Abstract
Purpose
To determine the dose–response of vitamin D3 supplementation on serum 25-hydroxyvitamin D [25(OH)D] among Chinese adults.
Methods
In this 5-arm, randomized, double-blinded controlled trial, 76 healthy participants were assigned to orally administrate 0, 400, 800, 1200 or 2000 IU/d of vitamin D3 for 16 weeks. Serum 25(OH)D, parathyroid hormone, calcium, biomarkers of liver and renal function were measured at multiple time points.
Results
The mean (SD) serum 25(OH)D at baseline was 31.6 (8.7) nmol/L, and the dose–response relationship was curvilinear with a plateau around 6 weeks for all doses. At week 16, 25(OH)D was increased by 6.0 (6.5), 21.7 (15.8), 26.3 (12.6), 32.0 (12.8) and 36.3 (26.0) nmol/L for 0, 400, 800, 1200 and 2000 IU/d (all P ≤ 0.002), corresponding to approximately 19, 53, 67, 77 and 80 % of reversion of vitamin D deficiency, respectively. Daily intake of 800 IU vitamin D3 reached a targeted 25(OH)D ≥ 30 nmol/L in at least 97.5 % of Chinese, but not a targeted 25(OH)D ≥ 50 nmol/L even with 2000 IU/d. Change of 25(OH)D was inversely associated with change of PTH concentration (r = −0.39, P < 0.001) after controlling for age and sex. No between-group differences were observed in terms of the change in serum calcium, alanine transaminase, aspartate aminotransferase, gamma-glutamyltransferase and creatinine (P ≥ 0.22).
Conclusions
Supplementation with 400, 800, 1200 or 2000 IU/d vitamin D could improve the vitamin D deficiency with various degrees. Whether 2000 IU/d vitamin D3 would generate a better result without side effect requires more studies with larger samples in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of major nutritional problems, about one billion world population suffered vitamin D deficiency [defined as circulating 25-hydroxyvitamin D: 25(OH)D < 50 nmol/L] or insufficiency [50 ≤ 25(OH)D < 75 nmol/L] [1]. The prevalence of vitamin D deficiency ranged from 40 to 100 % in American, European and Asian population [2–7]. Previously, we found that the prevalence of vitamin D deficiency and insufficiency was approximately 69.0 and 24.4 % among 3,262 Chinese aged 50–70 years from Beijing and Shanghai [2]. Besides its essential role in maintaining calcium hemostasis and bone health, such as lowering risks of rickets in children and osteopenia, osteoporosis or fractures in adults [8], poor vitamin D status was suggested to be associated with increased risks of type 2 diabetes, autoimmune diseases, hypertension and certain cancers [9–16]. Recently, the Institute of Medicine (IOM) raised the Recommended Dietary Allowance (RDA) of vitamin D to 15 µg (600 IU) and 20 µg (800 IU) for 97.5 % populations aged 1–70 years and ≥70 years, respectively, to achieve a target circulating 25(OH)D ≥ 50 nmol/L for maintaining bone health [17].
Human body can obtain vitamin D through skin photochemical synthesis and also consuming foods like oily fish, milk and eggs [8]. After being absorbed and bound to vitamin D-binding protein (DBP), vitamin D is transferred to liver and yield 25(OH)D (a well-accepted indicator of vitamin D status), by 25-hydroxylases. The resultant 25(OH)D is further hydroxylated in the kidney by 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) to 1,25(OH)2D, the most active form, which is then delivered to target tissues. The yield of 1,25(OH)2D could be stimulated by parathyroid hormone (PTH), but inhibited by calcium, phosphorus and itself. The 25(OH)D and 1,25(OH)2D are inactivated by 25-hydroxyvitamin D-24-hydroxylase (CYP24) [1, 8]. Vitamin D synthesis and degradation could be influenced by genetic variants involving vitamin D metabolism pathway [18]. Factors such as body weight (fat), age, baseline 25(OH)D levels, type and intervention duration were reported to modify dose–response relationships between vitamin D supplement and circulating 25(OH)D [19–21]. Increasing evidence also showed effect of ethnic differences on vitamin D metabolism and relationships of 25(OH)D with bone health, or cardiometabolic outcomes [22, 23]. For example, in a recent 4-arm randomized clinical trial among 328 African-Americans, a RDA of 1640 IU vitamin D3 was estimated to reach the 25(OH)D ≥ 50 nmol/L recommended by IOM [17, 24], whereas a RDA of 800 IU/d was estimated for 163 healthy postmenopausal white women according to the results from a 1-year trial [21]. However, the RDAs of vitamin D in Asian countries were largely based on the data from studies conducted in Caucasian populations, and no clinical trials have evaluated the efficacy of vitamin D3 supplement on circulating 25(OH)D, as well as the impact of circulating 25(OH)D on bone health outcomes in Asians. Unlike USA and European countries, there are few vitamin D fortified foods available and avoiding sun exposure is a common practice in Asians, especially among women [2]. Thus, these factors may attribute to poor vitamin D status in Asian populations.
In this 5-arm, randomized controlled clinical trial, we aimed to investigate the dose–response relationship between vitamin D3 supplementation and serum 25(OH)D or PTH concentrations in healthy Chinese adults. We also closely monitored changes in serum calcium and biomarkers of liver and renal function during the 16-week trial for potential adverse effects.
Subjects and methods
Participants
After responding to a recruitment advertisement, 117 of hospital employees in Shanghai were screened by a questionnaire and physical examination. Individuals were excluded if they met following criteria: (1) taking vitamin D supplements ≥200 IU/d or calcium supplements ≥800 mg/d in the past month or taking any medication interfering with vitamin D metabolism; (2) allergic to vitamin D; (3) with a history of diabetes, severe liver and renal diseases, kidney stones, cardiovascular disease, stroke, psychological disorders, cancer, peptic ulcers or other gastrointestinal conditions hindering absorption; hypo/hyper parathyroidism; (4) women during pregnancy or lactation; (5) having vacation ≥1 week during the 16-week trial; and (6) participating in other clinical studies within the previous 3 months. Following the screening, fasting blood samples were collected from 101 candidates to evaluate the concentrations of 25(OH)D, calcium, and biomarkers of liver and renal functions. Persons were eligible if they were 20–45 years old; 12.5 nmol/L ≤ 25(OH)D ≤ 50 nmol/L; and 18.5 kg/m2 ≤ BMI < 25 kg/m2, to minimize accumulation of this fat-soluble vitamin in excess fat mass [25]. Finally, a total of 76 eligible volunteers were enrolled in the trial (Fig. 1).
Study flow diagram. A total of 73 subjects (96 %) completed the 16-week trial, and three participants dropped out. * Two participants (one each from the 800 and 2000 IU/d arms) discontinued the study due to personal reasons (job transfer) at the run-in period and week 4, respectively, and one participant in the 1200 IU/d arm discontinued due to a kidney stone at week 10. The data of these participants were included in the analysis if available. 25(OH)D 25-hydroxyvitamin D
The study protocol was approved by Biological Research Ethics Committee of the Shanghai Institutes for Biological Research, and all participants provided written informed consents. The present trial was registered at ClinicalTrials.gov as NCT01817036.
Study design
A 5-arm randomized, double-blind, placebo-controlled clinical trial was conducted from January to May 2013 for 16 weeks. Following daily administrating placebo capsules for 1 week, 76 participants were randomly assigned to placebo or one of four doses of vitamin D3 (400, 800, 1200 and 2000 IU/d). The randomization was performed according to block randomization of age, sex, BMI and serum 25(OH)D during a screening by a statistician who was not involved in the conduct of the trial.
To ensure the double blinding, participants took 5 capsules/d with a combination of different numbers of placebos and/or 400 IU vitamin D3 capsules, which had a similar appearance and smell (provided by Xiamen Xingsha Pharmaceutical Group Co., Ltd). An independent laboratory (Royal DSM China Campus, Shanghai) evaluated the vitamin D3 contents in the five arms by sampling the combined capsules in each arm at three time points during the trial. The average doses of the three analyses were 0, 536, 980, 1359 or 2226 IU for the 0, 400, 800, 1200 and 2000 IU/d group, respectively.
The participants were asked to return all untaken capsules weekly. Adherence was assessed by capsule counts [(number of capsule supplied − number of capsule returned) / number of capsule supplied × 100 %]. During the trial, all participants were required (1) to maintain their habitual food intake and physical activity; (2) to limit sun exposure and vitamin D-rich foods such as fatty fish and cod liver oil as much as possible; and (3) to avoid taking non-trial vitamin D supplements.
Data collection
A face-to-face interview was conducted at baseline and at the end of the 16 weeks to collect information including health status, lifestyles, sun exposure, medical history and family history of diseases, medication log and intake of nutritional supplements by trained dieticians with standardized questionnaires. Total physical activity levels were categorized as low, moderate, or high based on the International Physical Activity Questionnaire (IPAQ, short last 7-day format), with minor modifications [26]. Dietary intake was obtained by using 3-day food records (2 weekdays + 1 weekend). During the intervention, all participants were asked to complete a brief questionnaire and a 3-day food record every 4 weeks. The brief questionnaire included information on compliance, sun exposure, travel history, any illnesses, or using non-trial vitamin D supplements, calcium supplements or any medications. Sun exposure levels were estimated by self-reported outdoor hours between 10:00 a.m and 3:00 p.m per week, and sun exposure protection score was calculated according to frequency of wearing hat, long sleeves and sunscreen within previous month.
Physical examinations were performed at baseline and the end of the 16-week period by trained medical professionals utilizing a standardized protocol. Body weight and height were measured in light indoor clothing without shoes to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as weight (kg) / height (m)2. Waist circumference was measured at the midpoint between the lowest rib and the iliac crest to the nearest 0.1 cm after exhalation using non-stretch tape measures. Blood pressure was measured three times by an electronic blood pressure monitor (Omron HEM-7000, OMRON Healthcare, Inc., Japan) on the left arm of the participant in a comfortable sitting position after resting at least 5 min, and the mean of the last two measurements was used for analyses. During the intervention, body weight and blood pressure were measured every 4 weeks.
Biochemical analyses
Overnight fasting blood and urine samples were collected at weeks 0, 1, 3, 6, 10 and 16. Serum 25(OH)D concentrations were assayed by utilizing a liquid chromatography–mass spectrometry with deuterated internal standards (regarded as the gold standard method) [27]. Serum PTH was measured by an enzyme-linked immunosorbent assay kit (MD Biosciences Inc, Zürich, Switzerland). Serum alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), creatinine and calcium were measured by an automatic biochemical analyzer (Hitachi 7080) with reagents purchased from Roche Diagnostics (Mannheim, Germany). All of the intra-assay and interassay coefficients of variation were <10 %.
Safety assessment
All participants were asked to report immediately to research team members if they experienced any adverse events such as diarrhea, cold and stomach discomfort. Serum calcium, biomarkers of liver and renal function (ALT, AST, GGT, and creatinine) and 25(OH)D concentrations were used to monitor occurrences of hypercalcemia (serum calcium > 2.6 mmol/L) and hypervitaminosis D [serum 25(OH)D ≥ 220 nmol/L, the no-observed-adverse-effect level (NOAEL)] [28]. Other abnormal parameters of laboratory assays were defined according to corresponding normal ranges: ALT > 40 IU/L; AST > 40 IU/L; GGT > 32 IU/L in women and >50 IU/L in men; or creatinine >84 umol/L in women and >104 umol/L in men.
Statistical analysis
Intention-to-treat analysis was applied. Normal or non-normal distribution quantitative and categorical variables, described as mean ± SDs or median (interquartile range) and proportions, were compared across five arms by using ANOVA or Kruskal–Wallis test and Fisher’s exact test, respectively. A mixed-effects model was used to assess the effects of vitamin D3 dose and treatment duration on serum 25(OH)D concentrations. The dose and visit time were included as fixed effects, and the participant was included as a random effect. Interactions between dose and time were also explored. ANCOVA was used to analyze overall group difference at weeks 0, 1, 3, 6, 10 and 16, followed by a Fisher’s least significant difference (LSD) multiple-comparisons test with adjustments for age, gender and baseline 25(OH)D concentrations (not for the comparison at week 0). RDA of vitamin D was defined as a sufficient dose of this nutrient to meet a targeted 25(OH)D value in at least 97.5 % healthy people. Considering the insufficiency cutoff of 50 nmol/L suggested by IOM, the RDA of vitamin D would be a given dose when 95 % prediction lower limit of circulating 25(OH)D ≥ 50 nmol/L [17, 21]. Since the relation between 25(OH)D and bone health outcomes has not been established in Asians, the RDA at a targeted level of ≥30 and 40 nmol/L was also explored, respectively. To estimate the 95 % prediction limits for serum 25(OH)D concentrations at week 16, one thousand bootstrapped samples were employed in each arm. Changes in all variables were calculated by subtracting the baseline values from the values at week 16. Multiple linear regression was applied to explore influencing factors related to serum 25(OH)D concentrations, including age, gender, BMI, treatment dose, baseline 25(OH)D, physical activity, smoking status, alcohol use, sun exposure, sun protection score and dietary calcium intake. A partial correlation coefficient was used to assess the correlation between change of PTH and change of 25(OH)D with adjustments for age and gender. The effects of vitamin D3 doses on changes of PTH, calcium, ALT, AST, GGT and creatinine were evaluated by general linear regression with adjustments for age and gender. PTH, ALT, AST and GGT were log-transformed before analyses to approximate the normal distribution. Occurrence of adverse events was counted as person*times for each arm at multiple time points (week 0, 1, 3, 6, 10 and 16). Data were analyzed using SAS, version 9.3 (SAS Institute, Cary, NC, USA), and P < 0.05 (two-sided) was considered statistically significant.
Results
Baseline characteristics
The mean (SD) baseline 25(OH)D concentration was 31.6 (8.7) nmol/L without gender difference (32.9 ± ± 9.04 nmol/L for men and 30.5 ± 8.43 nmol/L for women, P = 0.15). After randomization, participants in the five arms showed similar characteristics, except higher sun exposure in the placebo arm (Table 1). A total of 73 subjects (96 %) completed the 16-week trial, and three participants dropped out due to job transfer (in the 800 and 2000 IU/d groups) or having a kidney stone (in the 1200 IU/d group) (Fig. 1). The overall compliance rate was high with 98.9, 99.1, 97.6, 99.1 and 98.8 % for 0, 400, 800, 1200 and 2000 IU/d group, respectively.
Dose–response between vitamin D3 supplementation and serum 25(OH)D
As shown in Table 2, the mean serum 25(OH)D concentrations increased significantly from week 1 to week 16 in all vitamin D3 supplemented arms (all P < 0.001), and the mean ± SD 25(OH)D increments per 1 μg vitamin D3 (the slope of response, 1 μg = 40 IU) were 2.17 ± 1.58, 1.32 ± 0.63, 1.07 ± 0.43 and 0.73 ± 0.52 nmol/L/μg for 400, 800, 1200 and 2000 IU/d group (all P < 0.001), respectively. When considering between-group differences among four treatment arms at each visit, 25(OH)D was significantly higher only in the 2000 IU/d group than in the 400 IU/d group (P < 0.04) since week 3. The different significance became marginal when using Fisher’s LSD in multiple-comparisons test (P ≥ 0.07). The placebo group also showed seasonally increased 25(OH)D from winter to spring during the trial (P = 0.002).
A significant interaction between dose and time for 25(OH)D was observed (P for interaction <0.001), after adjusting for age and sex (Table 2). The interaction remained significant when further controlling for BMI, calcium intake, smoking status, alcohol use, sun exposure, using sunlight protection and physical activity (P < 0.001). At each given vitamin D3 dose, serum 25(OH)D rose steadily for the first few weeks and then leveled off at approximately week 6 (Supplemental Fig. 1), although larger slopes and higher plateau concentrations appeared in the groups with higher daily doses of vitamin D3.
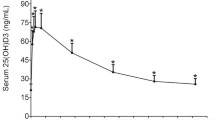
After the 16-week trial, 29.8 % (17/57) and 82.5 % (47/57) participants in four treatment arms had 25(OH)D < 50 and 75 nmol/L, respectively. The 95 % prediction intervals were 9.4–66.5 for 0 IU/d, 21.5–77.9 for 400 IU/d, 30.5–86.5 for 800 IU/d, 34.5–90.5 for 1200 IU/d and 34–92.5 nmol/L for 2000 IU/d group in the 1000 bootstrapped samples (Fig. 2), and no treated group attained 25(OH)D ≥ 50 or 40 nmol/L in at least 97.5 % of the participants, let alone 75 nmol/L. Estimated average requirements were 400 IU/d to achieve 25(OH)D ≥ 50 nmol/L. The RDA of vitamin D would be 800 IU/d at an targeted 25(OH)D ≥ 30 nmol/L. When potential confounding factors were examined by using multiple regression analysis, only vitamin D3 dose (P = 0.026) and initial 25(OH)D concentration (P = 0.01) significantly influenced serum 25(OH)D response (Supplemental Table 1). Participants with lower initial 25(OH)D tended to have a relatively greater response.
Serum 25(OH)D concentrations at week 16 with 95 % bootstrapped prediction intervals by arms. The solid triangle points represent all individuals’ 25(OH)D values at week 16. The dot shows the prediction value for each arm (blue dots), and vertical lines (blue) represent the 95 % prediction limits. The horizontal red dashed lines represent 75, 50, 40 and 30 nmol/L, respectively. 25(OH)D 25-hydroxyvitamin D
Serum PTH and its relation with 25(OH)D
The mean serum PTH concentrations in the five arms tended to decline from baseline to the end of the trial, but a significant reduction in this hormone was detected only in the 1200 IU/d [median and interquartile range: −8.7(−19.4, 4.0) pg/ml] and 2000 IU/d [−3.3 (−16.2, −0.24) pg/ml, both P = 0.03]. As indicated by Fig. 3, the change of PTH concentrations was inversely associated with 25(OH)D concentrations change (r s = −0.39, P < 0.001, adjusted for age and sex). No between-group differences in PTH were found among the five arms.
Plot of PTH, 25(OH)D concentration change of all subjects pre- and post-intervention. PTH was natural log-transformed before analysis. The correlation coefficient between change in ln-PTH and change in serum 25(OH)D (week 16–week 0) was −0.39 (P < 0.001, adjusted for age and sex). PTH parathyroid hormone, 25(OH)D 25-hydroxyvitamin D
Adverse events
During the 16-week trial, 25 participants reported getting a cold, or having stomach discomfort, and one of them (in the 1200 IU/d group) was diagnosed with a kidney stone at week 10 (Table 3). No hypervitaminosis D was detected with the highest value of 25(OH)D being 123.9 nmol/L, far below then 220 nmol/L defined as the no-observed-adverse-effect level NOAEL [27]. Except for a few occasions, serum calcium values in the five arms were generally lower than 2.6 mmol/L (the cutoff for hypercalcemia) at most time points during the study (Supplemental Fig. 2). Likewise, the markers of liver and renal function (ALT, AST, GGT and creatinine) also remained within the normal ranges with a few exceptions (Supplemental Fig. 2). No between-group differences were observed in the change in serum calcium, ALT, AST, GGT and creatinine (P ≥ 0.22).
Discussion
To the best of our knowledge, this was the first report of a randomized, placebo-controlled trial evaluating a dose–response relationship between vitamin D3 supplementation and serum 25(OH)D among Asian people. At each given dose, circulating 25(OH)D increased in a curvilinear fashion with a plateau around week 6. Our data suggested that 2000 IU/d could not achieve targeted 25(OH)D ≥ 50 nmol/L in at least 97.5 % Chinese, while no major adverse outcomes were observed even with the tolerable upper intake level (UL, 2000 IU/d) in China.
Although racial differences were suggested between white and African-Americans when considering the relationship between 25(OH)D and bone or cardiometabolic outcomes [9, 29, 30], there was no appreciable difference in our study and others in terms of threshold for efficacy, defined as the average increment of circulating 25(OH)D response to vitamin D3 supplementation (nmol/L/ug, 1 ug = 40 IU). For instance, we found that the efficacy at week 16 was from 2.2 to 0.73 nmol/L/ug for the 400–2000 IU/d groups, while increments at the same doses were about 3–0.5 nmol/L/μg in American whites [31, 32] and were 1.1 and 0.76 nmol/L/μg for 800 and 2000 IU/d vitamin D3 in African-American women [33]. Despite similar efficacy of vitamin supplementation, several risk factors for poor vitamin D status, including lower dietary intake, rare use of multivitamin supplementation, air pollution, limited outdoor activity and lower skin synthesis for vitamin D, are more pronounced in Asians than Caucasians [34]. On the other hand, the current RDAs and ULs for vitamin D are lower in China (400 and 2000 IU/d) and other Asian countries like Japan (200 and 2000 IU/d) and Koran (200 and 2400 IU/d) than those in the USA (600 and 4000 IU/d) [17, 35–37]. However, with 400–2000 IU/d vitamin D3 doses, we did not observe any major adverse events except that one person in 1200 IU/d group was diagnosed with kidney stone at week 10. Since we did not exclude this preexisting condition in this person, no causal relation could be inferred in this case.
In this study, the response of serum 25(OH)D to each dose of vitamin D3 reached a plateau at approximate week 6, though higher dose groups tended to have steeper slopes initially and a higher overall magnitudes of increase (Fig. 2). Asian Indians may have a faster clearance rate of 25(OH)D, owing to observed higher activity of a key enzyme in the catabolism, namely 25(OH)D-24-hydroxylase, in comparison with Caucasians [38]. Although few studies have evaluated the time course of changes of 25(OH)D concentrations with multiple doses of vitamin D3, our finding was similar to the results from some earlier trials in Western populations. In an 11-week trial conducted in 20 healthy Boston residents (42°N latitude), a 6-week plateau was observed after administrating 1000 IU/d vitamin D3 capsules [39]. Although factors such as smoking status, age, weight, BMI and multivitamin supplements intake may affect the response [19, 21, 24], only the treatment doses and baseline 25(OH)D values significantly influenced the response of vitamin D3 supplementation in our multivariate analyses (Supplemental Table 1). This may be due to the limited sample size and the narrow range of BMI (18.5 ≤ BMI < 25 kg/m2). Taken together, our data showed comparable time to achieve a steady state of circulating 25(OH)D in Chinese as in other ethnic groups.
According to our result, even with the UL for vitamin D (2000 IU) in China could not achieve the IOM recommended 25(OH)D of 50 nmol/L in Chinese adults with vitamin D deficiency. Previously, Gallagher et al. [21] estimated that RDAs were 800 IU/d for postmenopausal whites and elderly African-American women with vitamin D deficiency [40] and were 800–1600 IU/d for African-Americans aged 25–45 years [41]. Recently, Ng et al. [24] proposed even higher RDA (1640 IU/d) for African-American women with median age 51 years. The discrepancies in the estimated RDAs for vitamin D between IOM and the recent trials might be partially attributed to different latitude and/or baseline 25(OH)D values [19, 21, 40, 41]. It was noteworthy that the RDAs recommended by IOM did not take into account the between-individual variations at any given dose (only between-study variations), since the committee only summarized the mean (or median) responses of serum 25(OH)D values to total vitamin D intake from different studies by using a mixed effect model [42]. With the same approach, the estimated RDA in our study would be 800 IU/d, if the individual responses were replaced with the mean response. This suggests that the current RDA for vitamin D may be seriously underestimated by the IOM committee. Furthermore, the RDA recommended by IOM was targeted 25(OH)D ≥ 50 nmol/L to maximize bone health and muscle function, according to the data mainly from white and Africans-Americans [17]. As ethnic differences were noticed in the relation between bone mineral density and fractures [29], it is important to determine the relations not only between vitamin D intake and circulating 25(OH)D concentrations, but also between the targeted 25(OH)D values and bone health in Asians.
Consistent with earlier studies [43], we also observed an inverse association between change in serum 25(OH)D and change in PTH concentrations. Previously, Holick et al. [1] found that the inverse association between 25(OH)D and PTH values appeared when circulating 25(OH)D < 75 nmol/L, the concentration at which PTH began to level off. In our study, serum PTH concentrations were reduced significantly when 25(OH)D values were 64.1 (13.8) nmol/L for 1200 IU/d and 65.2 (23.7) nmol/L for 2000 IU/d group. Low 25(OH)D concentration may suppress calcium absorption and lower circulating calcium concentration, which could promote secretion of PTH and eventually stimulate kidney producing 1,25-dihydroxyvitamin D [1,25(OH)2D], a more biologically active form of vitamin D [8, 44].
Our study had several strengths: (1) the compliance rate was very high and sun exposure and travel history were closely monitored; (2) serum 25(OH)D, calcium and biomarkers of liver and renal function were measured at multiple time points to evaluate the time course and potential side effects. Admittedly, our study also had some limitations: (1) the sample size was small, which might have limited power to detect the between-group differences; (2) we were not allowed to examine the efficacy and safety beyond the current UL for vitamin D in China; and (3) with relatively young participants (20–45 years) in the study, it is unknown whether our results could be generalized to older age groups.
In conclusion, daily supplementation with 2000 IU vitamin D3 for 16-week improved 80 % of vitamin D deficiency in healthy Chinese without major adverse reactions. However, even with the UL dose in China, it was still insufficient to achieve the 25(OH)D ≥ 50 nmol/L recommended by the IOM. More studies with larger dose range of vitamin D and larger sample sizes are urgently needed to determine proper vitamin D intake and also the relation between 25(OH)D and bone health in Asian populations.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- GGT:
-
Gamma-glutamyltransferase
- PTH:
-
Parathyroid hormone
- SBP:
-
Systolic blood pressure
References
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281. doi:10.1056/NEJMc072359
Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X (2009) Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 32:1278–1283. doi:10.2337/dc09-0209
Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim S-K (2011) Vitamin D insufficiency in Korea-a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 96:643–651. doi:10.1210/jc.2010-2133
Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS (1998) Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783. doi:10.1056/NEJM199803193381201
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373. doi:10.4065/81.3.353
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443. doi:10.1007/s001980050030
Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P (2006) Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int 17:441–446. doi:10.1007/s00198-005-0006-9
Lips P (2006) Vitamin D physiology. Prog Biophys Mol Bio 92:4–8. doi:10.1016/j.pbiomolbio.2006.02.016
Scragg R, Sowers M, Bell C (2004) Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care 27:2813–2818. doi:10.2337/diacare.27.12.2813
Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P (2011) Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Investig 40:627–639. doi:10.3109/08820139.2011.573041
Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM (2012) Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab 97:1953–1961. doi:10.1210/jc.2011-3187
Forouhi NG, Luan Ja, Cooper A, Boucher BJ, Wareham NJ (2008) Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the medical research council ely prospective study 1990–2000. Diabetes 57:2619–2625. doi:10.2337/db08-0593
Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, Giovannucci EL, Fuchs CS, Chan AT (2013) Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 61:779–785. doi:10.1161/HYPERTENSIONAHA.111.00659
Chiu KC, Chu A, Go VLW, Saad MF (2004) Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79:820–825
Chacko SA, Song Y, Manson JE, Van Horn L, Eaton C, Martin LW, McTiernan A, Curb JD, Wylie-Rosett J, Phillips LS, Plodkowski RA, Liu S (2011) Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. Am J Clin Nutr 94:209–217. doi:10.3945/ajcn.110.010272
Choi HS, Kim K-A, Lim C-Y, Rhee SY, Hwang Y-C, Kim KM, Kim KJ, Rhee Y, Lim S-K (2011) Low serum vitamin D is associated with high risk of diabetes in Korean adults. J Nutr 141:1524–1528. doi:10.3945/jn.111.139121
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference Intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58. doi:10.1210/jc.2010-2704
Wang TJ, Zhang F, Richards JB et al (2010) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376:180–188. doi:10.1016/S0140-6736(10)60588-0
Zittermann A, Ernst J, Gummert J, Borgermann J (2014) Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr 53:367–374. doi:10.1007/s00394-013-0634-3
Raimundo F, Lang M, Scopel L, Marcondes N, Araújo M, Faulhaber G, Furlanetto T (2014) Effect of fat on serum 25-hydroxyvitamin D levels after a single oral dose of vitamin D in young healthy adults: a double-blind randomized placebo-controlled study. Eur J Nutr. doi:10.1007/s00394-014-0718-8
Gallagher JC, Sai A, Templin T 2nd, Smith L (2012) Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 156:425–437. doi:10.7326/0003-4819-156-6-201203200-00005
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511. doi:10.1161/CIRCULATIONAHA.107.706127
Kendrick J, Targher G, Smits G, Chonchol M (2009) 25-hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the third national health and nutrition examination survey. Atherosclerosis 205:255–260. doi:10.1016/j.atherosclerosis.2008.10.033
Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, Emmons KM, Fuchs CS (2014) Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr 99:587–598. doi:10.3945/ajcn.113.067777
Blum M, Dolnikowski G, Seyoum E, Harris S, Booth S, Peterson J, Saltzman E, Dawson-Hughes B (2008) Vitamin D3 in fat tissue. Endocrine 33:90–94. doi:10.1007/s12020-008-9051-4
Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X (2007) Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol 49:1798–1805. doi:10.1016/j.jacc.2007.01.065
Lai JKC, Lucas RM, Banks E, Ponsonby AL, Ausimmune Investigator G (2012) Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern Med J 42:43–50. doi:10.1111/j.1445-5994.2011.02471.x
Hathcock JN, Shao A, Vieth R, Heaney R (2007) Risk assessment for vitamin D. Am J Clin Nutr 85:6–18
Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM (2005) Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194. doi:10.1359/JBMR.041007
Gutierrez OM, Farwell WR, Kermah D, Taylor EN (2011) Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the national health and nutrition examination survey. Osteoporos Int 22:1745–1753. doi:10.1007/s00198-010-1383-2
Jarrett F, Michelle Ducasa G, David BB, Berwick M (2014) The effect of oral supplementation of vitamin D3 on serum levels of vitamin D: a review. Epidemiology 4:148. doi:10.4172/2161-1165.1000148
Smith SM, Gardner KK, Locke J, Zwart SR (2009) Vitamin D supplementation during Antarctic winter. Am J Clin Nutr 89:1092–1098. doi:10.3945/ajcn.2008.27189
Talwar SA, Aloia JF, Pollack S, Yeh JK (2007) Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 86:1657–1662
Mitchell D, Henao M, Finkelstein J, Burnett-Bowie S-A (2012) Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract 18:914–923. doi:10.4158/EP12072.OR
Sasaki S (2008) Dietary reference intakes (DRIs) in Japan. Asia Pac J Clin Nutr 17:420–444
Paik HY (2008) Dietary reference intakes for Koreans (KDRIs). Asia Pac J Clin Nutr 17:416–419
Chinese Nutrition Society (2014) Dietary Reference intakes (DRIs) in Chinese (2013). Science Press (CN)
Awumey EMK, Mitra DA, Hollis BW, Kumar R, Bell NH (1998) Vitamin D metabolism is altered in Asian Indians in the Southern United States: a clinical research center study. J Clin Endocrinol Metab 83:169–173. doi:10.1210/jc.2007-2308
Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD (2008) Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681. doi:10.1210/jc.2007-2308
Gallagher JC, Peacock M, Yalamanchili V, Smith LM (2013) Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab 98:1137–1146. doi:10.1210/jc.2012-3106
Gallagher JC, Jindal PS, Smith LM (2014) Vitamin D supplementation in young white and african american women. J Bone Miner Res 29:173–181. doi:10.1002/jbmr.2010
Ross AC, Taylor CL, Yaktine AL, Valle. HBD (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press (US)
Holick MF (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Acknowledgments
This study was supported by Ministry of Science and Technology of China [2013BAI04B03], and the National Natural Science Foundation of China [81170734, 81321062]. The authors’ responsibilities were as follows: P. Y. and L. L., conducted research, performed statistical analysis and wrote the paper; Y. H., G. L., X. C., L. S., X. Y. and H. Z. conducted research; Y. C. and F. B. H contributed to data analysis and interpretation and edited the paper; H. L. and X. L. designed the study, conducted research and had primary responsibility for the final content. All authors reviewed and approved the final manuscript. We give our sincere thanks to Lixin Tang for the devotion during field work, Yiqing Song for advice during the manuscript preparation and especially to Dr. Walter Willett for his helpful comments. We further appreciate the strong support shown by Xiamen Xingsha Pharmaceutical Group Co., Ltd. Finally, the greatest thanks should be given to all the participants involved in this study.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pang Yao and Ling Lu have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, P., Lu, L., Hu, Y. et al. A dose–response study of vitamin D3 supplementation in healthy Chinese: a 5-arm randomized, placebo-controlled trial. Eur J Nutr 55, 383–392 (2016). https://doi.org/10.1007/s00394-015-0859-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0859-4