Abstract
Background
Both loop diuretics (LDs) and congestion have been related to worse heart failure (HF) outcome. The relationship between the cause and effect is unknown. The aim of this study was to investigate the interaction between congestion, diuretic use and HF outcome.
Methods
Six hundred and twenty-two chronic HF patients from TIME-CHF were studied. Congestion was measured by means of a clinical congestion index (CCI). Loop diuretic dose was considered at baseline and month 6. Treatment intensification was defined as the increase in LD dose over 6 months or loop diuretic and thiazide or thiazide-like diuretic co-administration. The end-points were survival and HF hospitalisation-free survival.
Results
High-LD dose at baseline and month 6 (≥ 80 mg of furosemide per day) was not identified as an independent predictor of outcome. CCI at baseline remained independently associated with impaired survival [hazard ratio (HR) 1.34, (95% confidence interval) (95% CI) (1.20–1.50), p < 0.001] and HF hospitalisation-free survival [HR 1.09, 95% CI (1.02–1.17), p = 0.015]. CCI at month 6 was independently associated with HF hospitalisation-free survival [HR 1.24, 95% CI (1.11–1.38), p < 0.001]. Treatment intensification was independently associated with survival [HR 1.75, 95% CI (1.19–1.38), p = 0.004] and HF hospitalisation-free survival [HR 1.69, 95% CI (1.22–2.35), p = 0.002]. Patients undergoing treatment intensification resulting in decongestion had better outcome than patients with persistent (worsening) congestion despite LD dose up-titration (p < 0.001).
Conclusion
Intensification of pharmacological decongestion but not the actual LD dose was related to poor outcome in chronic HF. If treatment intensification translated into clinical decongestion, outcome was better than in case of persistent or worsening congestion.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although never properly tested to improve prognosis of chronic heart failure (CHF) [1], loop diuretics (LDs) are an important part of the complex treatment for the vast majority of CHF patients [2]. On the one hand, such an approach seems to be justified, given the high prevalence of fluid accumulation in CHF patients and the well-established relationship between congestion, symptoms, quality of life and unfavourable prognosis [3,4,5,6,7,8]. In addition, invasively monitored pressure-triggered up-titration of medication in patients with advanced heart failure (HF) has been shown to improve outcome, which was primarily based on up-titration of LD therapy [9]. On the other hand, the safety of LD is being questioned, since the use of high doses of LD has been related to worsening renal function (WRF) and worse outcome in a large number of observational trials [1]. Still, data about congestion status were not available for comprehensive adjustment of adequate diuretic therapy in most databases previously used to analyse the safety profile of LD. Hence, the assumption that high doses of LD are harmful may be biased, as patients with advanced CHF are more likely to be congested and to have worse renal function [10]. As a consequence, they receive more often and higher doses of LD, sometimes co-administered with thiazide or thiazide-like diuretics [11,12,13,14,15]. Thus, high-dose LD therapy may be a surrogate for advanced disease and thereby a marker of poor outcome despite attempts to adjust for confounders in previous studies. In the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF), extensive phenotyping and detailed information on medication are available from multiple time points [16], which makes this trial ideally suited to study the prognostic impact of decongestion. Therefore, this post hoc analysis was designed to investigate the interaction between diuretic use, congestion and outcome in CHF.
Methods
Data source and study population
This is a post hoc analysis of TIME-CHF. The design [17] and main results [16] of the trial have been previously reported. Briefly, TIME-CHF was a randomised, controlled multicentre trial conducted in Switzerland and Germany that compared an NT-proBNP-guided vs a symptom-guided management in patients with CHF (n = 622), age ≥ 60 years, symptoms corresponding to New York Heart Association (NYHA) functional class ≥ II, HF hospitalisation within 12 months prior to inclusion, and an age-adjusted elevated NT-proBNP level (> 400 ng/L in those < 75 years, > 800 ng/L in those ≥ 75 years). Patients with both reduced (HFrEF) (n = 499) and preserved (n = 123) left ventricular ejection fraction were included between January 2003 and December 2006 and followed up clinically for 18 months. The investigation conforms with the principles outlined in the Declaration of Helsinki, was approved by the local ethics committees, and all participants provided their written informed consent.
Patients were clinically evaluated at baseline and after 1, 3, 6, 12 and 18 months. At each visit, history was taken and patients underwent a detailed clinical examination to determine the presence and extent of congestion by means of a clinical congestion index (CCI) as previously described [3, 18]. Briefly, CCI is a composite clinical marker of congestion taking into account the presence of hepatomegaly, NYHA ≥ III, peripheral oedema, jugular venous distension, orthopnoea, rales and paroxysmal nocturnal dyspnoea. The CCI value for each patient can vary from 0 (no congestion) to 7 (severe congestion) [3].
Information on all drugs including doses and changes between the visits was collected. LD dose is expressed as furosemide equivalent, where 10-mg torasemide and 1-mg bumetanide, respectively, are converted to 40-mg furosemide. Difference in LD dose between baseline and month 6 was used for investigation of changes in LD doses over time. The only thiazide and thiazide-like drugs used in TIME-CHF were hydrochlorothiazide and metolazone. Patients taking any of the two were considered thiazide users. Given the fact that in clinical practice thiazides are usually co-administered with LD for short courses, the use of thiazides of any duration was considered as treatment intensification during a certain time frame. Intensification of pharmacological decongestion was described as an increase in LD dose during the first 6 months of follow-up or a co-administration of a thiazide or thiazide-like drug with a LD. WRF was defined as an increase in serum creatinine by ≥ 44.2 μmol/L (0.5 mg/dL) over 6 months [19].
Outcome events
Death except cancer-related was the primary outcome event for this study, with death or HF hospitalisation as a secondary outcome. Although the study duration was 18 months, the subjects underwent a systematic long-term follow-up up to 5½ years, based on medical records or phone calls to patients and/or their general practitioners every 6 months.
Haemodialysis or haemofiltration was not used for the purpose of mechanical fluid extraction in TIME-CHF. Overall, three patients received temporary haemodialysis and one haemofiltration due to worsening renal failure. They were included as WRF in the analysis.
Statistical analysis
Descriptive statistics are expressed as median [interquartile range] for continuous variables, as the distribution of all continues variables was not normal (Kolmogorov–Smirnov test), and as numbers (percentages) for categorical variables. The groups were compared using Mann–Whitney U tests for continuous variables and Chi-squared test for categorical variables. To test the association between LD use (low vs high dose) and intensification of pharmacological decongestion and outcome, Cox regression was performed. Independence of these associations was tested using multivariable Cox regression analysis. When testing the prognostic significance of intensification of pharmacological decongestion, only the events taking place after the month 6 follow-up visit were considered. A stepwise forward model was used (inclusion p ≤ 0.05, exclusion p > 0.1). The Kaplan–Meier method was used to construct the survival curves, with the log-rank test used for comparison among groups. All analyses using both baseline and month 6 values included only patients who survived and remained in the study. For all the other analyses, all patients were considered. A two-sided p value of 0.05 or less was considered to be statistically significant. Statistical analysis was performed using the IBM® SPSS® for Windows® software (version 23.0, SPSS® Inc, Chicago, IL).
Results
Baseline characteristics
Baseline characteristics are presented in Table 1. The patients were elderly and severely symptomatic—three out of four were in NYHA ≥ III. The majority [n = 499 (80%)] had HFrEF with HF due to ischaemic heart disease being most prevalent. Most had significant comorbidities and poor disease-related quality of life. A high percentage of patients received evidence-based HF medications already at baseline.
The prevalence of congestion
The prevalence of congestion in TIME-CHF population was extensively analysed previously [3, 18]. Briefly, congestion was highly prevalent at baseline and decreased continuously during the first 6 months (CCI ≥ 3 in 53% vs. 19% of patients at baseline and month 6, respectively, of those who survived and remained in the study after 6 months).
The use of diuretics
The use of LD was high during the entire follow-up with 575 of 622 (92%), 509 of 567 (90%), 469 of 521 (90%), 440 of 489 (90%), 391 of 446 (88%), 358 of 406 (88%) patients using LD at baseline, month 1, 3, 6, 12, and 18, respectively. Median daily LD dose was 80 [40–100] at baseline, 40 [40–80] at month 1, 40 [25–80] at month 3, 40 [40–80] at month 6, 40 [20–80] at month 12, and 40 [20–80] mg of furosemide equivalent at month 18.
The use of thiazides was relatively low. Twenty-five (4%) patients used thiazides at baseline and 86 (14%) used them at any time point during the follow-up. Sixty (10%) patients received thiazides during the first 6 months, whereas 56 (12%) were on thiazides after month 6. There were only 21 (4%) 6-month survivors not on thiazides during the first 6 months, who received such treatment later during follow-up.
Prognostic relevance of diuretic therapy
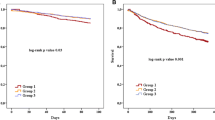
During the median total follow-up of 27 [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] months, 241 (39%) patients died; 317 (51%) either died or were hospitalised for HF. Patients using high-LD dose (≥ 80 mg of furosemide per day) at baseline (Fig. 1a, b), as well as a thiazide diuretic at baseline (Fig. 1c, d) and at any time point during follow-up (Fig. 1e, f) were at increased risk of dying or being hospitalised for worsening heart failure.
Kaplan–Meier curves of survival and heart failure hospitalisation-free survival comparing high loop diuretic dose (≥ 80 mg per day) users with low loop diuretic dose (< 80 mg per day) users at baseline (a, b), thiazide diuretic users and non-users at baseline (c, d), and thiazide diuretic users and non-users at any time point during follow-up (e, f). HF heart failure, TZD thiazide or thiazide-like diuretic
Univariable and multivariable predictors of death, and death or HF hospitalisation are shown in Table 2. High-LD dose at baseline (≥ 80 mg of furosemide per day) and the use of thiazides at baseline were not identified as independent predictors of outcome, whereas congestion remained strongly and independently associated with impaired survival and HF hospitalisation-free survival.
As congestion decreased significantly among patients surviving 6 months [3], we analysed the prognostic significance of LD use at month 6 and intensification of congestion treatment during the first 6 months of follow-up. Median daily LD dose (mg of furosemide equivalent) administered to patients surviving 6 months was as follows: 60 [40–80] at baseline, 40 [20–80] at month 1, 40 [20–80] at month 3, 40 [40–80] at month 6, 40 [20–80] at month 12, and 40 [20–80] at month 18. Of note, the difference between median daily dose of furosemide at baseline and month 6 was not statistically significant (p > 0.05).
A total of 489 patients, i.e. 79% of TIME-CHF participants survived the first 6 months and did not drop out. Of those, 316 (65%) required LD dose down-titration or remained on a stable LD dose and received no thiazides together with a LD (no treatment intensification group); 173 (35%) required LD dose up-titration or a co-administration of a thiazide drug (treatment intensification group). The comparison of patients receiving no intensification with patients undergoing intensification of congestion treatment is shown in Table 3. Patients in the intensification group were sicker: they had a higher comorbidity burden, their functional capacity was more impaired, their plasma NT-proBNP level at month 6 was higher, their haemoglobin was lower, their renal function was worse, and they were more likely to experience a WRF and to be congested.
The median follow-up of patients with survival ≥ 6 months was 30 [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] months. Univariable and multivariable predictors of death, and death or HF hospitalisation are shown in Table 4. LD dose administered at month 6 was not identified as an independent predictor of outcome, whilst treatment intensification remained an independent predictor of outcome. Although congestion at month 6 did not appear as an independent predictor of mortality, it remained a strong and independent predictor of HF hospitalisation-free survival after month 6.
Loop diuretic dose adjustment, congestion and outcome
A total of 256 (55%) patients had no or only mild congestion (CCI < 3) at month 6 without treatment intensification; 118 (25%) patients had no or only mild congestion (CCI < 3) at month 6 following treatment intensification within the first 6 months; 46 (10%) patients were congested (CCI ≥ 3) at month 6, but had not received treatment intensification; and 44 (10%) patients were congested (CCI ≥ 3) at month 6 despite treatment intensification. The best prognosis was noted if congestion had been manageable without treatment intensification (Fig. 2). If treatment intensification had been required to decongest, the prognosis was worse than without intensification. The worst prognosis was noted if obvious clinical congestion was present (CCI ≥ 3) despite treatment intensification. This was especially true for HF hospitalisation-free survival (Fig. 2b).
Changes in CCI were observed in 442 patients (90% of 6-month survivors). The decrease in CCI by at least 1 point without treatment intensification was noted in the majority of patients (265 (60%)). These patients had the best outcome (Fig. 2c, d). One hundred and twenty-three (28%) patients underwent treatment intensification, resulting in decreasing CCI, whereas 26 (6%) patients experienced progressive congestion (an increase in CCI by at least 1 point) without treatment intensification. The latter two groups had comparable outcome (Fig. 2c, d). Progressive congestion despite treatment intensification was noted in 28 (6%) patients. This subgroup demonstrated a very poor outcome (Fig. 2c, d).
Discussion
This study adds significantly to our understanding of the complex interaction between diuretic use, congestion and CHF outcome. (1) Intensification of pharmacological decongestion, but not the actual LD dose, was related to worse outcome in CHF. (2) The need of intensification of pharmacological decongestion is a marker of more advanced disease with worse clinical, biochemical and functional properties. (3) CHF outcome in patients with no or reduced congestion undergoing treatment intensification is at least as good as in patients with no treatment intensification but persistent congestion. These findings suggest that advanced CHF and congestion are the main drivers of poor outcome and not treatment with LDs or thiazides per se.
The role of loop diuretics in heart failure care
Cardiac dysfunction-mediated renin–angiotensin–aldosterone system (RAAS) activation with consecutive sodium and water retention is a key component of CHF pathophysiology [10, 20] and determines an unfavourable outcome [3, 9, 21, 22]. To date, no other decongestive means have been shown to be superior to LD both in acute and chronic HF care [1, 2, 23, 24]. The DOSE trial showed that LD are capable of reducing signs and symptoms of fluid accumulation in the setting of acute decompensation, [25]. However, their use, both acutely and long-term, has never been demonstrated to improve outcome in a well-designed prospective trial [1]. Despite that, this class of drugs is as often prescribed to CHF patients as evidence-based neurohormonal blockers [11]. Rohde et al. has recently demonstrated in a small though randomised, double-blinded clinical trial that LD withdrawal may be possible in stable CHF patients [26], but the trial was underpowered to assess hard outcome. Therefore, the clinical impact of such intervention needs to be further investigated. Still in daily clinical practice, decongestive interventions are rarely modified [27].
A post hoc analysis of the DIG trial showed that LD users with CHF are more likely to be rehospitalised or to die than patients not taking these drugs [28]. This association between LD use and death persisted after propensity matching. Similar results were obtained from the JCARE-CARD database by Hamaguchi et al., who found an independent association between LD use after discharge and long-term adverse events [29]. Besides, high-dose LD treatment has been demonstrated to limit the up-titration of ACE inhibitors [30]—the first-line drugs in HFrEF [2]. Other investigators analysed different HF populations and found similar relationship between LD use and mortality in a dose-dependent manner [12, 13, 31, 32].
However, these studies share similar limitations. First, the authors did not include LD dose changes over time, but analysed different doses at a certain time point only. Second, congestion status was not generally available for adjustment. Therefore, the most important parameter for the use of LD therapy and multivariable adjustment was often missing. In addition, co-administration of thiazides to boost natriuresis was often not taken into consideration. The TIME-CHF database provides a unique opportunity to analyse the prognostic significance of LD use and CHF outcome taking into account these important factors. Contrary to previous findings, our data show that the actual dose of LD is not an independent predictor of adverse outcome. Instead, intensification of pharmacological decongestion—most likely a marker of clinical deterioration and persistent congestion—but not the LD dose itself independently predicted outcome. In fact, patients requiring treatment intensification were still more congested than patients without this need during the first 6 months of follow-up. In addition, they were already at a more advanced state of HF prior to adaptation of diuretic therapy. In addition, if congestion was controlled with treatment intensification, the prognosis was better than in patients with obvious persistence of clinical congestion. Dini et al. have previously demonstrated that congested patients could potentially benefit from high-LD doses [33]. This is in line with the CHAMPION trial where adjustments based on filling pressure mainly concerned diuretic therapy which resulted in better outcome [9]. These findings together with the results of this study highlight the importance of effective decongestion in CHF. It may be hypothesised that higher doses of LD or higher rate of thiazides’ co-administration would have been effective to control congestion among patients with persistent congestion at month 6 and such interventions could have potentially translated into better outcome. The fact that many patients remained congested despite treatment intensification supports this hypothesis. Still, it needs to be tested in a prospective interventional trial to see if more aggressive decongestive therapy results in better outcome than the current, often cautious approach.
The importance of persistent congestion
Resistance to adequate LD doses is common [34]. Still, there is no general agreement regarding a universal definition of diuretic resistance, which may include the lack of diuretic response to an absolute high daily LD dose, or urinary output, weight loss, or urinary sodium excretion, as a response to a certain LD dose [35, 36]. Regardless of the definition, patients with impaired LD response are known to be at increased risk of adverse events [1, 34, 35, 37]. Still, the use of diuretic therapy differs significantly between medical centres [11] and the reluctance to increase diuretic therapy due to potential negative effects on the kidneys is common [3]. These facts indicate that interpretation of diuretic need in HF patients vary significantly and highlight the need for uniform recommendations on the use of decongestive therapies.
From a pharmacological point of view, increasing LD dose during chronic administration may be inevitable. If sodium reabsorption is inhibited in the distal loop of Henle, more sodium reaches the distal convoluted tubule, resulting in hypertrophy of the distal tubular cells [37]. These histological alterations lead to increased sodium reabsorption capacity of the distal part of the nephron [38]. Rao et al. have elegantly demonstrated that only approximately 35% of the LD-induced sodium delivered from the loop of Henle into the distal tubule ultimately ended up in the urine in HF patients, which is much less than in normal subjects [39]. Thus, distal nephron adaptation might be a reason for persistent congestion despite LD dose escalation. In this study, some patients were still congested at month 6, meaning that dose up-titration was not always sufficient. Co-administration of thiazides may be chosen to overcome distal tubular hypertrophy-mediated diuretic resistance. In TIME-CHF thiazide users were at a twofold increased risk of dying, but only 14% of patients were considered suitable for such therapy during the entire follow-up. Such adjustment was considered as treatment intensification and appeared as an independent predictor of outcome. Until interventional trials of thiazide and LD co-administration are conducted, the selection of potential candidates for such therapy relies on physician’s discretion.
Does the renal function matter?
Significant WRF was present in 16% of patients over 6-months [19, 40]. The decline in renal function identifies patients at high risk of rehospitalisation and mortality [10, 14, 19, 40]. It has been shown that renal function may be an even stronger predictor of mortality than cardiac function in CHF patients [41]. On the one hand, WRF can be caused by the failing heart, because of venous and intraabdominal hypertension, and arterial blood pressure drop [20, 42], called Type 2 cardiorenal syndrome [42]. On the other hand, treatment with LD can potentially lead to intravascular volume depletion and renal hypoperfusion, even in the presence of persistent interstitial fluid retention [1]. As WRF interacts with treatment, renal function and CHF prognosis, physicians are forced to modify treatment strategies. Still, there is no evidence-based consensus on how to react to WRF in CHF and how to adjust LD therapy. In the presence of acute kidney injury during acute decompensation, it was reported that LD dose down-titration or discontinuation was the most common treatment adjustment [43]. However, it is not clear whether this was justified, and in clinical practice it often remains uncertain, if renal function decline is related to hypo- or hypervolemia. It has been shown that WRF if accompanied with successful haemoconcentration may even predict a better outcome [5], meanwhile the occurrence of WRF in patients with persistent congestion indicate a worse prognosis [44] in the setting of acute heart failure. The present study shows that remaining congestion is a clinically important problem in CHF patients, i.e. even in the chronic setting with the aim of target up-titration of medication and regular clinical controls. We previously reported that the need of chronically high doses of LD during the first months was related to poor outcome in TIME-CHF only if WRF was present [40]. Together with the findings of the present analysis, it may be hypothesised that reluctance to sufficiently decongest patients, e.g. in case of WRF, may result in poor outcome. However, the precise interaction between these three factors has not yet been prospectively studied, and our study has not the statistical power for full adjustment of all these factors including interactions. These complex interactions may be further complicated by the influence of non-haemodynamic factors on renal filtration, such as activation of RAAS, sympathetic nervous system, inflammation and endothelial dysfunction [45]. In addition, clinical congestion has been recognised as a late manifestation of fluid retention in HF [4], meaning that a certain proportion of CHF patients may have no signs/symptoms of congestion, despite significant haemodynamic congestion [46].
Limitations
We acknowledge a number of potential limitations of our study. The results of the present analysis are based on an elderly CHF population, thus potentially limiting their generalizability. In addition, the estimation of LD dose change took into account only the doses administered at baseline and month 6, excluding the effect of possible fluctuations and day-by-day variations. Still, considering daily medication changes as done in a previous analysis or intermediate changes between the different visits did not change the main findings of the study (data not shown) [40]. Since there is no known formula to calculate the diuretic effects of thiazides in combination with LD we did not consider the dose, although thiazide use-related potential harms might be dose dependent. In addition, information on intracardiac filling pressures was not available; therefore, we had to rely on clinical congestion, which does not always reflect intravascular (haemodynamic) volume overload. However, we have previously shown that clinical congestion is a highly prevalent and powerful marker of outcome, potentially serving as a target for treatment with LD [3]. TIME-CHF participants were recruited from 2003 till 2008, i.e. before the introduction of angiotensin receptor–neprilysin inhibitor (ARNi) into CHF care. A recent secondary analysis of the PARADIGM-HF trial showed that treatment with ARNi can potentially reduce the need of LD [15]; still, the reduction in LD use 6 months after randomisation was only 2% [15]. In addition, patients were not treated with sodium/glucose cotransporter 2 (sGLT-2) inhibitors, which very recently have been shown to improve outcome in HF patients with reduced ejection fraction [47, 48]. The use of sGLT-2 inhibitors may change the role of diuretics in HF significantly, as they have a significant diuretic effect [49]. In addition, TIME-CHF participants received close monitoring and strict follow-up regimen with effective escalation of evidence-based medications, making the intervention different from those usually seen in the real-world population. Besides, there were no data about the potential increase in tubular damage markers of patients with WRF. Finally, the size of the study was not sufficient for full adjustment of all relevant factors including interactions.
In summary, treatment intensification but not the actual dose of LD was related to poor outcome in CHF. If treatment intensification translated into clinical decongestion, outcome was better than in case of persistent or progressing congestion. These findings suggest that HF and congestion are the main drivers of poor outcome and not the LD dose per se. There is an urgent need for prospective testing whether liberal use of LD or thiazide co-administration to completely decongest HF patients improves outcome.
Data availability
Raw data are available upon request.
References
Simonavičius J, Knackstedt C, Brunner-La Rocca H-P (2019) Loop diuretics in chronic heart failure: how to manage congestion? Heart Fail Rev 24:17–30. https://doi.org/10.1007/s10741-018-9735-7
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureThe Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Simonavičius J, Sanders van-Wijk S, Rickenbacher P et al (2019) Prognostic significance of longitudinal clinical congestion pattern in chronic heart failure: insights from TIME-CHF trial. Am J Med 132:e679–e692. https://doi.org/10.1016/j.amjmed.2019.04.010
Zile MR, Bennett TD, St John Sutton M et al (2008) Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 118:1433–1441. https://doi.org/10.1161/CIRCULATIONAHA.108.783910
Testani JM, Chen J, McCauley BD et al (2010) Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122:265–272. https://doi.org/10.1161/CIRCULATIONAHA.109.933275
Palazzuoli A, Ruocco G, Franci B et al (2020) Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01642-9
Kobayashi M, Girerd N, Duarte K et al (2020) Prognostic impact of plasma volume estimated from hemoglobin and hematocrit in heart failure with preserved ejection fraction. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01639-4
Kobayashi M, Rossignol P, Ferreira JP et al (2019) Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol 108:549–561. https://doi.org/10.1007/s00392-018-1385-1
Abraham WT, Adamson PB, Bourge RC et al (2011) Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377:658–666. https://doi.org/10.1016/S0140-6736(11)60101-3
Mullens W, Damman K, Testani JM et al (2020) Evaluation of kidney function throughout the heart failure trajectory: a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail 22:584–603. https://doi.org/10.1002/ejhf.1697
Brunner-La Rocca H-P, Linssen GC, Smeele FJ et al (2019) Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK-HF registry. JACC Heart Fail 7:13–21. https://doi.org/10.1016/j.jchf.2018.10.010
Eshaghian S, Horwich TB, Fonarow GC (2006) Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 97:1759–1764. https://doi.org/10.1016/j.amjcard.2005.12.072
Damman K, Kjekshus J, Wikstrand J et al (2016) Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 18:328–336. https://doi.org/10.1002/ejhf.462
Lawson CA, Testani JM, Mamas M et al (2018) Chronic kidney disease, worsening renal function and outcomes in a heart failure community setting: a UK national study. Int J Cardiol 267:120–127. https://doi.org/10.1016/j.ijcard.2018.04.090
Vardeny O, Claggett B, Kachadourian J et al (2019) Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail 21:337–341. https://doi.org/10.1002/ejhf.1402
Pfisterer M, Buser P, Rickli H et al (2009) BNP-guided vs symptom-guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA 301:383–392. https://doi.org/10.1001/jama.2009.2
Brunner-La Rocca HP, Buser PT, Schindler R et al (2006) Management of elderly patients with congestive heart failure–design of the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF). Am Heart J 151:949–955. https://doi.org/10.1016/j.ahj.2005.10.022
Simonavicius J, Puronaite R, Brunner-La Rocca H-P (2020) The reply. Am J Med 133:e330–e332. https://doi.org/10.1016/j.amjmed.2019.12.011
Maeder MT, Rickli H, Pfisterer ME et al (2012) Incidence, clinical predictors, and prognostic impact of worsening renal function in elderly patients with chronic heart failure on intensive medical therapy. Am Heart J 163(407–414):414.e1. https://doi.org/10.1016/j.ahj.2011.12.003
Mullens W, Verbrugge FH, Nijst P, Tang WHW (2017) Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 38:1872–1882. https://doi.org/10.1093/eurheartj/ehx035
Anuradha L, McNulty SE, Mentz RJ et al (2015) Relief and recurrence of congestion during and after hospitalization for acute heart failure. Circ Heart Failure 8:741–748. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001957
Selvaraj S, Claggett B, Pozzi A et al (2019) prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM-HF. Circulation 140:1369–1379. https://doi.org/10.1161/CIRCULATIONAHA.119.039920
Yancy CW, Jessup M, Bozkurt B et al (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 128:e240–e327. https://doi.org/10.1161/CIR.0b013e31829e8776
Ellison DH, Felker GM (2017) Diuretic treatment in heart failure. N Engl J Med 377:1964–1975. https://doi.org/10.1056/NEJMra1703100
Felker GM, Lee KL, Bull DA et al (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364:797–805. https://doi.org/10.1056/NEJMoa1005419
Rohde LE, Rover MM, Figueiredo Neto JA et al (2019) Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J 40:3605–3612. https://doi.org/10.1093/eurheartj/ehz554
Simonavicius J, Brunner-La Rocca H-P (2020) Do chronic heart failure patients receive optimal decongestive interventions in a real-life setting? Eur J Heart Fail. https://doi.org/10.1002/ejhf.1839
Ahmed A, Husain A, Love TE et al (2006) Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 27:1431–1439. https://doi.org/10.1093/eurheartj/ehi890
Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M et al (2012) Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure: a report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). Circ J 76:1920–1927
Jm TM, M P, D K et al (2020) Higher doses of loop diuretics limit uptitration of angiotensin-converting enzyme inhibitors in patients with heart failure and reduced ejection fraction. Clin Res Cardiol 109:1048–1059. https://doi.org/10.1007/s00392-020-01598-w
Neuberg GW, Miller AB, O’Connor CM et al (2002) Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J 144:31–38
Abdel-Qadir HM, Tu JV, Yun L et al (2010) Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J 160:264-271.e1. https://doi.org/10.1016/j.ahj.2010.05.032
Dini FL, Guglin M, Simioniuc A et al (2012) Association of furosemide dose with clinical status, left ventricular dysfunction, natriuretic peptides, and outcome in clinically stable patients with chronic systolic heart failure. Congest Heart Fail 18:98–106. https://doi.org/10.1111/j.1751-7133.2011.00252.x
Trullàs J-C, Casado J, Morales-Rull J-L et al (2019) Prevalence and outcome of diuretic resistance in heart failure. Intern Emerg Med 14:529–537. https://doi.org/10.1007/s11739-018-02019-7
Jardim SI, Ramos Dos Santos L, Araújo I et al (2018) A 2018 overview of diuretic resistance in heart failure. Rev Port Cardiol 37:935–945. https://doi.org/10.1016/j.repc.2018.03.014
Galluzzo A, Frea S, Boretto P et al (2020) Spot urinary sodium in acute decompensation of advanced heart failure and dilutional hyponatremia: insights from DRAIN trial. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01617-w
Shah N, Madanieh R, Alkan M et al (2017) A perspective on diuretic resistance in chronic congestive heart failure. Ther Adv Cardiovasc Dis 11:271–278. https://doi.org/10.1177/1753944717718717
Kaissling B, Bachmann S, Kriz W (1985) Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol 248:F374-381. https://doi.org/10.1152/ajprenal.1985.248.3.F374
Rao VS, Planavsky N, Hanberg JS et al (2017) Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol 28:3414–3424. https://doi.org/10.1681/ASN.2016111178
Brunner-La Rocca H-P, Knackstedt C, Eurlings L et al (2015) Impact of worsening renal function related to medication in heart failure. Eur J Heart Fail 17:159–168. https://doi.org/10.1002/ejhf.210
Hillege Hans L, Girbes Armand RJ, de Kam Pieter et al (2000) Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102:203–210. https://doi.org/10.1161/01.CIR.102.2.203
Rangaswami J, Bhalla V, Blair JEA et al (2019) Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 139:e840–e878. https://doi.org/10.1161/CIR.0000000000000664
Boulos J, Darawsha W, Abassi ZA et al (2019) Treatment patterns of patients with acute heart failure who develop acute kidney injury. ESC Heart Fail 6:45–52. https://doi.org/10.1002/ehf2.12364
Metra M, Davison B, Bettari L et al (2012) Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 5:54–62. https://doi.org/10.1161/CIRCHEARTFAILURE.111.963413
Damman K, Testani JM (2015) The kidney in heart failure: an update. Eur Heart J 36:1437–1444. https://doi.org/10.1093/eurheartj/ehv010
Stevenson LW, Perloff JK (1989) The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261:884–888
McMurray JJV, Solomon SD, Inzucchi SE et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med. https://doi.org/10.1056/NEJMoa1911303 (0:null)
Packer M, Anker SD, Butler J et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. New Engl J Med. https://doi.org/10.1056/NEJMoa2022190 (0:null)
Mordi NA, Mordi IR, Singh JS et al (2020) Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with Type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.048739
Acknowledgements
The authors would like to thank the participants and investigators of the TIME-CHF.
Funding
This substudy has no specific funding. The TIME-CHF study was sponsored by the Helmut Horten Foundation (Lugano, Switzerland), and by smaller unrestricted grants from Roche Diagnostics, AstraZeneca, Novartis, Menarini, Pfizer, Servier, Roche Pharma, and Merck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest/competing interests to declare.
Ethics approval
The investigation was approved by the local ethics committees.
Consent to participate
All the participants provided their written informed consent.
Consent for publication
The consent for publication was a part of the written informed consent provided by each participant.
Rights and permissions
About this article
Cite this article
Simonavičius, J., Maeder, M.T., Eurlings, C.G.M.J. et al. Intensification of pharmacological decongestion but not the actual daily loop diuretic dose predicts worse chronic heart failure outcome: insights from TIME-CHF. Clin Res Cardiol 110, 1221–1233 (2021). https://doi.org/10.1007/s00392-020-01779-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-020-01779-7