Abstract
Background and aim
Rheumatoid arthritis is associated with increased morbidity and mortality due to atherosclerotic cardiovascular diseases. Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme of phospholipase A2; it plays an important role in inflammation and atherosclerosis. Herein we aimed to investigate whether Lp-PLA2 activity is associated with atherosclerosis in patients with rheumatoid arthritis and compare the Lp-PLA2 activity with carotid intima media thickness (CIMT).
Patients and methods
25 patients with rheumatoid arthritis and 40 controls (20 patients with type 2 diabetes mellitus [DM] and 20 healthy controls) were included in the study. Frozen serum samples were used for analyzing Lp-PLA2 activity. Disease activity was calculated with DAS28 (Disease Activity Score 28) in the rheumatoid arthritis group. The mean CIMT was calculated in all participants.
Results
Lp-PLA2 activity was significantly higher in the DM group (p = 0.006) and LDL (Low density cholesterol levels) were lower in rheumatoid arthritis and healthy control groups compared with diabetics (p = 0.001 and p = 0.029, respectively). The mean CIMT was significantly higher in patients with type 2 DM (p = 0.047).
Conclusion
Lp-PLA2 activity was not increased in the rheumatoid arthritis group when compared with healthy controls and the DM group. This result may be associated with low disease activity scores in patients with rheumatoid arthritis.
Zusammenfassung
Hintergrund und Ziel
Die rheumatoide Arthritis geht mit erhöhter Morbidität und Mortalität aufgrund arteriosklerotisch bedingter kardiovaskulärer Erkrankungen einher. Die lipopoproteinassoziierte Phospholipase A2 (Lp-PLA2) stellt eines der Enzyme der Gruppe der Phospholipasen A2 dar und spielt eine bedeutende Rolle bei Entzündungsprozessen und Arteriosklerose. Ziel der vorliegenden Arbeit war es, einen möglichen Zusammenhang zwischen der Lp-PLA2-Aktivität mit einer Arteriosklerose bei Patienten mit rheumatoider Arthritis zu ermitteln und die Lp-PLA2-Aktivität mit der Intima-Media-Dicke der A. carotis („carotid intima media thickness“, CIMT) zu vergleichen.
Patienten und Methoden
In die vorliegende Studie wurden 25 Patienten mit rheumatoider Arthritis und 40 Kontrollen – 20 Patienten mit Diabetes mellitus (DM) Typ 2 und 20 gesunde Kontrollen – aufgenommen. Zur Analyse der Lp-PLA2-Aktivität wurden gefrorene Serumproben verwendet. Die Krankheitsaktivität wurde anhand des DAS28 (Disease Activity Score) in der Gruppe mit rheumatoider Arthritis bestimmt. Bei sämtlichen Teilnehmern wurde die durchschnittliche CIMT berechnet.
Ergebnisse
In der Gruppe mit DM war die Lp-PLA2-Aktivität signifikant höher als bei den übrigen Teilnehmern (p = 0,006), und der Wert für das Low-Density-Lipoprotein(LDL)-Cholesterin war bei Patienten mit rheumatoider Arthritis und den gesunden Kontrollen im Vergleich zu den Patienten mit DM niedriger (p = 0,001 bzw. p = 0,029). Die durchschnittliche CIMT war bei Patienten mit DM vom Typ 2 signifikant höher als bei den anderen Teilnehmern (p = 0,047).
Schlussfolgerung
Im Vergleich zu gesunden Kontrollen und der Gruppe mit DM war die Lp-PLA2-Aktivität in der Gruppe mit rheumatoider Arthritis nicht erhöht. Dieses Ergebnis steht möglicherweise mit niedrigeren Werten für die Krankheitsaktivität bei Patienten mit rheumatoider Arthritis in Zusammenhang.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mortality and comorbidity due to cardiovascular disease are increased in patients with rheumatoid arthritis (RA), which is claimed to be associated with accelerated atherosclerosis [1]. Many factors such as age, gender, sedentary lifestyle, smoking, diabetes mellitus, family history, and hyperlipidemia contribute to this process [2]. There are many noninvasive methods for determination of atherosclerosis [3]. Recent studies have shown that oxidized-LDL (Ox-LDL [Low density cholesterol]) has a critical role in development of atherosclerosis and inflammation [4]. Ox-LDL was shown in synovial liquid samples of patients with systemic lupus erythematosus (SLE), RA, and antiphospholipid antibody syndrome [5]. Moreover, ox-LDL levels are determined to be higher in active RA patients than in SLE patients or the normal population [6].
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a calcium independent lipase hydrolyzing phospholipids and is also named as platelet activating factor acetylhydrolase (PAF-AH) [7]. It’s mostly produced by monocytes and macrophages and carried bound with LDL in plasma. The key role that Lp-PLA2 plays in atherogenesis is hydroxylation of ox-LDL in blood circulation. This reaction results in production of oxidized fatty acids (oxFA) and lysophosphatidylcholine (LysPC), which is a proinflammatory and atherogenic cytokine. LysPC indicates cell death, plasma membrane damage, endothelial dysfunction, and apoptosis of smooth muscle cells and macrophages in atherogenesis [8]. There is another form of Lp-PLA2, the HDL(High Density Cholesterol)-bound form, which is accused of being anti-atherogenic. The Lp-PLA2 level can be measured by an immunoassay called the PLAC (Lipoprotein-associated phospholipase A2 activity) test or Lp-PLA2 activity dimension may be applied.
The purpose of this study was to compare carotid intima media thickness with Lp-PLA2 activity in terms of atherosclerosis.
Materials and methods
25 consecutive patients with rheumatoid arthritis who met the American College of Rheumatology and EULAR 2010 criteria for rheumatoid arthritis were investigated, as well as 20 apparently healthy nonsmoking volunteers and 20 patients with type 2 diabetes mellitus as the control groups. The exclusion criteria for the patients with RA were a history of prior coronary artery disease, hypothyroidism, hyperthyroidism, hyperlipidemia, diabetes mellitus (DM), and any malignancy. Disease activity was evaluated with the Disease Activity Score 28 (DAS28), and the Health Assessment Questionnaire (HAQ) score of each patient was calculated. Participants’ demographical data were registered and physical examinations were performed. Samples were centrifuged with a Micron (Cayman Chemical Company, Ann Arbor, MI, USA) centrifugal filter device under a centrifugal force of 14,000 g and temperature controlled at 4 ℃, to reach a cutoff molecular weight of 30,000 Daltons. The C Reactive Protein (CRP) was measured with an enzyme-linked immunosorbent assay (ELISA) based on purified protein and polyclonal anti-C-reactive protein antibodies with units of mg/dL. In addition, hemoglobin A1c (HbA1c) and postprandial glucose levels were measured in all diabetic patients.
Length and weight measurements of all participants were done, and body mass index (BMI) values were calculated. Informed consent forms were obtained from all subjects and the study protocol was approved by the Abant Izzet Baysal University ethics committee.
Measurement of plasma Lp-PLA2 activity
After an overnight fast of at least 12 h, venous blood samples were obtained from all subjects. Serum Lp-PLA2 activity was analyzed by dynamic assay with a platelet-activating factor acetylhydrolase assay kit in units of µmol/min/mL (Cayman Chemical Company, Ann Arbor, MI, USA) [9].
Evaluation of carotid intima media thickness
Measurement of mean CIMT was performed with Doppler ultrasound with Siemens’ Antares model USG implement (Erlangen, Germany), using a probe of 7.5 MHz. Measurement and evaluation were performed by a radiology specialist.
Statistical analysis
Statistical analysis and graphical presentation was performed using SPSS and GraphPad Prism 5.0 (ver. 17.0 for Windows; SPSS Inc., Chicago, IL, USA). Results are given as means with standard deviations or medians with ranges. Multiple group comparisons were performed by Analysis Of Variance (ANOVA) followed by Tukey–Kramer post hoc analysis. Regression coefficients were calculated based on Pearson or Spearman product moment.
Kolmogorov–Smirnov test was performed for the normal distribution of the data. Statistical significance was assumed for p < 0.05.
Results
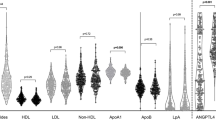
Mean Lp-PLA2 activity was 15.12 ± 6.2 ng/min/mL in the RA group, 20.65 ± 6.2 ng/min/mL in the DM group, and 16.50 ± 4 ng/min/mL in healthy controls (p < 0.001; one patient in the RA group was excluded because of faulty evaluation). Lp-PLA2 activity was significantly higher in the DM group when compared with RA and healthy groups (p =0.002 and p =0.023; respectively, ANOVA, post hoc Tukey test; Fig. 1). Demographical data and biochemical results of the all groups are given in Table 1.
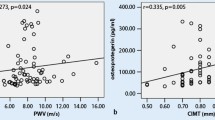
While Lp-PLA2 activity did not show an association with blood glucose levels; HDL-cholesterol, LDL-cholesterol, total cholesterol, BMI, HAQ score, and DAS28 were associated with Lp-PLA2 activity in patients with RA (Fig. 2).
CIMT was significantly higher in patients with DM when compared with healthy controls (p =0.018, ANOVA, Tukey test).
CRP levels were significantly lower in healthy controls and diabetics (p = 0.021 and p = 0.038, respectively). LDL-cholesterol levels were significantly lower in the RA and healthy control group than in diabetics (p = 0.001 and p = 0.029, respectively) and total cholesterol levels were higher in the DM patients’ group (p = 0.002 and p = 0.021, respectively).
Discussion
The increased cardiovascular risk is mainly associated with accelerated atherosclerosis [10]. The proatherogenic effect of Lp-PLA2 has been shown in many prospective studies and cohort analyses. Gong et al. [11] showed that Lp-PLA2 activity levels were higher in patients with metabolic syndrome, in addition to atheroma plaques in the carotid artery, when compared with the healthy control group. Another study showed that Lp-PLA2 activity was higher in the patients with type 1 diabetes mellitus [12]. A study on type 2 diabetics showed increased Lp-PLA2 activity in type 2 diabetes mellitus, whereas plasma Lp-PLA2 levels were normal [13]. Hatoum et al. [14] showed that an increased Lp-PLA2 level was an independent risk factor for atherosclerosis.
Average life expectancy is 5–7 years shorter in RA patients than in the normal population, due to cardiovascular diseases [15]. In the literature it’s possible to find a number of studies on Lp-PLA2 levels in diabetics, whereas Lp-PLA2 levels in RA have not been studied enough, although coincidence of RA and atherosclerosis is not rare [16, 17]. Lourida et al. [17] declared that total and HDL-related Lp-PLA2 activity was decreased in early RA patients and they explained that this result was induced by increased anti-oxLDL antibodies and reduced enzyme production. The same study showed a meaningful increase in anti-oxLDL antibody levels. After one year of (Disease Modifying Anti-Rheumatic Drug) DMARD therapy, a meaningful decrease in antibody levels and, as a result, an increase in HDL-related Lp-PLA2 activity was ascertained. HDL-cholesterol is lower in RA patients. In our study, measurement of total Lp-PLA2 activity was performed. According to our study, there was no significant increase in Lp-LA2 activity between RA patients and the controls. This result may be due to all RA patients being under therapy during the study. Tselepis et al. [18] observed that Lp-PLA2 activity in juvenile RA patients was lower than in the normal population (used HDL-related Lp-PLA2 activity) and this decrease was parallel for HDL2 and HDL3 subtypes and more evident in active disease. It’s a disadvantage that we were not able to test oxLDL, anti-oxLDL levels, or lipoprotein subtypes. A decrease in Lp-PLA2 activity in RA patients was ascertained. On the contrary, a meaningful increase was expected in Lp-PLA2 levels, like in the diabetics group. In our study, Lp-PLA2 activity was lower in the RA group than in diabetics and similar to that of healthy controls group (p =0.005). Comparison of the diabetics group and healthy controls showed higher Lp-PLA2 activity in the DM group, but the results were not statistically meaningful (p: 0.059). This can be explained by lower total cholesterol and LDL-cholesterol levels in RA patients. In addition, LDL-cholesterol levels were higher in the healthy control group than in the RA group. Lower LDL-cholesterol levels in the RA patients might be associated with hydroxychloroquine therapy. 80% of patients with RA used hydroxychloroquine.
The heterogeneity of lipid levels of healthy controls and the small number of participants are weak aspects of our research. The healthy group’s lipid profile heterogeneity may be one of the reasons why no significant difference was found between RA patients’ and healthy controls’ Lp-PLA2 activity. A study on patients with increased disease activity and homogeneous LDL-cholesterol profiles consisting of measurements before and after therapy might provide better results. Furthermore, when compared to previous studies we couldn’t find the expected elevation in Lp-PLA2 activity and CIMT measurements. That can be explained by lower average DAS28, short disease duration, and average age values in RA group.
Conclusion
Lp-PLA2 activity in RA patients was similar to that of the healthy control group but lower than in diabetics. No association was determined between CIMT and Lp-PLA2 activity, which may be associated with the fact that our RA patients had inactive disease and were under therapy. On the other hand, Lp-PLA2 activity was higher in the diabetic group. LpPLA2 activity and CIMT can be useful parameters for determining the tendency for atherosclerosis in RA patients. Measuring these parameters before and after treatment in patients with active disease will give much more valuable information. In the near feature, we think it can be done with a large cohort.
References
del Rincon I, O’Leary DH, Freeman GL, Escalante A (2007) Acceleration of atherosclerosis during the course of rheumatoid arthritis. Atherosclerosis 195(2):354–360
Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE et al (2003) Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 107:1303–1307
Celermajer DS (1998) Noninvasive detection of atherosclerosis. N Engl J Med 339(27):2014–2015
Holvoet P, Collen D (1998) Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis 137(Suppl):S33–S38
McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY et al (2006) Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 54(8):2541–2549
Kim SH, Lee CK, Lee EY, Park SY, Cho YS, Yoo B, Moon HB (2004) Serum oxidized low-density lipoproteins in rheumatoid arthritis. Rheumatol Int 24(4):230–233
Dada N, Kim NW, Wolfert RL (2002) Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn 2(1):17–22
MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA et al (1999) Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J 338(Pt 2):479–487
Aarsman AJ, Neys FW, Van den Bosch N (1991) Catabolism of platelet-activating factor and its acyl analog. Diffrentiation of the actvities of lysophospholipase and platelet-activating factor-acetlyhydrolase. Eur J Biochem 200:197–193
Fuchs HA, Sergent JS (1997) Rheumatoid arthritis: the clinical picture. In: Koopman WJ (ed) Arthritis and allied conditions, 13th edn. Williams and Wilkins, Pennsylvania, pp 1041–1070
Gong HP, Du YM, Zhong LN, Dong ZQ, Wang X, Mao Y, Lu QH (2011) Plasma lipoprotein-associated phospholipase A2 in patients with metabolic syndrome and carotid atherosclerosis. Lipids Health Dis 10:13
Gomes MB, Cobas RA, Nunes E, Castro-Faria-Neto HC, da Matta MF, Neves R, Tibirica E (2009) Plasma PAF-acetylhydrolase activity, inflammatory markers and susceptibility of LDL to in vitro oxidation in patients with type 1 diabetes mellitus. Diabetes Res Clin Pract 85(1):61–68
Nelson TL, Kamineni A, Psaty B, Cushman M, Jenny NS, Hokanson J, Furberg C, Mukamal KJ (2011) Lipoprotein-associated phospholipase A2 and future risk of subclinical disease and cardiovascular events in individuals with type 2 diabetes: the Cardiovascular Health Study. Diabetologia 54(2):329–333
Hatoum IJ, Hu FB, Nelson JJ, Rimm EB (2010) Lipoprotein-associated phospholipase A2 activity and incident coronary heart disease among men and women with type 2 diabetes. Diabetes 59(5):1239–1243
Theodorou DJ, Theodorou SJ, Resnick D (2003) Imaging of rheumatoid arthritis. In: Hochberg MC, Sılman AJ, Smolen JS, Weinblatt ME, Weisman MH (eds) Rheumatology. Mosby, St. Louis, pp 765–780
Lourida ES, Georgiadis AN, Papavasiliou EC, Papathanasiou AI, Drosos AA, Tselepis AD (2007) Patients with early rheumatoid arthritis exhibit elevated autoantibody titers against oxidized low-density lipoprotein and exhibit decreased activity of the lipoprotein-associated phospholipase A2. Arthritis Res Ther 9(1):R19
Hilliquin P, Houbaba H, Aissa J, Benveniste J, Menkes CJ (1995) Correlations between PAF-acether and tumor necrosis factor in rheumatoid arthritis. Influence of parenteral corticosteroids. Scand J Rheumatol 24(3):169–173
Tselepis AD, Elisaf M, Besis S, Karabina SA, Chapman MJ, Siamopoulou A (1999) Association of the inflammatory state in active juvenile rheumatoid arthritis with hypo-high-density lipoproteinemia and reduced lipoprotein-associated platelet-activating factor acetylhydrolase activity. Arthritis Rheum 42(2):373–383
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Bes, S. Gürel, G. Buğdaycı, O. Dikbaş, and M. Soy declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Bes, C., Gürel, S., Buğdaycı, G. et al. Atherosclerosis assessment and rheumatoid arthritis. Z Rheumatol 77, 330–334 (2018). https://doi.org/10.1007/s00393-016-0239-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-016-0239-3