Abstract
Purpose
Using ob/ob mice as a model of non-alcoholic fatty liver disease (NAFLD), we investigated the effect of moderate alcohol intake on the development of NAFLD and molecular mechanisms involved.
Methods
Ob/ob mice were fed water or ethanol solution (2.5 g/kg body weight/day) for 6 weeks, and markers of liver injury, insulin signalling and adiponectin in visceral adipose tissue were determined.
Results
Whereas bodyweight and the degree of liver steatosis did not differ among ob/ob mouse groups, those consuming ethanol had markedly less macrovesicular hepatic fat accumulation, inflammatory alterations and significantly lower transaminase levels. Despite similarly elevated protein levels of tumour necrosis factor α, protein concentrations of plasminogen activator inhibitor 1 were significantly lower in livers of ob/ob mice consuming ethanol in comparison with controls. The hepato-protective property of moderate alcohol ingestion in ob/ob mice was associated with an induction of the sirtuin-1/adiponectin-signalling cascade in visceral fat tissue and an activation of Akt in the liver. Similar effects of moderate alcohol exposure were also found in vitro in 3T3-L1 and AML-12 cells.
Conclusion
These data suggest that moderate alcohol intake may diminish the development of NAFLD through sirtuin-1/-adiponectin-dependent signalling cascades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By now, non-alcoholic fatty liver disease (NAFLD), a disease comprising a continuum of diseases ranging from simple steatosis to hepatic cirrhosis, is one of the most common liver diseases worldwide [1, 2]. NAFLD is commonly associated with metabolic co-morbidities like obesity, type 2 diabetes and the metabolic syndrome [3]. Indeed, NAFLD has repeatedly been considered as the hepatic manifestation of the metabolic syndrome [2, 3]. Results of studies performed in recent years suggest that not only genetic predisposition, alterations of the intestinal barrier function, a sedentary lifestyle and over-nutrition, but also certain dietary patterns (e.g. a diet rich in fat and sugar or iron/animal-derived protein) may be critical in the development of NAFLD (for overview see [4]). However, despite intense research effort, molecular mechanisms involved in the disease onset but even more so its progression and herein especially the role of the composition of diet have not yet been clarified.
Heavy alcohol consumption (e.g. an intake >50 g/day) is unequivocally associated with the development of liver steatosis [5]. In contrast, a recent population-based study suggests that moderate alcohol consumption (<20 g on 1–3 days/week) may even decrease the odds to develop NAFLD [6, 7]. These data are in line with the findings of others who reported that moderate drinkers (<20 g/day) had a lower risk of being diagnosed with non-alcoholic steatohepatitis (NASH) but also fibrosis than lifetime non-drinkers [8]. Results of human studies suggest that the beneficial effect of moderate alcohol intake may be associated with increased levels of adiponectin [9]; however, molecular mechanisms involved in the beneficial effects of moderate intake of alcoholic drinks have not yet been fully understood. Results obtained in animal experiments are inconsistent. Indeed, using ob/ob mice as a model of NAFLD, a protective effect of chronic alcohol consumption (≤21 g/kg body weight (b.w.)/day) was shown [10]. In contrast, results of other studies suggest that moderate to elevated consumption of plain ethanol may actually add to the progression of NAFLD [11, 12] and that this could even be dose dependent [13, 14]. Furthermore, protective effects found in human studies might not have resulted from the intake of ethanol itself but rather from other factors associated with the intake of alcohol (e.g. intake of resveratrol in red wine or hops ingredients in beer or even changes in lifestyle [15–18]).
Starting from this background, the primary aim of the present study was to determine the effects of moderate chronic intake of ethanol (2.5 g/kg b.w./day) on the development of NAFLD in ob/ob mice. Because moderate alcohol intake diminished the development of NAFLD, a secondary objective of the study was to identify potential mechanisms involved in the beneficial effects of the moderate intake of alcohol.
Materials and methods
Animals and treatments
Mice were housed in a specific pathogen-free barrier facility accredited by the ‘Association for Assessment and Accreditation of Laboratory Animal Care International’. All procedures were approved by the local ‘Institutional Animal Care and Use Committee’. Six-week-old male C57BL/6J mice used as naïve controls (n = 10), and ob/ob mice (n = 12) were obtained from Janvier (Janvier Labs, France). Animals had free access to chow during the entire experiment. Furthermore, mice had either free access to plain tap water or water enriched with 2.5 g/kg b.w./day ethanol for 6 weeks. Ethanol intake was assessed daily and adjusted to liquid intake. Body weight was assessed twice weekly. This dose of ethanol did not cause any behavioural changes in mice or any signs of drunkenness. Blood was collected from the portal vein just prior to killing, and portions of liver and adipose tissue were snap-frozen immediately, frozen-fixed in Tissue-Tek® O.C.T. mounting media (Sakura, Germany) or fixed in neutral-buffered formalin.
Cell culture
3T3-L1 cells, a model for adipocytes (DSMZ, Germany), were cultured at 37 °C in a humidified, 5 % carbon dioxide atmosphere. At 80 % confluence, differentiation of 3T3-L1 cells was started by exposing cells to DMEM medium containing 10 % foetal bovine serum, 0.5 mM 3-Isobutyl-methylxanthin, 2.5 µM dexamethasone, 850 nM insulin as well as 1 % penicillin and streptomycin (all PAN-Biotech, Germany). On day 9, cells were treated with 0.2 vol% ethanol for 6 h in the presence or absence of 1 mM 4-methylpyrazole in an alcohol vapour chamber as described previously by others [19].
Alpha mouse liver 12 (AML-12) cells obtained from American type culture collection (ATCC, USA) were grown in DMEM/F12 media (PAN-Biotech, Germany) supplemented with 10 % foetal bovine serum, 40 ng/mL dexamethasone, 0.005 mg/mL insulin, 5 ng/mL selenium and 0.005 mg/mL transferrin as well as 1 % penicillin and streptomycin (PAN-Biotech, Germany). At 70 % confluence, cells were serum starved for 18 h in starvation media supplemented with bovine serum albumin (0.01 %), insulin, selenium and transferrin as well as penicillin and streptomycin. Cells were then challenged with 10 ng/mL tumour necrosis factor (TNF) α (Sigma–Aldrich, Germany) ±2 µg/ml adiponectin (Bio Vendor, Germany) for 6 h. Cells were rinsed twice with ice-cold phosphate-buffered saline and used for RNA isolation.
Hepatic lipid accumulation, histological evaluation and clinical chemistry
To determine hepatic lipid accumulation, triglyceride levels were measured, and Oil Red O lipid staining was performed as previously detailed [20]. Representative pictures of Oil Red O staining were taken using a camera integrated in a microscope (DM4000 B LED, Leica, Germany) at 200× magnification. Paraffin-embedded livers were cut into 5-µm sections, stained with haematoxylin and eosin (H&E, Sigma, Germany), and histology was evaluated using the NAFLD activity scoring system (NAS) according to Kleiner et al. [21]. Neutrophil granulocytes were stained using a commercially available naphthol AS-D chloro-acetate-esterase staining kit (Sigma–Aldrich, Germany). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels as well as lipid profile (triglycerides, total cholesterol, HDL and LDL cholesterol and free fatty acids) were determined in heparinized plasma in a routine laboratory at the University Clinic of Jena (Beckman Coulter® Biomedical GmbH, Germany).
Enzyme-linked immunosorbent assays (ELISA) for TNFα and plasminogen activator inhibitor (PAI)-1
Protein concentration of TNFα and PAI-1 in whole liver lysates, extracted with a buffer containing 50 mM TRIS, 150 mM NaCl, 2 mM KCl, 0.5 % Triton-X 100 (Roth, Germany) and protease inhibitors (Sigma–Aldrich, Germany) were determined using commercially available ELISA kits according to the instructions of the manufacturers (TNFα: Alpco Diagnostics, USA; PAI-1: AssayPro, USA).
RNA isolation and real-time RT-PCR
Total RNA was extracted from liver and fat tissue as well as cells using peqGOLD TriFast™ (PEQLAB, Germany), and cDNA was synthetized as previously described [22]. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used for the detection of adiponectin, acyl-coenzyme A oxidase (ACOX1), BCL2-associated X protein (BAX), chemokine (c–c motif) ligand 2 (CCL2), fatty acid synthase (FAS), forkhead box O1 (FOXO1), glucokinase (GK), glucose transporter type 4 (GLUT4), insulin receptor substrate (IRS)-1 and IRS-2, phosphoenolpyruvate carboxykinase (PEPCK), perilipin 2 and 3, peroxisome proliferator-activated receptor gamma (PPARγ), sirtuin-1 (SIRT1), sterol regulatory element-binding protein 1c (SREBP-1c) and 18S (for primer sequences see Table 1) as described previously [22]. To determine the amount of target genes, the comparative CT method was used and normalized to an endogenous reference (18S) relative to a calibrator (2−ΔΔCt).
Caspase-9 activity
For this study, caspase-9 activity was measured using a luminescent Caspase-Glo® 9 assay (Promega, Germany).
Immunoblots
Cytosolic protein lysates were prepared by homogenizing liver tissue in a lysis buffer (1 mol/L HEPES, 1 mol/L MgCl2, 2 mol/L KCl, 1 mol/L DTT all Roth, Germany) containing a protease and phosphatase inhibitor mix (Sigma–Aldrich, Germany). Proteins (60 µg protein/well) were separated in 10 % SDS-polyacrylamide gels and transferred onto Hybond™-P polyvinylidene difluoride membranes (Amersham Biosciences, Germany). Blots were probed with antibodies against phospho-Akt (a protein kinase B) or total Akt (Cell Signalling Technology, USA). To ensure equal loading of blots, all blots were stained with Ponceau Red (Sigma–Aldrich, Germany). Bands were visualized using a Super Signal Western Dura kit (Thermo Scientific, USA), and blots were analysed using software integrated in the Chemi Doc™ MP System (Bio-Rad, Germany).
Statistical analysis
All results are reported as mean ± standard error of mean (SEM) (n = 4–6). Statistically significant differences between ob/ob groups were determined using Mann–Whitney U test included in the GraphPad Prism 6 software (GraphPad Prism Inc., USA). A p value <0.05 was considered to be significant. Grubbs test included in GraphPad Prism 6 software was used to identify outliers.
Results
Effect of moderate alcohol consumption on liver status of ob/ob mice
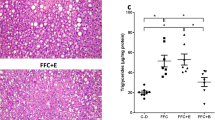
As no differences in regards to markers of liver damage, plasma lipid profile and selected other parameters (e.g. expression of insulin receptor substrates and PAI-1) between naïve wild-type mice and wild-type mice fed moderate amounts of ethanol (data not shown) were found, data from wild-type mice fed plain water are shown to represent both control groups. As expected, ob/ob mice fed plain water developed massive macrovesicular steatosis associated with beginning inflammatory alterations (see Fig. 1a and NAS score in Table 2). Despite a significantly higher overall caloric intake (+~6 kcal/mouse/week, p > 0.05) resulting from the alcohol added to the drinking water, body weight was similar between ob/ob groups. Furthermore, while liver-to-body weight ratio and number of fatty hepatocytes did not differ between groups, ob/ob mice chronically consuming moderate amounts of ethanol displayed only microvesicular fat accumulation in most of the hepatocytes along with markedly lower hepatic triglyceride levels (−17 %, p = 0.056) and less signs of inflammation when compared to ob/ob mice only consuming drinking water (see Table 2). In line with these findings, plasma levels of ALT and AST were significantly lower in ob/ob mice drinking moderate amounts of alcohol in comparison with ob/ob mice fed with tap water. However, both plasma AST and ALT levels were still higher than those of naïve wild-type mice (see Fig. 1b, c). Not only plasma levels of triglycerides, total cholesterol, HDL as well as LDL cholesterol but also free fatty acids were similar between ob/ob mouse groups (see Table 3). However, total cholesterol but also HDL and LDL cholesterol plasma levels were markedly higher than in naïve controls. In line with these findings, HDL/LDL ratios were similar between both ob/ob mouse groups. Furthermore, mRNA expression of perilipin 2 and 3 shown before to be involved in the formation, maintenance and involution of lipid droplets [23] was also significantly induced in livers of mice drinking plain water only (Fig. 1d, e). In contrast, in livers of mice consuming moderate amounts of ethanol, expression of perilipin 2 and 3 mRNA were at the level of naïve controls. Number of neutrophils and mRNA expression of CCL2 but also markers of apoptosis such as BAX mRNA expression and activity of caspase-9 were all markedly induced in livers of ob/ob mice fed with tab water. In livers of ob/ob mice consuming moderate amounts of alcohol, levels of these markers were almost at the level of controls; however, as some of these data varied considerably within the groups, results did not reach the level of significance for most of these parameters (Table 2).
Effect of moderate alcohol consumption on indices of liver damage and hepatic perilipin mRNA expression in ob/ob and control mice. a Representative photomicrographs of haematoxylin and eosin staining (×100 and ×400) and Oil Red O staining (×200) of liver sections. b, c Plasma ALT and AST levels d, e Perilipin 2 and 3 mRNA expression normalized to 18S mRNA in the liver. Data are shown as mean ± SEM (n = 4–6). *p < 0.05 in comparison with ob/ob mice treated with ethanol
Effect of moderate alcohol consumption on protein levels of TNFα, PAI-1, markers of insulin signalling cascade as well as carbohydrate metabolism and lipogenesis in the liver
Neither protein levels of TNFα, nor expression levels of IRS-1 and 2 mRNA differed between livers of ob/ob mice fed plain water and those consuming moderate amounts of ethanol (Fig. 2a–c). However, TNFα protein and IRS-2 mRNA expression in liver tissue were markedly different in ob/ob mice regardless of additional treatment in comparison with naïve control mice. As data varied considerably, IRS-1 mRNA expression did not differ between groups. In contrast, protein levels of phosphorylated Akt were markedly higher in livers of mice drinking moderate amounts of alcohol in comparison with naïve controls and ob/ob mice fed plain water (Fig. 2e, f); however, as levels of phosphorylated Akt varied considerable within groups, differences did not reach the level of significance between ob/ob groups. Expression of PEPCK, GK and GLUT4 mRNA in the liver, respectively, was similar between ob/ob groups. However, expression of GLUT4 was higher in livers of ob/ob mice regardless of additional treatments when compared to naïve control mice. In contrast, mRNA expression of FAS (p < 0.05) and to a lesser extend SREBP-1c (p = 0.07) were both lower in livers of ob/ob mice fed moderate amounts of ethanol in comparison with ob/ob mice fed plain water. Hepatic mRNA expression of ACOX-1 and PPARγ did not differ between ob/ob mouse groups; however, with the exception of SREBP-1c, expressions of genes involved in lipogenesis were markedly higher in livers of ob/ob mice than in livers of naïve lean control mice (Table 4). Furthermore, the elevated protein levels of phosphorylated Akt were associated with a marked protection against the induction of PAI-1 protein levels found in livers of ob/ob mice fed plain water (Fig. 2d).
Effect of moderate alcohol consumption on TNFα protein, markers of insulin signalling, PAI-1 protein levels and phosphorylation status of Akt protein in livers of ob/ob and control mice. a TNFα protein levels and b, c IRS-1 and 2 mRNA expression normalized to 18S mRNA. d Liver protein levels of PAI-1 and e representative blots of phospho-Akt and total Akt as well as densitometric analysis of the blots. Data are shown as mean ± SEM (n = 4–6). *p < 0.05 in comparison with ob/ob mice treated with ethanol
Effect of moderate alcohol consumption on the SIRT1-/adiponectin-signalling cascade in visceral adipose tissue
Adiponectin expression in visceral adipose tissue was significantly lower in ob/ob mice fed plain water when compared to those chronically drinking moderate amounts of alcohol (Fig. 3a). Furthermore, expression of SIRT1, shown to be a key regulator of adiponectin expression in adipocytes [24], was significantly induced only in visceral adipose tissue of ob/ob mice drinking moderate amounts of alcohol (~threefold) when compared between groups (Fig. 3b).
Effect of moderate alcohol intake on adiponectin and SIRT1 mRNA expression in visceral adipose tissue in mice. a Adiponectin and b SIRT1 mRNA expression in adipose tissue in mice normalized to 18S mRNA (n = 4–6). Data are shown as mean ± SEM. *p < 0.05 in comparison with ob/ob mice treated with ethanol
Effect of moderate alcohol exposure on 3T3-L1 cells challenged with 4-methylpyrazole
To further delineate whether the positive effects found in ob/ob mice exposed to moderate alcohol levels may have resulted from a direct effect of ethanol on adipose tissue, differentiated 3T3-L1 cells were challenged with 0.2 vol% of ethanol in an alcohol vapour chamber. Adiponectin mRNA expression was significantly increased in differentiated 3T3-L1 cells treated with 0.2 vol% of ethanol in comparison with naïve cells (Fig. 4a). Furthermore, expressions of SIRT1 and FOXO1 mRNA were also significantly higher in 3T3-L1 cells exposed to 0.2 vol% of ethanol for 6 h in comparison with naïve cells (Fig. 4b, c). These effects of ethanol were almost completely blunted when cells were concomitantly exposed to 4-methylpyrazole, an inhibitor of alcohol dehydrogenase (ADH) [25].
Effect of moderate alcohol exposure on adiponectin, SIRT1 and FOXO1 mRNA expression in 3T3-L1 cells and effect of TNFα and adiponectin on AML 12 cells. a Adiponectin, b SIRT1, and c FOXO1 mRNA expression in 3T3-L1 cells treated with 0.2 vol% ethanol and 4-methylpyrazole (4-MP; n = 3). d PAI-1 mRNA expression in AML 12 cells treated with TNFα and adiponectin (n = 4). mRNA expression was normalized to 18S mRNA. Data are shown as mean ± SEM. *Data are significantly different, p < 0.05
Effect of adiponectin treatment on PAI-1 expression in AML-12 cells challenged with TNFα
To further address the role of adiponectin in regulating PAI-1 expression in hepatocytes, AML-12 cells, a model of mouse hepatocytes [26], were challenged with TNFα. AML-12 cells challenged with TNFα for 6 h expressed significantly higher (~+3.7-fold) levels of PAI-1 mRNA. This effect of TNFα was completely blunted in cells concomitantly treated with adiponectin when exposed to TNFα (Fig. 4c, d).
Discussion
Results of several epidemiological studies suggest that the effect on general health and herein also the liver may substantially differ between moderate (e.g. <20 g/d for men and <10 g/d for women) and elevated alcohol intake (e.g. >50 g/d for men and >40 g/d for women) (for overview also see [27–29]). Furthermore, in recent years results of epidemiological studies suggest that moderate alcohol consumption may actually possess beneficial effects on the development and also the progression of liver damage in patients with NAFLD. Indeed, in a large biopsy-proven NAFLD population study, daily consumption of <20 g alcohol was associated with lower odds of NASH as well as fibrosis [6]. In line with these findings, Moriya et al. [7] reported in a cross-sectional study that drinking <20 g on 1–3 days/week is associated with a lower prevalence of fatty liver. However, from the results of these epidemiological studies, it cannot be ruled out that compounds taken in along with alcoholic beverage (e.g. compounds derived from hops or grapes or lifestyle modifications associated with moderate alcohol intake) may contribute to the beneficial effects of alcohol consumption rather than the ethanol itself. Indeed, it has been shown that chronic intake of elevated amounts of beer and wine in comparison with spirits may have less harmful effects on the liver [15, 30]. Results of animal studies assessing the effect of plain ethanol on the liver in settings of NAFLD are inconsistent as doses and models used varied considerably between studies [10, 11, 14]. In the present study, the hypothesis that moderate alcohol consumption (e.g. 2.5 g/kg b.w. given for 6 weeks) protects against the development of NAFLD was tested in a genetic model of NAFLD (ob/ob mice). Indeed, concomitant intake of this comparably low dose of alcohol diminished the development of NAFLD as shown by markedly lower transaminases, less pronounced steatosis and inflammation and lower indices of apoptosis in livers of ob/ob mice fed ethanol. However, moderate intake of ethanol had no effects on plasma levels of free fatty acids, triglycerides, total as well as LDL and HDL cholesterol in ob/ob mice. These data are somewhat contrary to the findings of others reporting that moderate alcohol intake, e.g. resulting from beer or wine consumption, is associated with higher plasma HDL cholesterol levels in humans [31]. Differences between these studies and ours might have resulted from differences in the type of alcoholic beverages consumed (plain ethanol vs. beer or wine) but also differences in species (mice vs. humans).
An increased release of TNFα, mainly from Kupffer cells, and alterations of insulin signalling have been proposed to be key factors in the development of NAFLD (for overview see [32, 33]). Furthermore, several studies have shown that chronic intake of alcohol is associated with an induction of TNFα and impaired insulin signalling in the liver as reviewed by Cohen et al. and Purohit et al. [34, 35]. Indeed, decreased expressions of IRS-1 and IRS-2 have repeatedly been shown to be associated with impairments of insulin signalling and insulin resistance [36–39]. Furthermore, results of studies in humans suggest, that moderate alcohol consumption is associated with improved insulin sensitivity in non-diabetic humans [18, 40]. Here, neither TNFα nor IRS-1 or 2 levels were altered between livers of ob/ob mice only fed drinking water and those drinking moderate amounts of ethanol. In contrast, serine phosphorylation of Akt which was repeatedly shown to be a mediator of the cellular effects of insulin and to be negatively regulated by TNFα [41, 42] was markedly higher in livers of ob/ob mice exposed to moderate amounts of alcohol. However, mRNA expression of markers of carbohydrate metabolism, shown before to be regulated at least in part through Akt-dependent signalling pathways (for overview see [43, 44]), did neither differ between ob/ob groups nor lean control mice. In contrast, expressions of markers of lipid metabolism, with the exception of SRBEP-1c, were markedly higher in livers of ob/ob mice than in lean controls. However, only FAS and to a lesser extend SREBP-1c expression was found to be significantly affected by the moderate intake of alcohol. There are only limited data available on the effects of moderate alcohol intake on hepatic lipogenesis yet. However, our data suggest that FAS expression may be markedly suppressed through an adiponectin-dependent down-regulation of SRBEP-1c in livers of ob/ob mice ingesting moderate amounts of alcohol. This in turn may also add to the less pronounced lipid accumulation found in these mice when compared to ob/ob mice ingesting only plain water. Indeed, it has been shown that adiponcetin, probably through SREBP-1c-dependent signalling pathways, may alter FAS expression in the liver [45]. Differences between the studies of others and our own in regards to expression of markers of carbohydrate but also lipid metabolism may have resulted from the different Akt subtypes determined. Indeed, in the present study, an antibody detecting phosphorylation status of Akt 1–3 was used to determine phosphorylation status of Akt in liver, whereas for instance, others reported that Akt 2 is the subtype of Akts primarily involved in GLUT4-mediated glucose uptake [44]. Furthermore, several studies suggest that Akt also may affect glucose metabolism through rather direct effects, e.g. through an induction of GLUT4 translocation to cell membranes [46, 47]. Therefore, as in the present study, only mRNA expression of markers of carbohydrate metabolism in the liver was determined, it cannot be ruled out that alterations found in Akt phosphorylation affected carbohydrate metabolism in the liver through other mechanisms. Furthermore, PAI-1 levels but also markers of apoptosis were only found to be increased in livers of ob/ob mice fed with plain water. Taken together, these data suggest that the protective effects of moderate alcohol intake under the present conditions did not result from alterations at the level of TNFα- and insulin receptor signalling. Rather, our data suggest that moderate alcohol intake “normalizes” Akt-dependent pathways downstream of TNFα and IRS-1/IRS-2. These findings by no means preclude that moderate alcohol consumption may also affect insulin release in the pancreas or insulin receptor levels in other tissue like the muscle but imply that additional pathways may contribute to the beneficial effects of moderate alcohol intake on the development of NAFLD.
Previous studies have indicated that the protective effects of adiponectin on the development of NAFLD are at least in part mediated through an induction of adenosine monophosphate-activated protein kinase (AMPK) in the liver subsequently leading to a “normalization” of Akt- and PAI-1-dependent pathways [22, 48, 49], while others could not confirm these findings [50]. Results of human studies suggest that moderate alcohol intake is associated with increased circulating adiponectin levels (for overview see [31]). Several animal studies also suggest that alcohol intake might modulate adiponectin release from adipose tissue and subsequently AMPK signalling in the liver [13, 14, 51, 52]; however, alcohol levels given in these studies were rather high (>27.5 % of calories derived from alcohol). In the present study, we found that moderate alcohol intake was associated with a marked induction of SIRT1 and adiponectin in visceral adipose tissue in ob/ob mice. In line with these findings, adiponectin levels were markedly higher in differentiated adipocytes exposed to moderate alcohol concentrations (e.g. 0.2 vol %) than in naïve control cells. This was associated with an induction of FOXO1 and SIRT1, shown to be regulators of adiponectin expression in visceral adipose tissue [24]. These effects of alcohol were almost completely blocked when cells were treated with 4-methylpyrazol, an inhibitor of ADH. Our results suggest that an altered NAD+/NADH + H+ ratio, associated with the metabolism of ethanol, might be the trigger of the induction of SIRT1/FOXO1 and subsequently adiponectin; however, this will have to be addressed in future studies. Furthermore, in AML-12 cells, used as a model of hepatocytes, the challenge with TNFα resulted in a significant induction of PAI-1, an effect almost completely attenuated when cells were concomitantly exposed to adiponectin. Taken together, these data suggest that moderate alcohol intake leads through SIRT1-/FOXO1-dependent signalling pathways to an induction of adiponectin in visceral adipose tissue, which in turn modulates AMPK/Akt signalling in the liver thereby attenuating the TNFα-dependent induction of PAI-1. Earlier studies employing models of chronic high doses of ethanol suggest that adiponectin may also modulate Kupffer cell signalling and herein especially toll-like receptor 4/myeloid differentiation primary response gene 88-dependent signalling cascades [9]. Differences between the findings of the present study and those of others might have resulted from differences in general dietary composition (e.g. standard chow vs. Lieber DeCarli diet [53]), in duration of feeding and in the employed alcohol concentrations.
Conclusion
Taken together, our data suggest that similar to the findings of recent epidemiological studies, moderate alcohol intake in mice diminishes the development of NAFLD. Our data further indicate that these “protective” effects depend upon an induction of the SIRT1-/adiponectin-signalling pathway in visceral adipose tissue subsequently leading to a modulation of the adiponectin/AKT/PAI-1 signalling cascade in the liver. However, when interpreting our data, it has to be kept in mind that ob/ob mice develop hyperinsulinemia, insulin resistance but also NAFLD due to their inherited leptin deficiency [54]. This lack of leptin has been shown to be associated with markedly reduced adiponectin levels [55], which in turn may result in alternations not found in other dietary or genetic models of NAFLD or even patients suffering from NAFLD. Therefore, further studies are necessary to determine whether similar molecular mechanisms are also involved in the lower severity of NAFLD found in moderate alcohol consumers versus alcohol abstainers. Furthermore, our data by no means should encourage abstainers to drink alcohol for health reasons and the choice to consume alcohol should always be based on individual considerations (e.g. taking into account life circumstances and general health).
Abbreviations
- ACOX1:
-
Acyl-coenzyme A oxidase
- ADH:
-
Alcohol dehydrogenase
- Akt:
-
Protein kinase B
- AML-12:
-
Alpha mouse liver 12 cells
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BAX:
-
BCL2-associated X protein
- b.w.:
-
Body weight
- CCL2:
-
Chemokine (c–c motif) ligand 2
- ELISA:
-
Enzyme-linked immunosorbent assay
- FAS:
-
Fatty acid synthase
- FOXO1:
-
Forkhead box O1
- GK:
-
Glucokinase
- GLUT4:
-
Glucose transporter type 4
- H&E:
-
Haematoxylin and eosin
- IRS-1/2:
-
Insulin receptor substrate 1/2
- NAFLD:
-
Non-alcoholic fatty liver disease
- NAS:
-
NAFLD activity score
- NASH:
-
Non-alcoholic steatohepatitis
- PAI-1:
-
Plasminogen activator inhibitor 1
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- SIRT1:
-
Sirtuin-1
- SREBP-1c:
-
Sterol regulatory element-binding protein 1c
- TNFα:
-
Tumour necrosis factor alpha
References
Adams LA, Lymp JF, St SJ, Sanderson SO, Lindor KD, Feldstein A, Angulo P (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121
Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 42:44–52
Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI (2006) Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 21:138–143
Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52:1836–1846
Lelbach WK (1975) Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann N Y Acad Sci 252:85–105
Dunn W, Sanyal AJ, Brunt EM, Unalp-Arida A, Donohue M, McCullough AJ, Schwimmer JB (2012) Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol 57:384–391
Moriya A, Iwasaki Y, Ohguchi S, Kayashima E, Mitsumune T, Taniguchi H, Ikeda F, Shiratori Y, Yamamoto K (2011) Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment Pharmacol Ther 33:378–388
White IR, Altmann DR, Nanchahal K (2002) Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ 325:191
Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141:1572–1585
Fromenty B, Vadrot N, Massart J, Turlin B, Barri-Ova N, Letteron P, Fautrel A, Robin MA (2009) Chronic ethanol consumption lessens the gain of body weight, liver triglycerides, and diabetes in obese ob/ob mice. J Pharmacol Exp Ther 331:23–34
Wang Y, Seitz HK, Wang XD (2010) Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res 34:567–573
You M, Considine RV, Leone TC, Kelly DP, Crabb DW (2005) Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42:568–577
Shearn CT, Smathers RL, Jiang H, Orlicky DJ, Maclean KN, Petersen DR (2013) Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J Nutr Biochem 24:1436–1445
Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H (2011) Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol 55:673–682
Assuncao M, Santos-Marques MJ, Monteiro R, Azevedo I, Andrade JP, Carvalho F, Martins MJ (2009) Red wine protects against ethanol-induced oxidative stress in rat liver. J Agric Food Chem 57:6066–6073
Chiva-Blanch G, Urpi-Sarda M, Ros E, Valderas-Martinez P, Casas R, Arranz S, Guillen M, Lamuela-Raventos RM, Llorach R, Andres-Lacueva C, Estruch R (2013) Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr 32:200–206
Gronbaek M, Jensen MK, Johansen D, Sorensen TI, Becker U (2004) Intake of beer, wine and spirits and risk of heavy drinking and alcoholic cirrhosis. Biol Res 37:195–200
Konrat C, Mennen LI, Caces E, Lepinay P, Rakotozafy F, Forhan A, Balkau B (2002) Alcohol intake and fasting insulin in French men and women. The D.E.S.I.R study. Diabetes Metab 28:116–123
Rodriguez FD, Simonsson P, Alling C (1992) A method for maintaining constant ethanol concentrations in cell culture media. Alcohol Alcohol 27:309–313
Kanuri G, Weber S, Volynets V, Spruss A, Bischoff SC, Bergheim I (2009) Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J Nutr 139:482–487
Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Spruss A, Henkel J, Kanuri G, Blank D, Puschel GP, Bischoff SC, Bergheim I (2012) Female mice are more susceptible to non-alcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase—plasminogen activator inhibitor 1-cascade but not the hepatic endotoxin response. Mol Med 18:1346–1355
Orlicky DJ, Roede JR, Bales E, Greenwood C, Greenberg A, Petersen D, McManaman JL (2011) Chronic ethanol consumption in mice alters hepatocyte lipid droplet properties. Alcohol Clin Exp Res 35:1020–1033
Qiao L, Shao J (2006) SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281:39915–39924
Blomstrand R, Theorell H (1970) Inhibitory effect on ethanol oxidation in man after administration of 4-methylpyrazole. Life Sci II 9:631–640
Wu JC, Merlino G, Fausto N (1994) Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci USA 91:674–678
Cleophas TJ (1999) Wine, beer and spirits and the risk of myocardial infarction: a systematic review. Biomed Pharmacother 53:417–423
Nova E, Baccan GC, Veses A, Zapatera B, Marcos A (2012) Potential health benefits of moderate alcohol consumption: current perspectives in research. Proc Nutr Soc 71:307–315
Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B (2010) The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 105:817–843
Hidestrand M, Shankar K, Ronis MJ, Badger TM (2005) Effects of light and dark beer on hepatic cytochrome P-450 expression in male rats receiving alcoholic beverages as part of total enteral nutrition. Alcohol Clin Exp Res 29:888–895
Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA (2011) Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342:d636
Baffy G (2009) Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 51:212–223
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871
Cohen JC, Horton JD, Hobbs HH (2011) Human fatty liver disease: old questions and new insights. Science 332:1519–1523
Purohit V, Gao B, Song BJ (2009) Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res 33:191–205
Araki E, Lipes MA, Patti ME, Bruning JC, Haag B III, Johnson RS, Kahn CR (1994) Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186–190
Gonzalez-Rodriguez A, Mas Gutierrez JA, Sanz-Gonzalez S, Ros M, Burks DJ, Valverde AM (2010) Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 59:588–599
Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T (2000) Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49:1880–1889
Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904
Bonnet F, Disse E, Laville M, Mari A, Hojlund K, Anderwald CH, Piatti P, Balkau B (2012) Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 55:3228–3237
Cintra DE, Pauli JR, Araujo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA (2008) Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol 48:628–637
Qin B, Anderson RA, Adeli K (2008) Tumor necrosis factor-alpha directly stimulates the overproduction of hepatic apolipoprotein B100-containing VLDL via impairment of hepatic insulin signaling. Am J Physiol Gastrointest Liver Physiol 294:G1120–G1129
Cheng Z, White MF (2011) Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal 14:649–661
Satoh T (2014) Rho GTPases in insulin-stimulated glucose uptake. Small GTPases 5:e28102
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112:91–100
Ji L, Zhang X, Liu W, Huang Q, Yang W, Fu F, Ma H, Su H, Wang H, Wang J, Zhang H, Gao F (2013) AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS ONE 8:e69910
Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, Bagchi M, Bagchi D, Maulik N (2009) Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim Biophys Acta 1792:39–48
Adachi M, Brenner DA (2008) High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47:677–685
Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, Tokuyama K, Noda T, Terauchi Y, Ueki K, Kadowaki T (2008) Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J 55:515–522
Nascimento AF, Ip BC, Luvizotto RA, Seitz HK, Wang XD (2013) Aggravation of nonalcoholic steatohepatitis by moderate alcohol consumption is associated with decreased SIRT1 activity in rats. Hepatobiliary Surg Nutr 2:252–259
Liang X, Hu M, Rogers CQ, Shen Z, You M (2011) Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid Redox Signal 15:425–435
Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE (2010) Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol 185:4928–4937
DeCarli LM, Lieber CS (1967) Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr 91:331–336
Campfield LA, Smith FJ, Burn P (1996) The OB protein (leptin) pathway–a link between adipose tissue mass and central neural networks. Horm Metab Res 28:619–632
Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703
Acknowledgments
This work was in part supported by a Grant from ‘Zentrum für Ernährungsmedizin (ZEM)’ and ‘BMBF (FKZ: 01EA1305)’ (IB).
Conflict of interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanuri, G., Landmann, M., Priebs, J. et al. Moderate alcohol consumption diminishes the development of non-alcoholic fatty liver disease (NAFLD) in ob/ob mice. Eur J Nutr 55, 1153–1164 (2016). https://doi.org/10.1007/s00394-015-0929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0929-7