Abstract
Background
Implantable cardioverter- defibrillator (ICD) therapy is established for the prevention of sudden cardiac death (SCD) in different entities. However, data from large patient cohorts with electrical heart disease are rare. Therefore, we investigated these patients as well as patients with hypertrophic cardiomyopathy by analyzing registry data from a multi-center ‘real-life’ registry.
Methods
The German Device Registry (DEVICE) is a nationwide, prospective registry with one-year follow-up investigating 5450 patients receiving device implantations in 50 German centers. The present analysis of DEVICE focussed on patients with electrical heart disease or HCM who received an ICD for primary or secondary prevention.
Results
174 patients with HCM and 112 patients with electrical heart disease (long-QT syndrome, Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy) were compared with 5164 other ICD patients. Median follow-up was 17.0 months. Patients in the control group were significantly older. Of note, overall mortality after 1 year was 1.8% in HCM patients, 6.6% in patients with electrical heart disease and 7.3% in the control group. Patients in the control group presented significantly more severe comorbidities. In contrast to HCM patients and the control group where primary prevention was the major indication for ICD implantation, 77.5% of patients with electrical heart disease received an ICD for secondary prevention. The number of surgical revisions was higher in patients with electrical heart disease.
Conclusion
Data from the present registry display a surprisingly high mortality in patients with electrical heart disease equivalent to the control group. A high proportion of patients who received an ICD for secondary prevention may be regarded as a major determinant for these results, while severe comorbidities such as diabetes, hypertension, and renal failure are major determinants for mortality in the control cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantable cardioverter-defibrillators (ICD) are the established therapy for primary and secondary prevention of sudden cardiac death (SCD) [1]. ICD therapy significantly reduces the rate of sudden cardiac death as well as overall mortality in patients with ischemic cardiomyopathy compared with the sole antiarrhythmic drug therapy [2,3,4]. Regarding the prevention of SCD in non-ischemic cardiomyopathy the available data are not as convincing as for the ischemic cardiomyopathy. However, the “Prophylactic defibrillator implantation in patients with non-ischemic dilated cardiomyopathy”—trial (DEFINITE) presented a significant reduction of the risk of sudden cardiac death as well as a non-significant reduction of overall mortality [5]. A reduction of overall mortality in patients with non-ischemic cardiomyopathy was observed in the “Sudden cardiac death in heart failure”-trial (SCD-HeFT) [4].
In patients with hypertrophic cardiomyopathy (HCM), ICD implantation is indicated in patients with survived SCD or with documented ventricular tachycardia for secondary prevention [1]. ICD implantation for primary prevention depends on the risk estimation employing the HCM risk calculator, which should be regularly performed [1]. Regarding the inherited primary arrhythmia syndromes which are regularly referred to as electrical heart disease evidence and follow-up data are sparse. Apart from secondary prevention after survived SCD history of documented arrhythmias as well as history of syncope play an important role in long-QT-syndrome, Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia (CPVT) [1].
In the present study, data from a multi-center real-world registry were analyzed and patients with electrical heart disease (long-QT syndrome, Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy) and HCM were compared with patients with structural heart disease.
The German Device Registry is a nationwide, prospective database of ICD or CRT implantations and revisions which is organised by the Stiftung Institut für Herzinfarktforschung Ludwigshafen, Germany (IHF) [6,7,8]. In 50 participating centers, information on demographic data, indication for the device, implantation procedure, as well as peri-interventional complications were documented at the time of device operation by the individual operator. Recruitment of patients for the German Device Registry started in March 2007 until March 2011, then continued as Device II registry and was terminated in February 2014. After written informed consent, data were entered into an internet-based electronic case report form by the centers. Case report forms were thereafter transmitted, encrypted with a secure socket layer and the IHF took responsibility for data management and monitoring. The present study focuses on patients with hypertrophic cardiomyopathy and patients with electrical heart disease and compares these cohorts with other ICD recipients. Follow-up contacts were scheduled at least 1 year after implantation or revision by telephone. The follow-up was performed by the IHF. During telephone contact, questions on arrhythmias (e.g. syncope, resuscitation, ablation), cardiac events (e.g. myocardial infarction, revascularization), complications, medication, and heart failure symptoms were posed. In case of an ineffective call, further information was gathered from other caring physicians or civil registration offices.
Statistical analysis
The patient population is characterized by descriptive statistical measures. Categorical data are presented as percentages, metrical data as medians with 25th and 75th percentiles. The distribution of binary variables was compared between age groups by Pearson Chi-square test, that of metrical variables by Mann–Whitney test. The shown baseline data are 99% complete except where indicated. The descriptive statistics are based on the available cases.
Observation time was calculated as the time span from the index intervention to the last follow-up contact. One-year mortality during 366 days was estimated using the Kaplan–Meier method.
The statistical computations were performed using SAS release 9.3 on a personal computer (SAS Institute, Inc., Cary, NC. USA).
Results
Patients’ characteristics/demographics
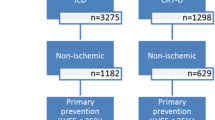
Demographic characteristics are summarized in Table 1. Out of 5450 patients registered in DEVICE I and DEVICE II in 174 patients (3.2%) HCM was the main diagnosis while electrical heart disease was present in 112 patients (2.1%). This group includes patients with Brugada syndrome (n = 25, 22.3%), long-QT-syndrome (n = 45, 40.2%), and arrhythmogenic right ventricular cardiomyopathy (ARVC, n = 25, 22.3%) as well as patients with idiopathic ventricular fibrillation. These patients were compared to 5164 patients (94.8%) who received an ICD due to other indications (Table 1). Patients with HCM or electrical heart disease were significantly younger than patients in the control group (Table 1). Defibrillator systems for cardiac resynchronization therapy (CRT) were implanted more frequently in the control group. Of note, the majority of patients with electrical heart disease received an ICD for secondary prevention while in patients with HCM and in the control group, primary prevention represented the major indication for ICD implantation.
Implantation procedure
Perioperative defibrillation testing was conducted in 79.8% of patients in the HCM group (99.3% successful), in 86.7% of patients with electrical heart disease (100% successful) and in 73.8% of patients in the control group (99.4% successful). Regarding severe peri-interventional complications pericardial effusion was observed more often in patients with electrical heart disease (2.7% vs. 0.6% in HCM patients and 0.7% in the control group, p = 0.046). Similar results were observed for pneumothorax (2.6% vs. 0% in HCM patients and 0.4% in the control group, p = 0.002). In-hospital death was reported for 0.3% of patients in the control group while no patients with HCM or electrical heart disease died in the hospital. Relevant cardiovascular medication is displayed in Table 2.
Follow-up
Follow-up information was obtained for 98.6% of the 5450 patients at a median observation time of 17.0 months (13.1; 23.2). For the remaining 1.4% of included patients observation time was restricted to hospital stay. Major outcome parameters are summarized in Table 3. one-year mortality was 1.8% in the HCM group, 6.6% in patients with electrical heart disease and 7.3% in the control group. A detailed comparison of deceased patients in the different study groups revealed that in particular, patient age of deceased patients was similar in patients with HCM (71.6 ± 8.3 years); with electrical heart disease (73.5 ± 20.8); and in the control group (71.1 ± 10.7%, p = 0.17). Unfortunately, direct cause of death was unknown in the majority of patients.
The rate of major adverse cardiac and cerebrovascular events (MACCE; including death, myocardial infarction and stroke) displayed similar results (Table 3). The incidence of shock deliveries did not significantly differ between study groups (Table 3).
Discussion
The present study displays an important outcome from data on patients with HCM or electrical heart disease, who received an ICD for primary and secondary prevention.
Patient collective and demographics
Patient characteristics of the present registry underline important differences between the examined study groups. First, patients with HCM or electrical heart disease are significantly younger as compared with the control group which is typical for these patient cohorts. As expected, left ventricular function was normal in patients with HCM or electrical heart disease while it was severely impaired in the control group. In accordance, left bundle branch block was more common in the control group. In addition, further risk factors such as atrial fibrillation, diabetes, hypertension, renal insufficiency or peripheral artery disease were reported significantly, more frequently, in the control group. In contrast, the majority of patients with electrical heart disease received a defibrillator system for secondary prevention while primary prevention was the major indication in HCM patients and in the control group. These demographic characteristics are representative for patient cohorts with HCM [9, 10] or electrical heart disease [11] and underline important differences regarding comorbidities compared with patient with ischemic or non-ischemic heart failure. This is also mirrored in the data on heart failure medication that is only rarely administered in patients with electrical heart disease. Of note, the rate of prescription of class III antiarrhythmic agents was similar in all three groups.
Follow-up
Of note, mortality as well as the rate of major cardiovascular and cerebrovascular events was significantly lower in patients with HCM but was almost equivalent in patients with electrical heart disease and in the control group. This is surprising as in particular patients with electrical heart disease present significantly less severe comorbidities that may influence overall prognosis. The majority of patients with electrical heart disease received a defibrillator system for secondary prevention and presented history of cardiopulmonary resuscitation. Of note, this may be interpreted as a major determinant for the increased mortality in this group as an association between secondary prophylaxis of sudden cardiac death and an increased event rate has been described before [12]. However, the observed event rates are higher than in previously published single center experiences in patients with electrical heart disease [13, 14].
In contrast to patients with electrical heart disease, the majority of patients with HCM and of the control group received a defibrillator system for primary prevention. In the patients of the control group, severe comorbidities such as diabetes or renal failure as well as heart failure represent major determinants for a deleterious outcome. This has already been reported in previous publications of the present registry [6, 8]. Of note, occurrence of appropriate therapy delivery did not differ between study groups. Significant differences were observed regarding the number of surgical revisions which was higher in patients with electrical heart disease. A possible explanation might be a more active lifestyle in this younger patient cohort that could explain an increased rate of lead problems. This aspect has for example been pointed out in a long-term analysis of defibrillator performance in children and young adults [15].
Potential value of the subcutaneous ICD
The data from the present registry only include transvenous ICD systems as it is derived from the beginning of the S-ICD era where implantation of subcutaneous ICD systems was not very common. The amount of peri-procedural complications in the group of patients with electrical heart disease is rather high. Nowadays, the majority of patients of the electrical heart disease group as well as a significant amount of the control group would most likely have been implanted with S-ICD systems as positive experiences with this system have been published in patients with electrical heart disease [11], HCM [9] and even coronary artery disease [16].
Limitations
The present data are derived from a registry. Therefore, several limitations are obvious. According to the design of the registry, a selection bias is probable as patient selection may not have been as strict as in randomized clinical trials. In particular, the group of patients with electrical heart disease is heterogeneous and lacks a matched control group.
In addition, collection of complementary data such as relevant comorbidities is not as thorough as it usually is in randomized trials as this data were assessed at the discretion of the individual operator. Furthermore, unfortunately the direct cause of death remained unknown in the majority of the deceased patients. The present registry also lacks detailed information on device programming as well as on potential inappropriate therapy delivery. Nonetheless, the results of the present study represent ‘real-life’ data on patients with ICD systems.
Conclusion
Data from the present registry display a surprisingly high mortality in patients with electrical heart disease equivalent to the control group. A high proportion of patients who received an ICD for secondary prevention may be regarded as a major determinant for these results while severe comorbidities such as diabetes, hypertension and renal failure are major determinants for mortality in the control cohort.
References
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J et al (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC) endorsed by: association for european paediatric and congenital cardiology (AEPC). Europace. 17:1601–1687
Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H et al (1996) Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 335:1933–1940
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 346:877–883
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 352:225–237
Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP et al (2004) Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 350:2151–2158
Kobe J, Andresen D, Maier S, Stellbrink C, Kleemann T, Gonska BD et al (2017) Complications and 1-year benefit of cardiac resynchronization therapy in patients over 75 years of age—Insights from the German device registry. Int J Cardiol 228:784–789
D'Ancona G, Safak E, Senges J, Hochadel M, Nguyen VL, Perings C et al (2017) Activation of remote monitoring for cardiac implantable electronic devices: small dog for tall weeds. Clin Res Cardiol 106:833–839
Frommeyer G, Andresen D, Ince H, Maier S, Stellbrink C, Kleemann T et al (2019) Can we rely on Danish? real-world data on patients with nonischemic cardiomyopathy from the German device registry. Heart Vessels 34(7):1196–1202
Frommeyer G, Dechering DG, Zumhagen S, Loher A, Kobe J, Eckardt L et al (2016) Long-term follow-up of subcutaneous ICD systems in patients with hypertrophic cardiomyopathy: a single-center experience. Clin Res Cardiol 105:89–93
Vamos M, Healey JS, Wang J, Connolly SJ, Mabo P, Van Erven L et al (2018) Implantable cardioverter-defibrillator therapy in hypertrophic cardiomyopathy: a simple substudy. Heart rhythm. 15:386–392
Frommeyer G, Dechering DG, Kochhauser S, Bettin M, Kobe J, Eckardt L et al (2016) Long-time "real-life" performance of the subcutaneous ICD in patients with electrical heart disease or idiopathic ventricular fibrillation. J Interventional Cardiac Electrophysiol. 47:185–188
Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL et al (2010) Long-term prognosis of patients diagnosed with Brugada syndrome: results from the Finger Brugada syndrome registry. Circulation 121:635–643
Monnig G, Kobe J, Loher A, Wasmer K, Milberg P, Zellerhoff S et al (2012) Role of implantable cardioverter defibrillator therapy in patients with acquired long QT syndrome: a long-term follow-up. Europace 14:396–401
Schuler PK, Haegeli LM, Saguner AM, Wolber T, Tanner FC, Jenni R et al (2012) Predictors of appropriate ICD therapy in patients with arrhythmogenic right ventricular cardiomyopathy: long term experience of a tertiary care center. PLoS ONE 7:e39584
Frommeyer G, Feder S, Bettin M, Debus V, Kobe J, Reinke F et al (2018) Long-term single-center experience of defibrillator therapy in children and adolescents. Int J Cardiol 271:105–108
Willy K, Bettin M, Reinke F, Bogeholz N, Ellermann C, Rath B et al (2019) Feasibility of entirely subcutaneous ICD systems in patients with coronary artery disease. Clin Res Cardiol. https://doi.org/10.1007/s00392-019-01455-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no relevant conflict of interest.
Rights and permissions
About this article
Cite this article
Frommeyer, G., Reinke, F., Andresen, D. et al. Implantable cardioverter defibrillators in patients with electrical heart disease and hypertrophic cardiomyopathy: data from the German device registry. Clin Res Cardiol 109, 508–512 (2020). https://doi.org/10.1007/s00392-019-01532-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-019-01532-9