Abstract
Diuretic resistance (DR) is common in patients with decompensated heart failure (HF), and is associated with adverse outcomes. To determine the prevalence of DR and its impact on survival among patients with decompensated HF, we prospectively evaluated the prevalence and influence on prognosis of DR (defined as persistent congestion despite ≥ 80 mg of furosemide per day) in a cohort of elderly patients from the Spanish HF registry (RICA) admitted for an acute decompensation of HF. Patients with new-onset HF were excluded. From the global cohort of 2067 patients, 435 (21%; 95% CI 19.3%–22.7%) patients met criteria for DR. Patients with DR had more comorbidities (hypercholesterolemia, diabetes mellitus, valvular disease, chronic kidney disease, and cancer) and a worse functional status compared to patients without DR. In addition, patients with DR had a higher proportion of ischemic etiology, more advanced functional class and lower left ventricular ejection fraction values. After 1 year of follow-up, all-cause mortality was higher in patients with DR with an adjusted hazard ratio of 1.37 (95% CI 1.06–1.79; p = 0.018). The prevalence of DR in a cohort of elderly patients admitted for acute HF decompensation is 21%. DR is an independent predictor of 1-year mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute heart failure (AHF) is one of the leading causes of hospital admission worldwide, and loop diuretics are administered in up to 90% of patients hospitalized for AHF. Loop diuretics provide symptomatic benefit improving congestive symptoms, but do not improve long-term morbidity and mortality [1]. Poor response to diuretic therapy (that is, persistent signs and symptoms despite increasing doses of diuretic drug, known as diuretic resistance [DR]) occurs frequently in patients during hospitalization for AHF, although the exact rate is unknown owing to the lack of a standard definition [2]. A poorer diuretic response has been associated with advanced heart failure (HF) and renal impairment and predicts mortality and HF rehospitalization [3].
A single accepted definition of DR is not available. Of the several definitions proposed, the most commonly used is “failure to decongest despite adequate and escalating doses of diuretics” [2]. Other definitions of DR have also been suggested: amount of sodium excreted as a percentage of filtered load < 0.2% [4]; failure to excrete at least 90 mmol of sodium within 72 h of a 160-mg oral furosemide dose [5]; and persistent congestion despite adequate doses of diuretic (> 80 mg furosemide per day) [6]. Not all these definitions include variables easy to determine in routine clinical practice.
The purpose of this study was to determine the prevalence of DR and to analyse its impact on survival among a real-world prospective cohort of elderly patients with chronic decompensated HF.
Methods
Study population (the RICA registry)
Patient data were collected from the Spanish National Registry on HF (Registro Nacional de Insuficiencia Cardiaca—RICA) supported by the Spanish Heart Failure Working Group of the Spanish Society of Internal Medicine. The RICA is a prospective, multicenter, cohort study including patients consecutively admitted to Spanish internal medicine departments for AHF. The registry began in 2008, and its organization and design have been extensively described elsewhere [7]. The RICA registry includes patients over the age of 50 years admitted for AHF according to the criteria of the European Society of Cardiology [8]. All discharged patients are registered, while those who die during the index admission are excluded. Patients are followed for 12 months after the index episode to determine mortality and re-admission rates. The registry complies with the Declaration of Helsinki. The local ethics committee from the University Hospital “Reina Sofia”, Córdoba, Spain approved the study protocols, and informed consent was obtained from all participating subjects.
Inclusion criteria
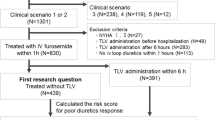
For this study, we included all patients enrolled in the RICA registry with an acute decompensation of previously diagnosed chronic HF (chronic decompensated HF). Patients with a first decompensation of AHF (new-onset HF) or with incomplete baseline information were excluded.
Study variables
The variables selected for this study were the following: past medical history related to HF, comorbidities (Charlson Comorbidity Index) [9], baseline functional status (using the Barthel Index scale for basic activities of daily living) [10], clinical parameters on admission (blood pressure, heart rate, weight and height), HF characteristics, and drug prescription before admission and at discharge. HF was characterized in more detail according to New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF) determined by 2-D echocardiography, and electrocardiogram. Blood chemistry values included kidney function, lipid and glucose profile, uric acid, and troponin and natriuretic peptides (when available). Glomerular filtration was estimated with the MDRD equation, and chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (eGRF) lower than 60 ml/min/1.73 m2.
Diuretic resistance
For the purposes of this study, we define DR as persistent congestion requiring hospitalization despite adequate doses of loop diuretic (≥ 80 mg furosemide per day). The assessment of congestion is not defined in the RICA protocol, is performed at investigator discretion, and according to their usual clinical practice (including clinical assessment, chest X-ray and/or lung ultrasound). We used this definition because it includes variables routinely collected in the RICA registry, and it is easily applicable in clinical practice.
In addition, a similar approach has been used in other studies. The DOSE trial enrolled patients with history of chronic HF with acute decompensation and a receipt of an oral loop diuretic before hospitalization at a dose between 80 and 240 mg of furosemide [11]. In a different setting, (stable patients with advanced chronic HF), a retrospective analysis of the PRAISE study (Prospective Randomized Amlodipine Survival Evaluation) used a similar “cut-off” of furosemide dose (80 mg) to compare high versus low diuretic dose [6].
Using this definition, we divided patients into three categories according to loop diuretic treatment before hospital admission. Patients were classified as having DR (group 1) if the daily dose of oral furosemide prior to admission was at least 80 mg (or equivalent doses of torasemide or bumetanide). Patients were classified as group 2 when their daily dose of furosemide was lower than 80 mg, and group 3 if they were not receiving loop diuretics before admission.
Follow-up and outcome
Follow-up consisted of four compulsory visits scheduled at 1, 3, and 6 months, and 1 year. Additional visits were allowed whenever necessary and according to clinical judgement. Primary end-points were first readmission due to acute decompensation of HF, and death for any cause, and survival time was the number of days between inclusion date and death. Readmissions during the follow-up period did not imply exclusion from the registry.
Statistical methods
Quantitative variables are expressed as median and interquartile range [IQR]. Qualitative variables are expressed as the number of patients and percentages. The Chi square or Fisher exact tests were used to compare qualitative variables. The Kolmogorov–Smirnov test was used to determine whether quantitative variables were normally distributed. The t test was used to compare normally distributed quantitative variables and the non-parametric Mann–Whitney U test was used when distribution was not normal. The one-way analysis of variance (ANOVA) and the non-parametric Kruskall–Wallis test were used to compare quantitative variables in more than two independent groups, and a Kaplan–Meier analysis was performed to compare survival between groups. Survival curves or distributions were compared using the log-rank test. The presence of DR was considered in a multivariate-adjusted logistic regression model that included those variables whose differences were significant in the univariate analysis: age, blood pressure, comorbidities (Charlson index), Barthel index, hemoglobin, creatinine, sodium, NYHA functional class, HF etiology, LVEF, and previous treatment with ACE inhibitors or beta-blockers. Finally, to analyse the independence of predictor variables we performed a collinearity analysis calculating the variance inflation factor (VIF) and tolerance statistics. The threshold to consider collinearity was set at > 10 and < 0.1 for VIF and tolerance statistics, respectively. Statistical significance was set at 0.05. Analyses were performed with the software Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS, Inc., Chicago, Illinois, USA).
Sample size
The required sample size for the present study was calculated based on previous findings in which prevalence of DR was 24–35%. With this assumption, a random sample of 779–972 individuals would be enough to estimate, with a confidence interval of 95% and a precision of ± 3 percentage units, a population percentage expected to be around 30%.
Results
Of the 2037 patients enrolled in this study, 435 were included in group 1 (daily dose of oral furosemide prior to admission ≥ 80 mg). Thus, the prevalence of DR in the RICA registry was 21% (95% confidence interval 19.3%–22.7%). The remaining 1632 patients were included in group 2 (740 patients with daily dose of furosemide lower than 80 mg), and group 3 (892 patients not receiving loop diuretics before admission).
The baseline clinical characteristics for each of these groups are shown in Table 1. Some clinically significant differences are observed among the demographic characteristics. Patients with DR have lower blood pressure values, more comorbidities (hypercholesterolemia, diabetes mellitus, ischemic heart disease, CKD and cancer) and worse functional status (lower Barthel index scores). The analytical values also show differences, the most relevant being lower levels of hemoglobin, GFR, and sodium in patients with DR. In contrast, uric acid is higher in patients with DR, and glucose is higher in patients receiving diuretics (groups 1 and 2). We find no significant differences in natriuretic peptides and troponin levels. With regard to HF-related variables, the DR group has a higher proportion of ischemic etiology, more advanced functional class, higher systolic pulmonary artery pressure, and lower LVEF values. We find no relevant differences in factors triggering decompensation, except that anemia is more common in patients receiving diuretic treatment. Finally, radiographic alterations are more frequent in patients with DR, but none of these differences reach significance in the statistical analysis.
Pharmacological treatment prior to admission is shown in Table 2. Furosemide is the most frequently prescribed loop diuretic, followed far behind by torasemide. The proportion of patients receiving ACE inhibitors and digoxin is lower in the DR group. In contrast, these patients more frequently receive beta-blockers, ivabradin, and other types of diuretics.
After 1 year of follow-up, 634 (30.7%) deaths were reported. Mortality is significantly higher in patients with DR (163/453; 37%) in comparison with groups 2 (206/740; 28%) and 3 (265/892; 30%). The unadjusted hazard ratio for all-cause death in group 1 versus groups 2 and 3 is 1.47 (95% CI 1.18–1.84; p = 0.002). We also find differences in rehospitalization rates between the groups (58.3%, 52.9% and 56% for groups 1, 2 and 3, respectively) but these differences are not statistically significant (p = 0.72). Kaplan–Meier survival curves for all-cause mortality and rehospitalization are shown in Figs. 1 and 2. Using the log-rank test for comparison, we find differences in short- and long-term mortality (log-rank p = 0.001), but differences in rehospitalization are only apparent and significant after long-term follow-up (log-rank p value after 365 days of follow-up = 0.028).
The presence of DR is considered in a multivariate logistic regression model adjusted for the variables that are different among the three groups. After controlling for these variables, DR maintains statistical significance as a mortality predictor (adjusted hazard ratio for all-cause death: 1.37; 95% CI 1.06–1.79; p = 0.018). We also identify the following predictors of death, as described in Table 3: age, Charlson and Barthel index, haemoglobin and creatinine values, and NYHA functional class. Finally, we do find statistical significance when analysing the collinearity between these variables.
Discussion
This is the first study to describe the prevalence of DR in a large cohort of elderly patients admitted for acute decompensation of chronic HF in a real-world setting, revealing a rate of 21%. Furthermore, we observe that the presence of DR independently increases the risk of death by 1.37-fold.
The real prevalence of DR is probably unknown, given the lack of a consensus definition and studies focusing on this question. The retrospective analysis of the PRAISE study finds a higher prevalence (35%) of DR using the similar definition (daily furosemide dose > 80 mg). However, the PRAISE study was performed in a different setting (stable patients with advanced chronic HF), and the baseline characteristics of patients are very different from those of the RICA registry: their patients are younger (65 years of age) with ischaemic aetiology and severe systolic dysfunction (mean LVEF 20%) [6]. To our knowledge, no other studies have analysd the prevalence of DR using the same definition.
The most extensive AHF registry in the world, the ADHERE registry (Acute Decompensated Heart Failure National Registry), has analysed DR with a focus on insufficient symptom relief and loop diuretic dosing during the first 24 h after admission. The patients enrolled in the ADHERE registry have more in common with those included in the RICA registry: they have a median age of 75 years and a significant comorbidity burden, and a similar proportion (53%) of patients have preserved LVEF. Among more than 50,000 patients enrolled in this study, only 33% lost ≥ 2.27 kg and 16% gained weight during hospitalization [12]. As for loop diuretic dosing, 23.8% of patients required high-dose intravenous loop diuretics, defined as a total intravenous furosemide dose ≥ 160 mg, during the first 24 h [13].
In the light of these results, and regardless of the definition used, it seems clear that DR is a common problem in clinical practice.
We also find that patients with DR more often present an ischaemic aetiology of HF, lower values of LVEF, more advanced functional class, and worse kidney function. These findings are also described in patients receiving high doses of diuretics in the PRAISE study [8]. In addition, patients with DR have more comorbidities and a poor functional status measured with the Barthel index. The Barthel index is well known to be a key outcome predictor of mortality in patients with HF in the chronic [10] and the acute setting [14], but to our knowledge, it is the first time that it has been directly associated with DR.
Differences in treatment before admission are also found between groups, which can be justified, in part, by the above-mentioned differences in baseline characteristics. For example, the lower proportion of ACE inhibitors and digoxin could be explained by the greater prevalence of CKD in patients with DR. Moreover, the higher proportion of patients treated with beta-blockers and ivabradine can be justified by the predominant ischemic aetiology of HF. It is interesting and important to note that patients receiving loop diuretics before admission (groups 1 and 2) are more frequently treated with additional diuretics, including thiazides or potassium-sparing diuretics. Sequential nephron blockade by addition of a second diuretic class (combination diuretic therapy) is an attractive strategy to overcome DR, but the optimal timing of this sequential blockade in HF remains uncertain [15,16,17].
Finally, we find that DR was independently associated with overall mortality. Various observational studies have demonstrated an association between higher doses of diuretics and worse outcomes. These findings may be confounded by indication: patients who receive higher doses of diuretics may do so because of greater disease severity compared with patients who can be successfully treated with lower dose [18]. Although most of these studies have found a persistent adverse effect of loop diuretics even after multivariable adjustment for other known predictors of mortality, such adjustment may be insufficient to completely eliminate confounding [18]. In our study, after adjusting for possible confounders including comorbidities and functional status, DR persists as an independent risk factor for mortality. However, even these results do not confirm that diuretics are harmful per se. The pathophysiology of DR is a complex interaction between multiple factors (pharmacokinetic and pharmacodynamic factors, CKD, renal adaptation mechanisms with nephron remodeling, neurohormonal activation, etc.) that cannot be completely controlled in clinical studies [2, 19,20,21]. In the future, carefully controlled studies will be required to clarify whether there is a causal relationship between diuretic use and adverse outcomes, or alternatively, if diuretic dosage is just a surrogate for disease severity.
Early identification of a poor diuretic response might allow the initiation of therapies to modify this response. Different objective methods to evaluate diuretic response have been introduced (metrics of diuretic response) and suggest that diuretic response should be determined based on the effect of the diuretic dose administered (e.g. weight loss or net fluid loss per milligramme of loop diuretic). The use of such metrics of diuretic response could help identify patients who might benefit from alternative decongestive therapies and guide treatment selection [3, 22,23,24,25].
We are aware that our study has some limitations. First, the RICA registry does not include patients who died during the index hospitalization. Assuming that a high proportion of these patients had more severe HF and some degree of DR, their exclusion could lead to an underestimation of DR. Second, the RICA registry does not collect information regarding interventional procedures during hospitalization (like coronary revascularization) that might have influence on prognosis. Third, loop diuretic doses are probably a surrogate marker of DR, and it would be desirable to confirm and compare our results using other metrics of diuretic response that are not included in the RICA registry. Finally, the heterogeneity about the DR definition is a limitation for external validation of these results, and for this reason, we have to be cautious before extending these results to the general HF population.
In summary, we frequently find patients who require hospitalization for chronic heart failure decompensation despite receiving high dose (at least 80 mg) of oral furosemide. This clinical scenario could be a surrogate marker of diuretic resistance with a higher risk for mortality.
References
Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT et al (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100, 000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149:209–216
ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA (2015) Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol 12:184–192
Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O’Connor CM et al (2014) Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 35:1284–1293
Knauf H, Mutschler E (1997) Sequential nephron blockade breaks resistance to diuretics in edematous states. J Cardiovasc Pharmacol 29:367–372
Epstein M, Lepp B, Hoffman S et al (1997) Potentation of furosemide by metolazone in refractory edema. Curr Therap Res 21:656–667
Neuberg GW, Miller AB, O’Connor CM, Belkin RN, Carson PE, Cropp AB et al (2002) Prospective randomized amlodipine survival evaluation. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J 144:31–38
Trullàs JC, Miró Ò, Formiga F, Martín-Sánchez FJ, Montero-Pérez-Barquero M, Jacob J et al (2016) The utility of heart failure registries: a descriptive and comparative study of two heart failure registries. Postgrad Med J 92:260–266
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200
Ruiz-Laiglesia FJ, Sánchez-Marteles M, Pérez-Calvo JI, Formiga F, Bartolomé-Satué JA, Armengou-Arxé A et al (2014) Comorbidity in heart failure. Results of the Spanish RICA Registry. QJM 107:989–994
Formiga F, Chivite D, Conde A, Ruiz-Laiglesia F, Franco AG, Bocanegra CP et al (2014) Basal functional status predicts three-month mortality after a heart failure hospitalization in elderly patients—the prospective RICA study. Int J Cardiol 172:127–131
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR et al (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364:797–805
Gheorghiade M, Filippatos G (2005) Reassessing treatment of acute heart failure syndromes: the ADHERE registry. Eur Heart J 7:B13–B19
Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM et al (2009) Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology 113:12–19
Miró Ò, Rossello X, Gil V, Martín-Sánchez FJ, Llorens P, Herrero-Puente P et al (2017) Predicting 30-Day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann Intern Med 167:698–705
Trullàs JC, Morales-Rull JL, Casado J, Freitas Ramírez A, Manzano L, Formiga F (2016) Rationale and design of the “safety and efficacy of the combination of loop with thiazide-type diuretics in patients with decompensated heart failure (CLOROTIC) trial:” a double-blind, randomized, placebo-controlled study to determine the effect of combined diuretic therapy (loop diuretics with thiazide-type diuretics) among patients with decompensated heart failure. J Card Fail 22:529–536
Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H (2014) Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med 25:67–72
Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA et al (2017) Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2:950–958
Felker GM, O’Connor CM, Braunwald E (2009) Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail 2:56–62
De Bruyne LK (2003) Mechanisms and management of diuretic resistance in congestive heart failure. Postgrad Med J 79:268–271
Ellison DH, Felker GM (2018) Diuretic Treatment in Heart Failure. N Engl J Med 378:684–685
Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M et al (2017) Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail 19:1014–1022
Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR et al (2014) Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 7:261–270
Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G et al (2014) Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome-an analysis from RELAX-AHF. Eur J Heart Fail 16:1230–1240
Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W et al (2014) Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail 20:392–399
Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O’Connor CM, Metra M et al (2018) Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol 258:185–191
Acknowledgements
we gratefully acknowledge all investigators who form part of the RICA Registry. This project was possible thanks to an educational unrestricted scholarship granted by Boehringer Ingelheim. We would like to thank RICA’s Registry Coordinating Center “S&H Medical Science Service” for their quality control data, logistic support, and administrative work and Prof. Salvador Ortiz, Universidad Autónoma de Madrid and Statistical Advisor S&H Medical Science Service, for the statistical analysis of the data presented in this paper.
Funding
This work was supported by an educational unrestricted scholarship granted by Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no conflicts of interest.
Statements on human and animal rights
The registry complies with the Declaration of Helsinki. The local ethics committee from the University Hospital “Reina Sofia” (Córdoba, Spain) approved the study protocols.
Informed consent
Informed consent was obtained from all participating subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
RICA Registry members: Álvarez Rocha P, Anarte L, Aramburu-Bodas O, Arévalo-Lorido JC, Carrascosa S, Carrera Izquierdo M, Casado Cerrada J, Cepeda JM, Cerqueiro JM, Conde-Martel A, Díez-Manglano J, Epelde F, Formiga F, García Escrivá D, González Franco A, López-Castellanos G, Manzano L, Montero-Pérez-Barquero M, Morales-Rull JL, Ormaechea G, Pérez Calvo JI, Pérez-Silvestre J, Quirós López R, Ruiz Ortega R, Suárez-Pedreira I, Trullàs JC.
Rights and permissions
About this article
Cite this article
Trullàs, JC., Casado, J., Morales-Rull, JL. et al. Prevalence and outcome of diuretic resistance in heart failure. Intern Emerg Med 14, 529–537 (2019). https://doi.org/10.1007/s11739-018-02019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-02019-7