Abstract
Background
Cardiac involvement in myotonic dystrophy type 1 (MD1) includes conduction disease, arrhythmias, and left-ventricular (LV) systolic dysfunction leading to an increased sudden cardiac death risk. An understanding of the interplay between electrical and structural myocardial changes could improve the prediction of adverse cardiac events. We aimed to explore the relationship between signs of cardiomyopathy by conventional and advanced cardiovascular magnetic resonance (CMR), and electrical abnormalities in MD1.
Methods
Fifty-seven MD1 patients (43 ± 13 years, 46% male) and 15 matched controls (41 ± 7 years, 53% male) underwent CMR including cine-imaging with feature-tracking strain analysis, late gadolinium enhancement (LGE), and native/post-contrast T1-mapping with extracellular volume calculation. Standard 12-lead and long-term ECG monitoring were performed as screening for rhythm and/or conduction abnormalities.
Results
Abnormal ECGs were recorded in 40% of MD1; a pathologic CMR was found in 44%: 21% had an impaired LV–EF and 32% showed non-ischemic LGE. When looking at MD1 patients with available long-term ECG monitoring (n = 39), those with atrial fibrillation (Afib)/flutter(Afl) episodes had lower LV–EF (52 ± 7 vs. 60 ± 5%, p = 0.002), lower global longitudinal strain (− 17 ± 3 vs. − 20 ± 3%, p = 0.034), a trend to lower left atrial emptying fraction (LA–EF) (44 ± 14 vs. 55 ± 8%, p = 0.08), and higher prevalence of LGE (88% vs. 23%, p = 0.001) with an intramural (75% vs. 23%, p = 0.01) and septal (63% vs. 13%, p = 0.009) pattern. In a model including LV–EF (OR 0.8, 95% CI 0.7–1.0, p = NS) and LGE presence (OR 14.8, 95% CI 1.4–159.0, p = 0.026), only LGE was independently associated with the occurrence of Afib/Afl episodes.
Conclusion
Myocardial abnormalities depicted by non-ischemic LGE-CMR were the only independent predictor for the occurrence of Afib/Afl on ECG monitoring, previously shown to predict adverse cardiac events in MD1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myotonic dystrophy type 1 (MD1) is an autosomal dominant inherited multisystem disorder characterized by musculoskeletal symptoms, cardiac involvement, as well as the other manifestations. MD1 is caused by the expansion of an unstable trinucleotide repeat (CTG) on chromosome 19, in the myotonic dystrophy protein kinase gene, and represents one of the most common muscular dystrophies in adulthood [1].
Cardiac involvement in MD1 includes conduction disease, atrial and ventricular tachyarrhythmias, as well as left-ventricular (LV) systolic dysfunction, and is associated with a threefold increase in the risk of sudden cardiac death (SCD) [2,3,4,5,6]. The rate of cardiac disease progression is unclear and several risk factors for adverse events have been proposed, including age at onset, severity of muscular involvement, number of CTG repeats, atrial and ventricular tachyarrhythmias, atrioventricular block (AVB), abnormal signal-averaged electrocardiogram (ECG), and reduced heart rate turbulence [7, 8].
In addition, cardiac biomarkers have been shown to be increased in MD1 and a recent study suggests that hs-cTnT might be particularly helpful as a “cardiac risk factor” in these patients [9]. Fibro-fatty infiltration and degeneration of the myocardium, described in earlier autopsy studies, seem to represent the substrate for the cardiac manifestations in MD1 [6, 10,11,12,13]. Due to the increased cardiac morbidity and mortality, regular cardiac screening including cardiovascular magnetic resonance imaging (CMR) is required in these patients [3,4,5,6, 14]. Nevertheless, the exact relationship between electrical disturbances and pathological cardiac imaging findings is incompletely explored [3, 4, 11, 15].
CMR is a highly sensitive non-invasive tool for the detection of functional and structural myocardial abnormalities in both ischemic and non-ischemic cardiomyopathies [16]. In addition to depiction of focal myocardial damage by the conventional late gadolinium enhancement (LGE)-imaging, advanced equilibrium contrast CMR techniques for quantification of myocardial extracellular volume fraction (ECV) have been developed and validated for quantification of interstitial fibrosis [17]. Moreover, feature-tracking CMR using the standard acquired cine-images is a technique that allows quantification of myocardial deformation and strain to detect even subtle changes in LV function, and was validated against myocardial tagging [18]. A better understanding of the interplay between electrical and structural myocardial changes could improve the prediction of adverse cardiac events including SCD in MD1 patients. The aim of this study was to explore the relationship between signs of cardiomyopathy, as depicted by the conventional and advanced CMR methods, and electrical abnormalities in MD1 patients.
Methods
Study population
Fifty-seven patients with genetically confirmed MD1 were prospectively enrolled in two German centres (Robert Bosch Krankenhaus, Stuttgart and University Hospital Muenster, Muenster) between 2011 and 2016, and underwent comprehensive CMR. In addition, 15 age- and gender-matched healthy volunteers were enrolled as control group. The study protocol was approved by the local ethics committee and the patients gave written informed consent for participation in the study.
Patient evaluation
All MD1 patients underwent neurological and cardiac examinations. Known cardiac disease was noted positive whenever patients had a previous diagnosis corresponding to cardiac involvement by MD1 (cardiomyopathy and/or arrhythmia). Skeletal muscle symptoms included myotonia, muscle weakness, or pain.
All patients underwent resting standard 12-lead ECGs on the day of CMR. In addition, most MD1 patients (n = 39) underwent ambulatory monitoring (Holter-ECG) during a 5 day period using an external event loop recorder (SpiderFlash-t, Sorin Group) in a time window of ± 12 months to CMR [19].
Based on the rest and Holter tracings, an abnormal ECG indicative of conduction disease and/or arrhythmias was defined whenever any of the following transient/persistent abnormalities were detected: (A) rhythm other than sinus and (B) conduction system abnormalities: sinus node dysfunction (bradycardia and/or exit blocks), PR interval ≥ 240 ms, second- or third-degree AVB, and intraventricular conduction abnormalities (QRS duration ≥ 120 ms ± left/right bundle branch block morphology, LBBB/RBBB) [4].
In a subset of the MD1 patients, venous blood samples were taken for laboratory analysis, including cardiac biomarkers—high-sensitive Troponin T (hs-cTnT) and brain natriuretic peptide (NT-proBNP), and markers of skeletal muscle damage—total creatinine kinase (CK), also on the day of CMR.
CMR imaging protocol
ECG-gated CMR studies were performed on 1.5 T scanners (Aera, Siemens Medical Solutions, Erlangen, Germany and Achieva, Philips, Best, The Netherlands). Cine-imaging followed by intravenous contrast administration (0.10 mmol/kg Magnevist®) and LGE imaging was performed in the standard imaging planes. For ECV imaging, Modified Look-Locker inversion recovery (MOLLI) T1 mapping was performed before and 15–20 min after contrast (Supplemental Material).
CMR data analysis
CMR analysis was performed using the software cvi42 version 5.1.2 (Circle Cardiovascular Imaging Inc., Calgary, Canada). Ventricular volumes, ejection fraction (EF), and LV mass were quantified, and LGE presence and pattern were visually assessed as: ischemic and non-ischemic (Supplemental Material). Left atrial (LA) volumes at end-diastole (LA–EDV) and end-systole (LA–ESV) were measured in the 4- and 2-chamber cine-images using the biplane area-length method [20]. Total LA emptying fraction (LA–EF), a measure of the LA reservoir function and LV compliance, was additionally calculated as 100 × (LA–ESV − LA–EDV)/LA–ESV [20, 21]. According to the latest available published data, an abnormal CMR was defined by at least one of the following: (A) LV end-diastolic volume index > 105 mL/m2 in men and > 96 mL/m2 in women; (B) LV–EF < 55% and/or RV–EF < 45%; (C) maximal end-diastolic wall thickness > 12 mm in men and > 11 mm in women; (D) LA–ESV > 163 ml in men and > 131 ml in women; (E) LGE presence [22].
T1 and ECV measurements
Global native and post-contrast T1 times and ECV values were derived by averaging the measurements from the 6 (AHA) segments in the corresponding basal/mid-ventricular short-axis slice maps obtained as detailed in the Supplemental Material. Each of the six myocardial segments in the respective short-axis slice used for analysis was classified as LGE-positive or -negative by comparison with the corresponding LGE short-axis image. ECV values in LGE-negative and LGE-positive segments, respectively, were then averaged in each patient.
Feature-tracking analysis
The feature-tracking analysis was performed on the standard acquired cine-CMR images as detailed in the Supplemental Material. The short-axis slices were used to derive global peak radial (GRS) and global peak circumferential strains (GCS). Global peak longitudinal strain (GLS) was derived from the long-axis slices. Each global peak strain was derived by averaging the (n = 16, AHA segmentation) segmental values.
Statistical methods
Continuous variables are expressed as means and standard deviation (SD). Skewed variables are expressed as median and interquartile range (IQR). Categorical variables are expressed as frequency with percentage. Student’s t test was used for comparison of normally distributed characteristics between patients and controls. The Mann–Whitney U test was used for comparison of variables with a skewed distribution. One-way analysis of variance (ANOVA) with Bonferroni post hoc correction was used in case of multiple comparisons of normally distributed variables. Fisher’s exact tests were performed to compare categorical variables. To find independent predictors for electrical abnormalities, univariable regression analysis was performed. Second, the significant CMR parameters were introduced into multivariable regression analysis. Statistical analysis was performed with SPSS (version 24.0, IBM Corp., Armonk, NY). A p value ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 summarizes the general characteristics of MD1 patients (43 ± 13 years, 46% male) and controls (41 ± 7 years, 53% male). Thirty percent of the MD1 patients had a previous diagnosis of cardiomyopathy and/or arrhythmia. In 40 (70%) MD1 patients, blood biomarkers were determined: 82% had elevated hs-cTnT (26; 18–38 ng/L), while 63% had increased CK levels (222; 125–434 U/L) and only 3% an abnormal NT-proBNP (50; 35–96 ng/L).
ECG findings
In addition to 12-lead rest ECG, 68% (n = 39) of the MD1 underwent Holter-ECG monitoring. Based on these two methods, 40% of the total MD1 patients—representing 59% of the MD1 with available Holter-ECG, presented transient/persistent rhythm and/or conduction abnormalities (Table 1). Five patients (9%) had atrial fibrillation (Afib) and three patients (5%) had atrial flutter (Afl) episodes on ECG monitoring. Furthermore, 30% (n = 17) patients had conduction abnormalities: n = 6 (11%) sinus node dysfunction (n = 4 sinoatrial exit blocks and n = 2 sinus bradycardia episodes), n = 5 (9%) had AVB (n = 4 first-degree and n = 1 second-degree Wenkebach type), and n = 7 (12%) had intraventricular conduction abnormalities (n = 3 LBBB, n = 2 RBBB and n = 2 bifascicular blocks). Non-sustained ventricular tachycardia (nsVTs) was detected in three patients on ECG monitoring.
Conventional CMR findings in MD1 vs. control
The CMR results in patients and controls are shown in Table 2. Based on the defined conventional parameters, 42% (n = 24) of the MD1 patients presented an abnormal CMR. An impaired LV–EF (mild-to-moderate in all) was observed in 21% (n = 12) of MD1. However, there was no significant difference in LV–EF between patients and controls. None of the patients/controls showed LV dilatation. MD1 patients tended to have overall smaller hearts than controls (Table 2). There were no significant differences regarding LA dilatation and LV hypertrophy prevalence between MD1 and controls. Nevertheless, LA–EF was significantly lower in MD1 compared to controls (p = 0.001, Table 2).
Thirty-two percent of MD1 (n = 18) showed LGE—all non-ischemic—in a median of two segments (IQR 1–3) compared to none of controls. Among LGE-positive patients, LGE distribution patterns were as follows: isolated intramural in 12 patients (67%), isolated subepicardial in one patient (5%), and mixed (intramural and subepicardial) in five patients (28%). The basal septal (n = 9, 50%), basal inferior (n = 8, 44%), and inferolateral (n = 8, 44%) were the most frequently involved LV segments (Fig. 1). Notably, 42% (n = 5) of MD1 with a reduced LV–EF showed no LGE, while 61% (n = 11) of the LGE-positive patients had a normal LV–EF.
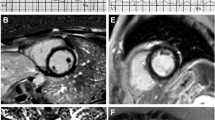
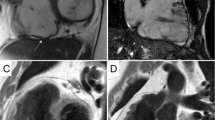
Illustrative CMR images of different LGE patterns found in MD1 patients: in the first patient (upper panels), in which atrial fibrillations episodes were detected in Holter-ECG, a mildly reduced left-ventricular systolic function (a, b; cine-CMR images in a basal short-axis view) together with the presence of an intramural LGE pattern (c; black arrows; basal short-axis view) was present in CMR. In the second patient (lower panels), in which a bifascicular block without atrial tachyarrhythmias was diagnosed, a normal left-ventricular systolic function (d, e; cine-CMR images in a basal short-axis view) together with the presence of a subepicardial LGE pattern (f; white arrows; basal short-axis view) was present in CMR
Advanced CMR imaging findings in MD1 vs. controls
Regarding myocardial deformation, we found a significantly lower GLS in MD1 compared to controls (− 19.9 ± 3 vs. 21.7 ± 2%, p = 0.002). There were no significant differences in GRS and GCS between the two groups (Table 2).
Significantly higher global native T1 values were measured in patients vs. controls (1001 ± 80 vs. 917 ± 48 ms, p < 0.001), but no significant differences were observed in global ECV (26 ± 3% vs. 26 ± 3%, p = 0.57) or post-contrast T1 (Table 2). In MD1 patients, averaged native T1 values in LGE-negative segments were significantly higher when compared to global native T1 in controls (1001 ± 79 vs. 917 ± 48 ms, p < 0.001).
Findings in MD1 with vs. without an abnormal CMR
MD1 patients with pathological CMR findings were more often male and tended to be older than the patients with normal examinations (Table 3). There were no significant differences regarding the prevalence of known cardiac disease and elevated cardiac/musculoskeletal biomarkers between MD1 with normal vs. abnormal CMR. MD1 patients with abnormal CMR showed more frequently an abnormal ECG (p = 0.013). When looking at the patients with available Holter-ECG (n = 39), the prevalence of both Afib/Afl (37% vs. 5%, p = 0.02) and conduction system abnormalities (53% vs. 15%, p = 0.02) was significantly higher in MD1 patients with abnormal CMR compared to those with normal CMR.
Advanced CMR imaging findings in MD1 with vs. without an abnormal CMR
Patients with pathological CMR findings in the conventional CMR techniques showed significantly lower global strain values compared to those with normal CMR (Table 3). On multiple comparison analysis, there were no significant differences in global strains between LGE-positive MD1 patients with normal LV–EF and those patients with completely normal CMR (GLS − 19.3 ± 3.8 vs. − 20.8 ± 3%, p = 0.90; GRS 49.4 ± 9.8 vs. 52.1 ± 10.6%, p = 1.00; GCS − 21.6 ± 5.7 vs. − 23.3 ± 2.5, p = 0.90).
There were no significant differences in global native- and post-contrast T1 as well as ECV between MD1 patients with normal and abnormal CMR findings (Table 3). Furthermore, there were no significant differences in T1-mapping parameters between MD1 patients with a normal vs. impaired LV–EF. Averaged ECV values in LGE-negative segments were lower in patients with vs. without abnormal CMR (25%±2 vs. 27 ± 3%, p = 0.022).
Relationship between electrical disturbances and CMR abnormalities
To avoid “the diluting effect” of missing Holter-ECG data, we examined the relationship between ECG abnormalities and CMR findings only in MD1 patients with an available Holter-ECG recording (Table 4). In this MD1 patient population (n = 39), those with rhythm and/or conduction abnormalities showed significantly more often an abnormal CMR finding (74% vs. 25%, p = 0.004) due to a significantly higher prevalence of LGE (63% vs. 10%, p = 0.001) (Fig. 2). Interestingly, despite no significant differences in LA size, LA––EF was reduced in MD1 with abnormal ECG when compared to those with normal ECG (p = 0.001; Table 4).
In a more detailed analysis, a significantly lower LV–EF was found in MD1 patients with vs. without Afib/Afl (52 ± 7 vs. 60 ± 5%, p = 0.002). These Afib/Afl-positive patients showed significantly more often presence of LGE (88% vs. 23%, p = 0.001) with an intramural (75% vs. 23%, p = 0.01) and septal (63% vs. 13%, p = 0.009), but not with a subepicardial pattern (25% vs. 10%, p = NS). LA––EF was also lower in these patients (44 ± 14 vs. 55 ± 8%, p = 0.08), but did not reach significance. Those MD1 patients with exclusively conduction abnormalities had no significant differences in LV–EF (58 ± 9 vs. 58 ± 5%, p = NS) but presented more often LGE (62% vs. 23%, p = 0.033) when compared to those without conduction abnormalities. No significant relationship between certain LGE patterns (intramural, septal, and subepicardial) and conduction abnormalities could be established in these latter patients. Moreover, LA–EF was also significantly lower in patients with exclusively conduction abnormalities (45 ± 12 vs. 56 ± 8%, p = 0.001). Among the three patients with documented nsVTs, two showed the presence of LGE, one of them with a reduced LV–EF. Regarding the advanced analyses, deformation parameters did not differ between MD1 patients with and without abnormal ECG. However, GLS was significantly lower in Afib/Afl-positive MD1 patients (− 17 ± 3 vs. − 20 ± 3%, p = 0.034). None of the T1-mapping parameters differed significantly in MD1 patients with vs. without abnormal ECGs. In Afib/Afl-positive patients, only global post-contrast T1 was significantly shorter (402 ± 50 vs. 457 ± 44 ms, p = 0.019) compared to patients without Afib/Afl.
Predictors for Afib/Afl occurrence in MD1
In the univariable analysis, age (OR 1.0, 95% CI 0.9–1.1, p = 0.42) and gender (OR 3.6, 95% CI 0.6–20.1, p = 0.15) were not significantly associated to the detection of Afib/Afl. Furthermore, LV–EF (OR 0.8, 95% CI 0.7–0.9, p = 0.008), LGE presence (OR 24.0, 95% CI 2.5–229.6, p = 0.006), LA–EF (OR 0.9, 95% CI 0.8–0.9, p = 0.021) GLS (OR 1.3, 95% CI 1.0–1.8, p = 0.046), and post-contrast global T1 (OR 0.97, 95% CI 0.94–0.99, p = 0.028) were all significantly related to Afib/Afl. To avoid overfitting, considering the low number of patients with Afib/Afl, we chose to introduce in the subsequent multivariable analysis the above significant parameters in a two-by-two manner, always including a functional and a structural parameter. Thus, in a model including LV–EF (OR 0.8, 95% CI 0.7–1.0, p = NS) and LGE presence (OR 14.8, 95% CI 1.4–159.0, p = 0.026), only LGE was independently associated with the occurrence of Afib/Afl episodes. In another model including GLS (OR 1.2, 95% CI 0.9–1.5, p = NS) and LGE (OR 17.5, 95% CI 1.7–180.3, p = 0.016), only LGE was an independent predictor for atrial tachyarrhythmia. In the model including LGE (OR 15.4, 95% CI 1.2–189.9, p = 0.033) and LA––EF (OR 0.9, 95% CI 0.9–1.0, p = NS), only LGE was an independent predictor for Afib/Afl. In the models including post-contrast global T1 and LV–EF/GLS/LA––EF, respectively, no independent predictors were found.
Predictors for conduction abnormalities occurrence in MD1
In the univariable analysis, only LGE presence (OR 5.3, 95% CI 1.3–22.6, p = 0.023) and LA–EF (OR 0.9, 95% CI 0.8–0.9, p = 0.008) were significantly related to the occurrence of conduction abnormalities. In the multivariable analysis, in the model including LGE (OR 1.8, 95% CI 0.3–10.4, p = NS) and LA–EF (OR 0.9, 95% CI 0.8–0.9, p = 0.04), only LA–EF was an independent predictor for conduction abnormalities. No significant relationship between different individual conduction abnormalities and CMR parameters was found.
Discussion
In the present study that focused on MD1 patients, we did not only find an association between structural and/or functional myocardial abnormalities and the presence of electrical disturbances, but—even more importantly—we were also able to detect a positive relationship between the presence of non-ischemic LGE in CMR (with a predominantly intramural septal pattern) and the occurrence of Afib/Afl. Moreover, in patients with exclusively conduction abnormalities, isolated signs of a LA dysfunction seem to be present.
CMR imaging of cardiac involvement in MD1 patients
In accordance with the previous studies reporting the findings of cardiomyopathy in up to three-quarter of MD1 patients, 42% of the present-study patients showed structural and/or functional abnormalities in CMR compared to none of the controls [3, 15]. Abnormal CMR findings were independent of the presence of musculoskeletal symptoms, of a previous (non-CMR based) diagnosis of cardiac involvement and of cardiac/musculoskeletal biomarker elevation. Elevated cardiac troponin that was suggested as a potential biomarker of cardiomyopathy was elevated in a substantial percentage of MD1 patients without any correlation to CMR or ECG findings [9]. This observation further emphasizes (a) a possibly limited specificity of this marker in MD1 patients and (b) the importance of CMR screening for the early detection of cardiac involvement in MD1 [15]. Focal myocardial fibrosis that can be depicted by LGE imaging is a well confirmed feature of MD1-associated cardiomyopathy across different studies, with a prevalence ranging from 13 to 40% [3, 14, 15].
Similarly, LGE—with a predominant intramural pattern involving the basal septum and inferior/inferolateral segments—was the most frequent CMR abnormality in the current study, being present in one-third of MD1 patients.
Relating structural and electrical abnormalities for a better risk assessment in MD1
More intriguing is the relationship between structural and electrical disturbances in MD1 patients. Myocardial fibrosis and degeneration seem to offer the substrate for development of conduction system disease, arrhythmias, and systolic dysfunction, but the exact interplay between these events is unclear [4, 6, 11]. While Petri et al. found no association between myocardial fibrosis on CMR and ECG abnormalities, the study of Hermans et al. reports a strong association between myocardial and conduction abnormalities in MD1 patients [3, 15]. Of course, differences in MD1 populations and the respective diagnostic approach might account for some differences between studies. In the present research, the prevalence of electrical abnormalities was more than double in MD1 patients with an abnormal CMR. Furthermore, among MD1 patients with electrical disturbances, those showing Afib/Afl episodes on Holter-ECG had a significantly higher prevalence of LV systolic dysfunction and LGE presence, while those with conduction abnormalities had only a higher prevalence of LGE. Despite no differences in LA size, left atrial function, a prognostic indicator for cardiovascular outcomes including incident Afib, was also significantly associated with the occurrence of atrial tachyarrhythmias and conduction disturbances, respectively [23, 24], in MD1. Interestingly, LGE was the best independent CMR predictor for the occurrence of Afib/Afl. Since it has already been shown that atrial fibrillation is an independent predictor of SCD and of development of major conduction defects in MD1 patients [4, 7], the present findings may have important clinical relevance. However, a careful interpretation of the weaker association between different types of conduction abnormalities and CMR findings in the present study is required and this somewhat unexpected observation may be due to low prevalence of conduction disease and exclusion of those MD1 patients having already undergone pacemaker/ICD implantation [5, 8, 15, 25].
Is there a potential role for advanced cardiac imaging using CMR in MD1?
Contrary to the other muscular dystrophies like Duchenne and Becker, no clear relationship between the degree of systolic impairment and the presence/extent of LGE has been described in MD1 [19, 26]. Thus, diffuse and rather subtle myocardial processes seem to be involved in the development of cardiomyopathy in MD1 and being characterized by a predominantly non-severe systolic dysfunction [19, 26]. Hence, we tested the ability of advanced CMR imaging parameters like feature-tracking derived global strain and T1-mapping-based quantification of interstitial fibrosis to depict these changes [17, 18].
In accordance with echocardiography data, we found reduced GLS values in MD1 patients compared to controls, even in the absence of significant differences in LV–EF [6, 27]. Unfortunately, GLS did not help in identifying MD1 patients with LGE and a preserved LV–EF or those patients with electrical abnormalities. Interestingly, Garcia et al. found speckle-tracking echocardiography derived GLS to be independently associated with the occurrence of adverse cardiac events, including new-onset Afib [27]. In the present CMR study, an impaired GLS was also associated with the detection of Afib/Afl on Holter-ECG based on univariable analysis. However, LGE presence was the only independent predictor for arrhythmic events on multivariable analyses.
Except for higher global native T1 in MD1 compared to controls, we did not find relevant changes in T1-mapping parameters—neither in MD1 patients with systolic dysfunction nor in those with electrical disturbances. Interestingly, global post-contrast T1 was the only T1-mapping parameter significantly modified in patients with Afib/Afl on Holter-ECG compared to those without. This isolated shortening of post-contrast T1 probably reflects the marked higher prevalence of LGE presence in patients with atrial tachyarrhythmias. Regarding the aforementioned lack of significant differences, it is unclear whether the T1-mapping technique used in the present study was not sensitive enough or whether we are measuring myocardial processes with opposing T1 effects (i.e., fibro-fatty infiltration) [28]. The previous data associating higher post-contrast T1 times in MD1 patients with more pronounced structural or electrical abnormalities in addition to our findings of lower ECV values in LGE-negative segments of patients with abnormal CMR could support the latter [29]. However, one can only speculate in the absence of corresponding histological data.
Possible clinical applications of the current findings
Our current findings further underline the importance of an initial CMR study and ambulatory Holter monitoring in all MD1 patients—in addition to standard 12-lead ECG and echocardiography [30]. Despite one-third of MD1 patients having abnormalities on Holter-ECG in the presence of normal resting tracings, need and frequency of subsequent Holter monitoring are yet not fully defined [30, 31]. Based on our findings, intensified monitoring for (atrial) tachyarrhythmias by ECG monitoring every 6 months should be indicated whenever non-ischemic LGE is detected on CMR—regardless of cardiac symptoms, 12-lead ECG abnormalities, and LV–EF [32]. Thus, an early detection of frequently asymptomatic Afib/Afl episodes could be enabled, with potential therapeutic and prognostic consequences, considering the burden associated with this often undetected arrhythmia [4, 6, 33,34,35]. The frequency of repeated CMR imaging and ECG monitoring in the presence of a completely normal baseline evaluation is not stipulated, but an interval of 3–5 years was suggested [30].
Limitations
The main limitation is the low prevalence of Afib/Afl in our study. Nevertheless, we were able to detect significant differences in CMR findings between patients with and without such episodes. A second limitation is the availability of good-quality T1 maps in only 77% of MD1 patients. There were no significant differences between MD1 with feasible vs. non-feasible T1-mapping analysis regarding prevalence of electrical or CMR abnormalities. The third limitation is the use of use T1 maps acquired on two scanners from different vendors, as variability between scanners, vendors, field strengths, and sequences is known for T1-Mapping. Nevertheless, the lack of a significant difference in T1-mapping parameters between MD1 and controls persisted in a per scanner sub-analysis [36].
Conclusion
Myocardial tissue abnormalities as depicted by the presence of non-ischemic LGE on CMR were the best independent predictor for the occurrence of Afib/Afl on long-term ECG monitoring. Advanced myocardial imaging parameters like feature-tracking derived systolic strain and T1-mapping showed no added value in relating myocardial disease and electrical disturbances in MD1.
References
Pelargonio G, Dello Russo A, Sanna T, Martino G de, Bellocci F (2002) Myotonic dystrophy and the heart. Heart 88:665–670
Lund M, Diaz LJ, Ranthe MF, Petri H, Duno M, Juncker I et al (2014) Cardiac involvement in myotonic dystrophy: a nationwide cohort study. Eur Heart J 35:2158–2164
Petri H, Ahtarovski KA, Vejlstrup N, Vissing J, Witting N, Køber L, Bundgaard H (2014) Myocardial fibrosis in patients with myotonic dystrophy type 1: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 16:59
Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E et al (2008) Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med 358:2688–2697
Petri H, Vissing J, Witting N, Bundgaard H, Køber L (2012) Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol 160:82–88
Petri H, Witting N, Ersbøll MK, Sajadieh A, Dunø M, Helweg-Larsen S et al (2014) High prevalence of cardiac involvement in patients with myotonic dystrophy type 1: a cross-sectional study. Int J Cardiol 174:31–36
Wahbi K, Babuty D, Probst V, Wissocque L, Labombarda F, Porcher R et al (2017) Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur Heart J 38:751–758
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A et al (2013) 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34:2281–2329
Valaperta R, Gaeta M, Cardani R, Lombardi F, Rampoldi B, Siena C de et al (2016) High-sensitive cardiac troponin T (hs-cTnT) assay as serum biomarker to predict cardiac risk in myotonic dystrophy: a case-control study. Clin Chim Acta 463:122–128
Bhakta D, Groh MR, Shen C, Pascuzzi RM, Groh WJ (2010) Increased mortality with left ventricular systolic dysfunction and heart failure in adults with myotonic dystrophy type 1. Am Heart J 160:1137–1141, 1141.e1
Bhakta D, Lowe MR, Groh WJ (2004) Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am Heart J 147:224–227
Nguyen HH, Wolfe JT, Holmes DR, Edwards WD (1988) Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J Am Coll Cardiol 11:662–671
Rakocević-Stojanović V, Pavlović S, Seferović P, Vasiljević J, Lavrnić D, Marinković Z, Apostolski S (1999) Pathohistological changes in endomyocardial biopsy specimens in patients with myotonic dystrophy. Panminerva Med 41:27–30
Choudhary P, Nandakumar R, Greig H, Broadhurst P, Dean J, Puranik R et al (2016) Structural and electrical cardiac abnormalities are prevalent in asymptomatic adults with myotonic dystrophy. Heart 102:1472–1478
Hermans MCE, Faber CG, Bekkers SCAM, Die-Smulders CEM de, Gerrits MM, Merkies ISJ et al (2012) Structural and functional cardiac changes in myotonic dystrophy type 1: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 14:48
Motwani M, Kidambi A, Greenwood JP, Plein S (2014) Advances in cardiovascular magnetic resonance in ischaemic heart disease and non-ischaemic cardiomyopathies. Heart 100:1722–1733
Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S et al (2013) Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging 6:373–383
Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B et al (2015) Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 16:871–881
Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, Yilmaz A (2014) Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson 16:81
Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S (2005) Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 7:775–782
Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TSM (2011) Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 12:421–430
Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R et al (2015) Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 17:29
Habibi M, Samiei S, Venkatesh BA, Opdahl A, Helle-Valle TM, Zareian M et al (2016) CMR-measured left atrial volume and function and incident atrial fibrillation: results from the multi-ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.115.004299
Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR et al (2014) Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging 7:570–579
Groh WJ, Lowe MR, Zipes DP (2002) Severity of cardiac conduction involvement and arrhythmias in myotonic dystrophy type 1 correlates with age and CTG repeat length. J Cardiovasc Electrophysiol 13:444–448
Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U, Yilmaz A (2014) Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging 15:1004–1012
Garcia R, Rehman M, Goujeau C, Degand B, Le Gal F, Stordeur B et al (2017) Left ventricular longitudinal strain impairment predicts cardiovascular events in asymptomatic type 1 myotonic dystrophy. Int J Cardiol 243:424–430
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G et al (2013) Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging 6:392–398
Turkbey EB, Gai N, Lima JAC, van der Geest RJ, Wagner KR, Tomaselli GF et al (2012) Assessment of cardiac involvement in myotonic muscular dystrophy by T1 mapping on magnetic resonance imaging. Heart Rhythm 9:1691–1697
Sommerville RB, Vincenti MG, Winborn K, Casey A, Stitziel NO, Connolly AM, Mann DL (2017) Diagnosis and management of adult hereditary cardio-neuromuscular disorders: a model for the multidisciplinary care of complex genetic disorders. Trends Cardiovasc Med 27:51–58
Merlevede K, Vermander D, Theys P, Legius E, Ector H, Robberecht W (2002) Cardiac involvement and CTG expansion in myotonic dystrophy. J Neurol 249:693–698
Russo V, Rago A, Ciardiello C, Russo MG, Calabrò P, Politano L, Nigro G (2016) The role of the atrial electromechanical delay in predicting atrial fibrillation in myotonic dystrophy type 1 patients. J Cardiovasc Electrophysiol 27:65–72
Haeusler KG, Gröschel K, Köhrmann M, Anker SD, Brachmann J, Böhm M et al (2018) Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol 107:871–880
Jobs A, Schwind J, Katalinic A, Babaev V, Tilz RR, Rausch S et al (2018) Prognostic significance of atrial fibrillation in acute decompensated heart failure with reduced versus preserved ejection fraction. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1321-4
Bettin M, Dechering D, Kochhäuser S, Bode N, Eckardt L, Frommeyer G, Reinke F (2018) Extended ECG monitoring with an implantable loop recorder in patients with cryptogenic stroke: time schedule, reasons for explantation and incidental findings (results from the TRACK-AF trial). Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1358-4
Puntmann VO, Peker E, Chandrashekhar Y, Nagel E (2016) T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res 119:277–299
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chmielewski, L., Bietenbeck, M., Patrascu, A. et al. Non-invasive evaluation of the relationship between electrical and structural cardiac abnormalities in patients with myotonic dystrophy type 1. Clin Res Cardiol 108, 857–867 (2019). https://doi.org/10.1007/s00392-019-01414-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-019-01414-0