Abstract
The literature describing vitamin D content of fat tissue is extremely limited. We conducted a pilot study that measured the concentrations of vitamin D3 in the fat tissue and serum of obese adults. These measurements were performed using a new liquid chromatography mass spectrometry (LC/MS) method. The objectives of this study were: to measure and report the vitamin D3 concentration in serum and subcutaneous fat samples from obese individuals and to examine the association of vitamin D3 in fat with vitamin D3 in serum. This cross-sectional study was conducted in 17 obese men and women who were scheduled to undergo gastric bypass surgery. The mean vitamin D3 concentration in subjects’ subcutaneous fat tissue samples was 102.8 ± 42.0 nmol/kg. The mean vitamin D3 concentration in serum was 7.78 ± 3.99 nmol/l. Vitamin D3 concentrations of fat tissue and serum were positively correlated (r = 0.68, P = 0.003). Consistent with previous findings in obese subjects, subjects in this study had suboptimal vitamin D status as demonstrated by a mean 25-hydroxyvitamin D concentration of 43.3 ± 15.4 nmol/l. In conclusion, fat tissue vitamin D3 can be measured by LC/MS and is detectable in obese subjects with suboptimal vitamin D status. Compatible with the long-standing concept that fat tissue is a storage site for vitamin D, fat tissue and serum vitamin D3 concentrations were positively correlated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
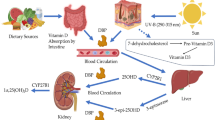
Prior studies have reported fat tissue to be a major repository in the body for vitamin D, but the studies performed in humans have only examined vitamin D concentrations in fat tissue of amputated limbs and in cadavers [1 , 2]. In subjects given intravenous injections of radioactively labeled vitamin D3, subsequent fat tissue samples obtained either from amputated limbs or at autopsy contained higher levels of the radioactive dose compared to other tissue types [1]. Similarly, vitamin D was found in fat tissue specimens obtained from 15 British subjects autopsied after sudden death [2]. So too, in studies done with rodents, fat tissue has been shown to contain vitamin D [3, 4].
Obese individuals have significantly lower circulating concentrations of 25-hydroxyvitamin D (25 (OH)D), the clinical indicator of vitamin D status, than do non-obese individuals [5–7]. Even in individuals who are not obese, body size, and adiposity are inversely associated with blood 25(OH)D concentrations [8, 9]. It has been suggested that this inverse association may be due to increased metabolic clearance of vitamin D through enhanced uptake in fat tissue [5] and/or decreased bioavailability of vitamin D once it is deposited in fat tissue [10]. These hypotheses have not been tested, perhaps in part because measurement of vitamin D in fat tissue is difficult.
In this paper, we describe a liquid chromatography mass spectrometry (LC/MS) method for measuring vitamin D in serum and fat tissue. We used this method to measure vitamin D3 in serum and in subcutaneous fat tissue samples collected from morbidly obese subjects undergoing gastric bypass surgery. We also report the association of the subcutaneous fat tissue D3 with serum D3 in the same individuals. These data will be useful for planning further studies that address the amount and location of vitamin D storage in fat tissue and the role of obesity in vitamin D deficiency.
Results
Preliminary analyses indicated that one subject who had recently traveled had a higher serum 25(OH)D level than the other 17 subjects (90 nmol/l compared with a range of 17.5–65.0 nmol/l in the other subjects) and a different ratio of vitamin D3 in subcutaneous fat to vitamin D3 in serum (1.7 nmol/kg:nmol/l) compared with a range of 7.4–36.4 in all other subjects; the fat and serum vitamin D3 measurements were 23.1 nmol/kg and 13.8 nmol/l, respectively; see Sect. “Discussion” for commentary). This subject was excluded from all subsequent analyses.
The clinical characteristics of the remaining 17 subjects are shown in Table 1. All the subjects were morbidly obese. Mean total vitamin D intake from food and supplements was 12.8 μg/day, at or close to the current recommended intakes for adults (5–15 μg/day depending on age) [11]. Nevertheless, all subjects had suboptimal vitamin D status as indicated by serum 25(OH)D concentrations <75 nmol/l.
The mean vitamin D3 concentration in subcutaneous fat tissue samples was 102.8 ± 42.0 nmol/kg, with values ranging widely among the subjects (from 28.1 to 186.6 nmol/kg). Mean serum vitamin D3 in the same subjects was 7.78 ± 3.99 nmol/l. The correlation of vitamin D3 concentrations in serum and fat was 0.68, P = 0.003 (Fig. 1).
Vitamin D3 concentrations in subcutaneous fat tissue and serum were inversely and similarly correlated with body weight (r = −0.38 and −0.42, respectively), but these correlations were not statistically significant in this small sample (P = 0.135 and 0.096, respectively).
Discussion
We have described a method for measuring vitamin D3 in fat samples by LC/MS and have added the resulting measurements to a very sparse literature concerning fat tissue content of vitamin D in humans. The mean subcutaneous abdominal fat D3 concentration that we obtained by this method (102.8 ± 42.0 nmol/kg) was fairly similar to that obtained by Lawson et al. [2] in perirenal (117 ± 57 nmol/kg) and pericardial (161 ± 81) fat tissue samples, but lower than that in axillary fat (301 ± 135) samples. In that study, an HPLC method was used to measure fat D3 in 15 humans autopsied after sudden death. Lawson et al.’s subjects probably weighed much less than ours (their weights are not given but they were not an exclusively obese sample as ours was), but it would be premature to draw conclusions about the relative vitamin D content in the fat of obese versus non-obese individuals. The method that we employed could be used in future studies, in which the fat tissue contents of lean and obese individuals are directly compared. An immediate priority however is to determine the variability in vitamin D content in fat tissue within given regions.
Our data suggest that there is a moderately strong positive association between serum and fat tissue content of vitamin D3, at least in obese individuals. We are aware of no other human data that address this association. However, for other fat-soluble vitamins, the positive correlations of 0.17 for retinol, 0.34 for α-tocopherol, and 0.56 for β-carotene have been reported [12]. The positive association of serum and fat tissue vitamin D3 concentrations is compatible with the long-standing concept that fat tissue is a storage site for vitamin D [1]. It is perhaps notable that the subject who was excluded because of an above-average level of serum vitamin D3 but a relatively low level of fat tissue D3 had traveled to Mexico for 2 weeks prior to bypass surgery. We speculate that the subject was vitamin D deficient prior to the trip and that sun exposure for 10 days in April produced enough vitamin D to raise the circulating vitamin D3 but who was not sufficient to have much impact on the vitamin D content of the large fat tissue compartment. A longer period of high sun exposure may have increased fat tissue D3 content as suggested by Lawson et al.’s demonstration in rats that prolonged irradiation raised fat D3 from 26.0 to 234 nmol/kg [2].
The low 25(OH)D status of our subjects is consistent with the previous work from our group and others showing an inverse association of 25(OH)D with body weight and adiposity [9, 13] and adds to the growing evidence for a higher vitamin D requirement in obesity [14].
In summary, this study demonstrates that vitamin D3 can be measured in fat tissue by LC/MS. It identifies a mean subcutaneous fat vitamin D3 concentration of 102.8 ± 42.0 nmol/kg in morbidly obese human subjects with suboptimal vitamin D status. In these subjects, serum and fat tissue vitamin D3 concentrations are positively correlated, consistent with fat tissue as a storage reservoir.
Materials and methods
Subjects
The subjects were obese men and women who were scheduled to undergo gastric bypass surgery. They were recruited from the obesity clinic at Tufts-New England Medical Center. Potential participants were excluded from the study if they used bile acid sequestering or anti-seizure medications and were excluded from the analysis if they were taking prescription vitamin D. As described in Sect. “Results,” an additional subject was excluded from the main analyses due to the apparent effect of recent travel on vitamin D3 measurements. The Institutional Review Board at Tufts-New England Medical Center approved the study, and written informed consent was obtained from each subject.
Experimental methods
Subjects were enrolled 1–2 weeks prior to their surgery at a pre-operative clinic visit. At this visit, dietary vitamin D intake over the last 6 months was estimated with the Fred Hutchinson food frequency questionnaire [15], supplemental vitamin D intake was recorded as part of a medical history interview, and non-fasting blood was drawn for the measurement of serum vitamin D3 and 25(OH)D. Serum 25(OH)D was measured with radioimmunoassay kits from DiaSorin Inc. (Stillwater, MN, USA). At the time of surgery, abdominal subcutaneous fat was collected, cut into pieces weighing ∼500 mg, placed in plastic vials, and stored frozen at −70°C until analysis.
Serum and subcutaneous fat vitamin D3 sample preparation for LC/MS analyses
Serum samples were prepared for LC/MS analysis as follows: a 1.0 ml aliquot of serum was placed in a glass tube and 200 μl of a 288 nmol/l solution of deuterated vitamin D3 internal standard was added. Tubes were vortexed for 1 min and allowed to stand 30 min at room temperature. A 2 ml aliquot absolute ethanol was added and the tubes were vortexed for 1 min. Two-milliliter water and 3 ml hexane were added and the tubes were again mixed for 5 min followed by centrifugation at 1,900g at 4°C for 15 min. The top (hexane) layer was aspirated and transferred to a clean test tube. Extracts were heated to 45°C and evaporated under N2. The dried extracts were reconstituted by adding 1 ml methanol and standing at room temperature for at least 30 min. The serum extracts were further purified using C-18 solid phase extraction. Columns were conditioned with: 5 ml of hexane, 5 ml of methanol, and finally with 5 ml of water. One milliliter of water was added to each sample and the tubes were vortexed. The contents of each tube were applied to individual C-18 columns. The columns were washed with 5 ml of water followed by two 5 ml washes with 70% methanol and last by 5 ml of acetonitrile. The vitamin D was eluted with 5 ml of methanol. The collected samples were evaporated as described above. The purified samples were reconstituted in methylene chloride and methanol and were analyzed by LC/MS as described below.
Subcutaneous fat tissue was prepared for LC/MS analysis as follows: a portion of fat tissue (0.2–0.25 g) was thawed, patted dry, and weighed. It was then added to a mixture of 1.3 ml of water and 1.3 ml of absolute ethanol and homogenized with a PowerGen 125 Homogenizer. Then 200 μl aliquots of tissue homogenate were placed in glass tubes and 200 μl of 288 nmol/l of deuterated vitamin D3 internal standard was added to each tube. The samples were evaporated under N2 at 45°. One milliliter of 30% KOH was added to each sample and 1 ml of water was added to the standard and blank; the tubes were mixed at room temperature on a reciprocal shaker at 250 rpm for a minimum of 3 h. Tubes were removed from the shaker and 2 ml of water was added, 3 ml of hexane was added, tubes were mixed, and 3 ml of absolute ethanol was added. The tubes were vortexed for 5 min and then centrifuged at 3,000 rpm at 4°C for 5 min. The top (hexane) layer was aspirated and transferred into a clean test tube. The extracts were then evaporated under N2 at 45°C. The extracts of fat tissue were further purified by C-18 Solid Phase Extraction as described above. The C-18 purified samples were reconstituted in 20 μl of methylene chloride followed by 180 μl of methanol. The reconstituted samples were analyzed by LC/MS.
LC/MS analysis
The instrument used for the vitamin D analyses was an Agilent 1100 HPLC and an Agilent 1100 single quadrupole MS. The HPLC column was a ProntoSIL 200-5-C30 obtained from MAC-MOD Analytical, Inc., Chadds Ford, PA, USA, manufactured by Bischoff Chromatography, Leonberg, Germany, with a 5-μm C-30 stationary phase. The column dimensions were 4.6 × 250 mm2. The column was maintained at 30°C during the analyses. The HPLC pump delivered the following mobile phase gradient: The flow rate was constant at 1 ml/min. Solvent A was methanol and solvent B was methylene chloride. For the first 10 min, 100% A was the mobile phase and then from 10.0 to 25.0 min a mixture of 50% A and 50% B was used. The solvent was switched to 100% A for the last 10 min of each cycle.
The mass spectrometer employed an atmospheric pressure chemical ionization source operated in positive ion mode. The quadrupole mass filter was operated in selected ion monitoring mode for ions at m/z 385 (vitamin D3) and m/z 388 (trideuterated vitamin D3 internal standard). The internal standard was provided by IsoSciences (King of Prussia, PA, USA); its purity was 99.6%.
As described above, we utilized isotope dilution, which is a method that internalizes the standard to the samples [16, 17]. We added a known quantity of a tri-deuterated vitamin D3 to each sample, then using the isotope ratio of the resulting solution, the known isotope ratio of the tri-deuterated vitamin D3 (99.6%) and natural abundance isotope ratio of tri-deuterated vitamin D3 in the sample (<0.1%), we calculated the concentration of the vitamin D3 in the sample. Recovery was 72%.
The following equation was used to calculate the vitamin D3 concentrations in adipose tissue:
where A s = LC/MS peak area of the vitamin D3 sample, A is = LC/MS peak area of the deuterated internal standard, C D3 = concentration of vitamin D3 (nmol/kg), C is = Concentration of the deuterated internal standard (nmol/l), W = weight of adipose tissue (kg).
The detection limit of vitamin D3 in fat tissue by this method is 0.081 pmol injected onto the column. The interassay variation of the LC/MS method for five vitamin D3 standards measured on five different days was 154 ± 3.38 nmol/l (CV = 2.1%). The interassay variation of the LC/MS method for eight vitamin D3 standards spiked into a pooled serum sample and measured on eight different days was 13.8 ± 1.3 nmol/l (CV = 9.1%).
Statistical methods
Linear associations between variables were described with Pearson correlation coefficients. Two-tailed P-values less than 0.05 were considered to indicate statistical significance. Analyses were conducted with SPSS version 15.0 (Chicago, IL, USA).
References
E.B. Mawer, J. Backhouse, C.A. Holman, G.A. Lumb, S.W. Stanbury, The distribution and storage of vitamin D and its metabolites in human tissues. Clin. Sci. 43, 413–431 (1972)
D.E. Lawson, J. Douglas, M. Lean, S. Sedrani, Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin. Chim. Acta. 157, 175–181 (1986)
S.J. Rosenstreich, C. Rich, W. Volwiler, Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J. Clin. Invest. 50, 679–687 (1971)
D.A. Brouwer, J. van Beek, H. Ferwerda, et al., Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br. J. Nutr. 79, 527–532 (1998)
Y. Liel, E. Ulmer, J. Shary, B.W. Hollis, N.H. Bell, Low circulating vitamin D in obesity. Calcif. Tiss. Int. 43, 199–201 (1988)
J.E. Compston, S. Vedi, J.E. Ledger, A. Webb, J.C. Gazet, T.R. Pilkington, Vitamin D status and bone histomorphometry in gross obesity. Am. J. Clin. Nutr. 34, 2359–2363 (1981)
N.H. Bell, A. Greene, S. Epstein, M.J. Oexmann, S. Shaw, J. Shary, Evidence for alteration of the vitamin D-endocrine system in blacks. J. Clin. Invest. 76, 470–473 (1985)
S. Arunabh, S. Pollack, J. Yeh, J.F. Aloia, Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 88, 157–161 (2003)
M. Blum, G. Dolnikowski, B. Dawson-Hughes, Body size and serum hydroxyvitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. (2007) (in press)
J. Wortsman, L.Y. Matsuoka, T.C. Chen, Z. Lu, M.F. Holick, Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 72, 690–693 (2000)
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes F. a. N. B. I. o. M, Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. (National Academy Press, Washington, D.C, 1997)
A.F. Kardinaal, P. van‘t Veer, H.A. Brants, H. van den Berg, J. van Schoonhoven, R.J. Hermus, Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am. J. Epidemiol. 141, 440–450 (1995)
L.Y. Matsuoka, J. Wortsman, T.C. Chen, M.F. Holick, Compensation for the interracial variance in the cutaneous synthesis of vitamin D. J. Lab. Clin. Med. 126, 452–457 (1995)
B. Dawson-Hughes R.P. Heaney, M.F. Holick, P. Lips, P.J. Meunier, R. Vieth, Estimates of optimal vitamin D status. Osteoporos. Int. 16, 713–716 (2005)
G. Block, M. Woods, A. Potosky, C. Clifford, Validation of a self-administered diet history questionnaire using multiple diet records. J. Clin. Epidemiol. 43, 1327–1335 (1990)
J.D. Fassett, Elemental isotope dilution analysis with radioactive and stable isotopes (Technical report). Pure Appl. Chem. 67, 1943–1949 (1995)
S. Friso, S.W. Choi, G.G. Dolnikowski, J. Selhub, A method to assess genomic DNA methylation using high-performance liquid chromatography—electrospray ionization mass spectrometry. Anal. Chem. 74, 4526–4531 (2002)
Acknowledgments
This research was supported by NIH grant number K23 AR47869 and by contract No. 58-1950-7-707 with the Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging at Tufts University. This article does not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blum, M., Dolnikowski, G., Seyoum, E. et al. Vitamin D3 in fat tissue. Endocr 33, 90–94 (2008). https://doi.org/10.1007/s12020-008-9051-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-008-9051-4