
Black LJ, Walton J, Flynn A, Kiely M (2014) Adequacy of vitamin D intakes in children and teenagers from the base diet, fortified foods and supplements. Public Health Nutr 17(4):721–731. doi:10.1017/s1368980013000359
Hennessy A, Browne F, Kiely M, Walton J, Flynn A (2016) The role of fortified foods and nutritional supplements in increasing vitamin D intake in Irish preschool children. Eur J Nutr. doi:10.1007/s00394-016-1171-7
Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF (2010) Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 140(4):817–822. doi:10.3945/jn.109.118539
Vatanparast H, Calvo MS, Green TJ, Whiting SJ (2010) Despite mandatory fortification of staple foods, vitamin D intakes of Canadian children and adults are inadequate. J Steroid Biochem Mol Biol 121(1–2):301–303. doi:10.1016/j.jsbmb.2010.03.079
Kiely M, Black LJ (2012) Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand J Clin Lab Invest Suppl 243:14–23. doi:10.3109/00365513.2012.681893
Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT (2011) Vitamin D status: United States, 2001–2006. NCHS Data Brief 59:1–8
Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS (2011) The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 94(1):128–135. doi:10.3945/ajcn.111.013268
El Hayek J, Pham TT, Finch S, Hazell TJ, Jean-Philippe S, Vanstone CA, Agellon S, Rodd C, Rauch F, Weiler HA (2013) Vitamin D status in Montreal preschoolers is satisfactory despite low vitamin D intake. J Nutr 143(2):154–160. doi:10.3945/jn.112.169144
Mortensen C, Damsgaard CT, Hauger H, Ritz C, Lanham-New SA, Smith TJ, Hennessy Á, Dowling K, Cashman KD, Kiely M, Mølgaard C (2016) Estimation of the dietary requirement for vitamin D in white children aged 4–8 y: a randomized, controlled, dose–response trial. Am J Clin Nutr. doi:10.3945/ajcn.116.136697
Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, McCowan LM, Simpson NA, Dekker GA, Roberts CT, Rodems K, Noland B, Raymundo M, Walker JJ, North RA (2014) Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension 64(3):644–652. doi:10.1161/hypertensionaha.114.03578
O’Donovan SM, Murray DM, Hourihane JO, Kenny LC, Irvine AD, Kiely M (2015) Cohort profile: the Cork BASELINE Birth Cohort Study: babies after SCOPE: evaluating the longitudinal impact on neurological and nutritional endpoints. Int J Epidemiol 44(3):764–775. doi:10.1093/ije/dyu157
Holland B, Welch A, Unwin ID, Buss DH, Paul A, Southgate D (1995) McCance and Widdowson’s the composition of foods, 5th edn. HMSO, London
Black LJ, Ireland J, Møller A, Roe M, Walton J, Flynn A, Finglas PM, Kiely M (2011) Development of an on-line Irish food composition database for nutrients. J Food Comp and Anal 24(7):1017–1023. doi:10.1016/j.jfca.2011.01.015
Irish Universities Nutrition Alliance (2012) National Preschool Nutrition Survey. http://www.iuna.net. Accessed May 2013
Krebs-Smith SM, Kott PS, Guenther PM (1989) Mean proportion and population proportion: two answers to the same question? J Am Diet Assoc 89(5):671–676
Wright CM, Williams AF, Elliman D, Bedford H, Birks E, Butler G, Sachs M, Moy RJ, Cole TJ (2010) Using the new UK-WHO growth charts. BMJ 340:c1140
SACN/RCPCH Expert Group (2012) Consideration of issues around the use of BMI centile thresholds for defining underweight, overweight and obesity in children aged 2–18 years in the UK. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/339411/SACN_RCPCH_defining_child_underweight__overweight_and_obesity_in_the_UK_2012.pdf. Accessed Aug 2013
Cashman KD, Kinsella M, Walton J, Flynn A, Hayes A, Lucey AJ, Seamans KM, Kiely M (2014) The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J Nutr 144(7):1050–1057. doi:10.3945/jn.114.192419
EFSA Panel on Dietetic Products Nutrition and Allergies (2012) Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J 10(7):2813
Norval M, Wulf HC (2009) Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol 161(4):732–736. doi:10.1111/j.1365-2133.2009.09332.x
Cashman KD, Fitzgerald AP, Kiely M, Seamans KM (2011) A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr 106(11):1638–1648. doi:10.1017/s0007114511005058
Grant CC, Wall CR, Crengle S, Scragg R (2009) Vitamin D deficiency in early childhood: prevalent in the sunny South Pacific. Public Health Nutr 12(10):1893–1901. doi:10.1017/s1368980009004820
Mansbach JM, Ginde AA, Camargo CA Jr (2009) Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124(5):1404–1410. doi:10.1542/peds.2008-2041
Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N (2011) Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS ONE 6(7):e22179. doi:10.1371/journal.pone.0022179
Davies PS, Bates CJ, Cole TJ, Prentice A, Clarke PC (1999) Vitamin D: seasonal and regional differences in preschool children in Great Britain. Eur J Clin Nutr 53(3):195–198
Maguire JL, Lebovic G, Kandasamy S, Khovratovich M, Mamdani M, Birken CS, Parkin PC (2013) The relationship between cow’s milk and stores of vitamin D and iron in early childhood. Pediatrics 131(1):e144–e151. doi:10.1542/peds.2012-1793
Black LJ, Walton J, Flynn A, Cashman KD, Kiely M (2015) Small increments in vitamin D intake by Irish adults over a decade show that strategic initiatives to fortify the food supply are needed. J Nutr 145(5):969–976. doi:10.3945/jn.114.209106
Spiro A, Buttriss JL (2014) Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull/BNF 39(4):322–350. doi:10.1111/nbu.12108
Hogler W (2015) Complications of vitamin D deficiency from the foetus to the infant: one cause, one prevention, but who’s responsibility? Best Pract Res Clin Endocrinol Metab 29(3):385–398. doi:10.1016/j.beem.2015.03.003
Food Safety Authority of Ireland (FSAI) (2007) Recommendations for a national policy on vitamin D supplementation for infants in Ireland. https://www.fsai.ie/workarea/downloadasset.aspx?id=1222. Accessed Jan 2013
European Commission (2007) Orientation paper on setting maximum and minimum amounts for vitamins and minerals in foodstuffs. SANCO/E4/FDA/bs D/540510. European Commission Health and Consumer Protection Directorate-General, Brussels, Belgium
Madsen KH, Rasmussen LB, Andersen R, Molgaard C, Jakobsen J, Bjerrum PJ, Andersen EW, Mejborn H, Tetens I (2013) Randomized controlled trial of the effects of vitamin D-fortified milk and bread on serum 25-hydroxyvitamin D concentrations in families in Denmark during winter: the VitmaD study. Am J Clin Nutr 98(2):374–382. doi:10.3945/ajcn.113.059469
Piirainen T, Laitinen K, Isolauri E (2007) Impact of national fortification of fluid milks and margarines with vitamin D on dietary intake and serum 25-hydroxyvitamin D concentration in 4-year-old children. Eur J Clin Nutr 61(1):123–128. doi:10.1038/sj.ejcn.1602506
Maguire Jl BCSKM et al (2013) Modifiable determinants of serum 25-hydroxyvitamin d status in early childhood: opportunities for prevention. JAMA Pediatr 167(3):230–235. doi:10.1001/2013.jamapediatrics.226
Houghton LA, Gray AR, Szymlek-Gay EA, Heath AL, Ferguson EL (2011) Vitamin D-fortified milk achieves the targeted serum 25-hydroxyvitamin D concentration without affecting that of parathyroid hormone in New Zealand toddlers. J Nutr 141(10):1840–1846. doi:10.3945/jn.111.145052
Hower J, Knoll A, Ritzenthaler KL, Steiner C, Berwind R (2013) Vitamin D fortification of growing up milk prevents decrease of serum 25-hydroxyvitamin D concentrations during winter: a clinical intervention study in Germany. Eur J Pediatr 172(12):1597–1605. doi:10.1007/s00431-013-2092-6
Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, Phinney KW, Coates PM, Picciano MF, Sempos CT (2013) Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 97(6):1235–1242. doi:10.3945/ajcn.112.057182
Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC (2016) Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 104(2):354–361. doi:10.3945/ajcn.116.130419
Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, Craig ME, Cutfield WS, Hofman PL, Taylor BJ, Grover SR, Pasco JA, Burgner D, Cowell CT (2006) Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 185(5):268–272
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M (2008) Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122(2):398–417. doi:10.1542/peds.2007-1894
Drincic AT, Armas LA, Van Diest EE, Heaney RP (2012) Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 20(7):1444–1448. doi:10.1038/oby.2011.404
Smith TJ, Tripkovic L, Damsgaard CT, Mølgaard C, Ritz C, Wilson-Barnes SL, Dowling KG, Hennessy Á, Cashman KD, Kiely M, Lanham-New SA, Hart KH (2016) Estimation of the dietary requirement for vitamin D in adolescents aged 14–18 y: a dose–response, double-blind, randomized placebo-controlled trial. Am J Clin Nutr. doi:10.3945/ajcn.116.138065
Acknowledgements
The National Children’s Research Centre is the primary funding source for the Cork BASELINE Birth Cohort Study. Additional support came from a grant from the UK Food Standards Agency to J.O’B.H., A.D.I. and D.M.M. and from Danone Nutricia Early Life Nutrition to M.K. This work and C. ní C. are supported by a grant to M.K. from the European Commission (ODIN, grant 613977 Food based solutions for optimal vitamin D nutrition and health through the life-cycle). None of the funding agencies had a role in the design, analysis or writing of this article.
The authors would like to thank Darina Sheehan, Emma Cobbe and the rest of the Cork BASELINE Birth Cohort Study research team and also the families for their dedication and continued support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
Research objectives and measurements in the Cork BASELINE Birth Cohort Study were conducted according to the guidelines laid down by the Declaration of Helsinki, and ethical approval was granted by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, ref ECM 5 (9) 01/07/2008. The study is registered with the United States National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov), ID: NCT01498965.
Rights and permissions
About this article
Cite this article
ní Chaoimh, C., McCarthy, E.K., Hourihane, J.O. et al. Low vitamin D deficiency in Irish toddlers despite northerly latitude and a high prevalence of inadequate intakes. Eur J Nutr 57, 783–794 (2018). https://doi.org/10.1007/s00394-016-1368-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1368-9