Abstract
Introduction
The aim of this study was to compare second-generation cryoballoon and contact-force radiofrequency point-by-point pulmonary vein isolation (PVI) in atrial fibrillation (AF) patients with regard to pulmonary vein reconnection and arrhythmia-free survival.
Methods and results
Altogether, 269 consecutive patients with drug-refractory AF undergoing PVI were included and randomly allocated to second-generation cryoballoon or contact-force point-by-point radiofrequency ablation. Median follow-up duration was 389 days (interquartile range 219–599). Mean age was 59 years (71% male); 136 patients underwent cryoballoon and 133 patients underwent radiofrequency ablation. Acute electrical PVI was 100% for both techniques. Procedure duration was significantly shorter in cryoballoon vs radiofrequency (166.5 vs 184.13 min P = 0.016). Complication rates were similar (6.0 vs 6.7%, P = 1.00). Single procedure freedom of atrial arrhythmias was significantly higher in cryoballoon as compared to radiofrequency (75.2 vs 57.4%, P = 0.013). In multivariate analysis, persistent AF, AF duration, and cryoballoon ablation were associated with freedom of atrial tachyarrhythmias. The number of repeat ablation procedures was significantly lower in the cryoballoon compared to radiofrequency (15.0 vs 24.3%, P = 0.045). At repeat ablation, pulmonary vein reconnection rate was significantly lower after cryoballoon as compared to radiofrequency ablation (36.8 vs 58.1%, P = 0.003).
Conclusions
Improved arrhythmia-free survival and more durable pulmonary vein isolation is seen after PVI using second-generation cryoballoon as compared to contact-force radiofrequency, in patients with drug-refractory paroxysmal AF. Complication rates for both ablation techniques are low.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary vein isolation (PVI) has become the cornerstone of catheter-based treatment for paroxysmal and early persistent atrial fibrillation [1, 2]. Since the introduction of PVI, several ablation technologies have been developed including point-by-point radiofrequency (RF) ablation, multi-electrode radiofrequency ablation, and cryoballoon ablation [1, 3, 4]. In conventional RF PVI, the RF energy is delivered to the tissue in a point-by-point force-controlled manner resulting in cellular necrosis by heating [2]. In cryoballoon (CB) ablation, PVI is achieved by freezing tissue in a “single shot” manner resulting in circumferential cell necrosis [3]. RF PVI guided by 3D electro-anatomic mapping techniques is an effective treatment modality and requires only limited use of fluoroscopy. However, the technique is also considered technically challenging, especially for less experienced operators [2, 5]. CB PVI can be performed without 3D navigation systems and seems to be more accessible, however is associated with a higher risk of phrenic nerve injury [3, 6]. In a recent randomized trial, CB and RF ablation were shown to be equally effective for the treatment of paroxysmal atrial fibrillation. Furthermore, there were no significant differences in overall safety [6]. Importantly, different generation cryoballoons and RF-ablation catheters with and without contact force were used in that study. Although a post-hoc analysis was performed for newer generation catheters, only a small proportion constituted contact-force RF ablation, and the study was not powered to detect differences. We therefore aimed to report single center ablation success, incidence of repeat ablation, extent and rate of pulmonary vein (PV) reconnection, and safety outcomes after second-generation CB and contact-force-guided RF ablation for drug-refractory paroxysmal atrial fibrillation.
Methods
Patient population
Patients who were suitable for primo PVI for drug-refractory paroxysmal or early persistent atrial fibrillation were randomly allocated to cryoballoon or contact-force radiofrequency catheter ablation and included in the Isala Heart Centre AF database. Procedures in which additional ablation strategies [e.g., complex fractionated atrial electrograms (CFAE), linear ablations, right atrium ablations] were applied, were excluded from the present study.
Pre-ablation protocol
All patients underwent cardiac computed tomography (CT) to assess left atrial and pulmonary vein (PV) anatomy. Transthoracic echocardiography was routinely performed to assess left ventricular function, valve abnormalities, and atrial dimensions. Patients were admitted 1 day before the ablation procedure. During hospitalization, cardiac rhythm was continuously monitored in all patients. On admission and after ablation, routine blood samples were taken for screening purposes. Pre-ablation transoesophageal cardiac echocardiography was performed to assess the interatrial septum and rule out left atrial thrombus.
Ablation protocol
The PVI ablation procedures were performed under conscious sedation or under general anaesthesia supervised by a cardiovascular anesthesiologist, in accordance with HRS/EHRA/ECAS expert consensus on AF ablation. The ablation catheter was introduced in the left atrium via a transseptal puncture under pressure and fluoroscopic guidance, using a Brockenbrough needle. During the procedure, anticoagulation was checked at intervals of 30 min, with a targeted activated clotting time between 300 and 350 s.
Catheter ablation technique
Cryoablation
Cryoballoon ablations were performed using the second-generation Artic Front cryoballoon catheter (Medtronic, Inc., Minneapolis, MN, USA). This catheter has a bidirectional steering mechanism and an over-the-wire design. The 28-mm diameter balloon was used to create “single shot” circular antral lesions at all four PV ostia [left superior PV (LSPV), left inferior PV (LIPV), right superior PV (RSPV), right inferior PV (RIPV)] in order to create electrical PV isolation. Balloon selection was based on the PV size assessed by pre-ablation cardiac CT. Catheter placement and PV occlusion were checked by fluoroscopy. Cryo-energy applications were performed up to 240 s, with an additional number of Cryo applications at the operator’s discretion. After all ablations were performed, electrical PV isolation of PVs was checked with a standard circular mapping catheter. Adenosine testing was not performed. Ablation of the RSPV and RIPV was performed under continuous phrenic nerve stimulation with palpation of diaphragmatic excursions and direct fluoroscopic visualization of the diaphragm in case of any diminished activity. Cryoablation was immediately terminated on any signs of phrenic nerve palsy.
Radiofrequency ablation
A steerable circular multipolar catheter (Lasso™, Biosense Webster Inc., Diamond Bar, CA, USA) and an irrigated RF contact-force ablation catheter with a 3.5-mm tip (Smarttouch Thermocool™, Biosense Webster Inc., Diamond Bar, CA, USA) were introduced in the left atrium through two transseptal sheaths. Electro-anatomical mapping of the left atrium and PV signal recordings were then performed, using 3D navigation system (Carto3™, Biosense Webster Inc., Diamond Bar, CA, USA). RF energy was applied in a point-by-point manner to create continuous circular lesions in order to achieve electrical PV isolation. Power setting was adjusted between 30 and 40 W with a continuous 0.9% NaCl flow rate of 2 ml/min and 20–30 ml/min during RF energy application. RF energy was applied with a targeted force–time integral (FTI) of > 400 g with an RF application duration of 20 to 40 s, targeting an impedance drop of at least 10 Ohm, or until elimination of the local electrogram. The endpoint of the ablation procedure was defined as the absence or dissociation of PV potentials within the PVs ≥ 30 min post-ablation.
Post-ablation protocol
Patients were hospitalized for at least 24 h with continuous telemetry monitoring. Anticoagulation was continued after the procedure with a target international normalization ratio (INR) of 2.0–3.0 for at least 3 months after ablation. In patients using non-vitamin K antagonist oral anticoagulation (NOAC), this was resumed after the ablation procedure. After 3 months, anticoagulation therapy was (dis)continued at the discretion of the referring physician and in accordance with atrial fibrillation guidelines. Complications were defined in accordance with HRS/EHRA/ECAS expert consensus on AF ablation [2].
Follow-up
A blanking period of 90 days was defined after PVI. Outpatient clinic visits were planned at 3, 6, and 12 months after PVI. Follow-up included physical examination, electrocardiography (all visits), event monitoring and 24–72 h Holter ECG (at 6 and 12 months). In case of symptoms, patients were immediately referred to the emergency department. Additional Holter ECG monitoring was performed if no arrhythmia could be detected. AF occurrence in the blanking period was not considered a relapse. After the blanking period antiarrhythmic drugs were discontinued. The need for a repeat ablation for recurrent symptomatic atrial arrhythmias was left at the discretion of the physician.
Redo procedures
Patients with recurrences, not sufficiently suppressed by AAD or not willing to use AAD, and with sufficient symptoms, underwent a redo procedure. The redo procedure was performed with Carto 3D electro-anatomical mapping using a circular decapolar mapping catheter (Lasso™, Biosense Webster Inc., Diamond Bar, CA, USA) and RF ablation with an irrigated contact-force ablation catheter with a 3.5-mm tip (Smarttouch Thermocool™, Biosense Webster Inc., Diamond Bar, CA, USA). After the creation of a 3D electro-anatomical map and voltage map of the left atrium and pulmonary veins, the antra were ablated with the primary goal of pulmonary vein isolation. Additional ablation was left to the discretion of the operator.
Study endpoints
The primary effectiveness endpoint was single procedure arrhythmia-free survival, defined as patients without AF/atrial flutter (AFl)/atrial tachycardia (AT) recurrence, after the blanking period of 90 days after PVI. Arrhythmia recurrence was defined as AF/AFl/AT recorded on an ECG or a 30 s telemetry strip, in accordance with HRS/EHRA/ECAS expert consensus statement on AF ablation.
Statistical analysis
Data are mentioned as mean ± standard deviation (SD), median with interquartile range (IQR), or percentage where appropriate. Statistical significance between groups was calculated using Student’s t test for continuous variables and Chi-square test for categorical variables. Arrhythmia-free survival differences between catheter groups were assessed with a log-rank test. Univariate and multivariate analysis was performed using Cox regression. A P value of ≤ 0.05 was considered statistically significant. Statistical analysis was conducted using IBM Statistics version 22.0 (IBM SPSS Statistics for Macinthosh, 2013: IBM Corp., Armonk, NY, USA).
Results
Patient population
Altogether, 269 consecutive patients with drug-refractory paroxysmal or early persistent AF undergoing PVI were included and randomly allocated to cryoballoon or radiofrequency ablation. Mean age was 58.9 ± 10.4 years, 191 (71%) were men; 136 patients underwent CB and 133 patients underwent RF ablation. General patients’ characteristics were similar in both treatment groups. Baseline characteristics are presented in Table 1.
Acute PVI
Acute PVI was achieved in all patients. A total number of 1103 PVs were ablated [1068 standard PVs, 4 left common PVs, 2 left middle PVs (LMPV), 29 right middle PVs (RMPV)]. Acute PVI results are described in Table 2.
Procedural characteristics
Mean procedure duration was significantly shorter in CB ablation as compared to RF ablation (166.5 ± 36.8 vs 184.1 ± 40.0 min, P = 0.016). Procedural characteristics are shown in Table 2. Fluoroscopy times of the ablation groups were not significantly different (11.5 ± 8.5 vs 9.1 ± 14.3 min, P = 0.26).
Complications
There were no significant differences with regard to overall complication rate between CB and RF ablation (6.0 vs 6.7%, P = 1.00). Temporary phrenic nerve palsy did occur more often in the CB group as compared to RF, although this did not reach statistical significance (2.9 vs 0%, P = 0.058). All complications were of a temporary nature. Complication characteristics are shown in Table 3.
Arrhythmia-free survival
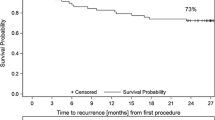
Median follow-up was 389 days (IQR 219–599) and did not differ in the CB and RF groups, respectively (P = 0.98). In the CB group, arrhythmia-free survival after a single PVI procedure was 75.2% with a median follow-up duration of 381 days (IQR 292–563). In the RF group, arrhythmia-free survival after a single PVI procedure was 57.4% with a median follow-up duration of 394 days (IQR 189–622). There was a significant difference in arrhythmia-free survival between catheter groups (log-rank test, P = 0.013) favoring CB. Arrhythmia-free survival among catheter groups is shown in Fig. 1. In univariate and multivariate analysis, RF ablation, persistent AF, and AF duration were significantly associated with decreased arrhythmia-free survival. Univariate and multivariate analysis results are displayed in Table 4. Analysis in paroxysmal AF patients only shows arrhythmia-free survival of 76.9% in CB group vs 60.7% in RF group, log-rank P = 0.019. There was no significant difference in type of atrial arrhythmia (AF/AFl/AT) recurrence between treatment groups (Chi-square test: P = 0.90), as described in Table 5.
Repeat ablation and PV reconnection
In total, 53 patients (19.7%) required a repeat ablation for recurrent atrial arrhythmias. Incidence of repeat ablation procedures was significantly lower after CB ablation as compared to RF ablation (14.7 vs 24.8%, P = 0.045). As mentioned, in all patients, all PVs were isolated in the index procedure. A total number of 211 PVs were assessed for electrical PV reconnection. In 49 of 53 patients (92.5%), PV reconnection was found, without a significant difference between CB vs RF (85.0 vs 97.0%, P = 0.15).
However, the number of reconnected PVs at repeat ablations was lower after CB ablation as compared to RF (36.8 vs 58.1%, P < 0.01), as shown in Figs. 2 and 3. The reconnection rate of the right-sided PVs was significantly higher after RF ablation as compared to CB (90.9 vs 50.0%, P < 0.01). PV reconnection is shown in Table 6.
Sensitivity analysis
We performed Cox regression analysis and plotted a Kaplan–Meier curve (Fig. 4) in patients with paroxysmal AF only, and similar results were observed, i.e., a higher arrhythmia-free survival rate in patients undergoing cryoablation. Additionally, our multivariate analysis provided insight into the independent association of the sort of ablation (cryoablation/RF ablation) and the type of AF (paroxysmal/persistent) and with arrhythmia-free survival after ablation.
Discussion
This study reports single center data on single ablation success, safety outcomes, and pulmonary vein reconnection patterns after PVI, comparing second-generation cryoballoon ablation to point-by-point contact-force radiofrequency ablation. Acute PVI was always reached using both ablation strategies. However, higher arrhythmia-free survival, lower rate of repeat ablation and a lower rate of reconnected PVs per patient were found after CB as compared to RF ablation. Both techniques had a high safety profile, and all observed complications were of a temporary nature.
Procedural characteristics
High, acute ablation success (100%) was achieved using both ablation strategies. Procedure duration was significantly shorter in CB ablation as compared to RF, in accordance with a recently reported randomized multicenter trial [6]. This might be explained by easier catheter handling and the “single shot” ablation strategy of the CB. However, adequate CB placement was checked by using fluoroscopy after contrast injections, whereas RF ablations were guided by electro-anatomic and force-controlled mapping and RF ablation. In our study, fluoroscopy time in CB ablation was comparable to RF ablation. This is in contrast with the results of a recent trial that showed a higher fluoroscopy time in the CB arm [6]. Although a trend to lower radiation dose was observed in RF ablation, we did not find a statistical significant difference as compared to CB, possibly due to a lower number of patients that were involved in our study.
Complications
The reported overall complication rate of 6.3% is in line with previous reports [6, 7]. We found no significant difference in complication rate between both ablation strategies. Furthermore, all observed complications were of temporary nature. The most common complication in the CB group was temporary phrenic nerve palsy, with a rate of 2.9%, which is considerably lower than reported in the sustained treatment of paroxysmal atrial fibrillation (STOP AF) trial (13.5%) and in line with the Fire&Ice trial (2.7%) [3, 6]. The most reported complication in the RF group was vascular access complication (2.2%). Although not different from CB ablation (2.2%), the groin complication rate was lower than the reported 4.3% in the Fire&Ice trial. In this study, there was one (0.8%) transient ischemic attack (TIA), which is comparable to other PVI techniques.
Arrhythmia-free survival
Our main finding of improved success rate after a single procedure CB ablation compared with point-by-point RF, is in line with earlier reports [8, 9]. However, in a recent multi-center randomized trial (Fire&Ice), CB ablation was shown to be non-inferior to point-by-point RF ablation regarding efficacy [6]. Success of the CB might have been compromised in this trial, due to inclusion of first-generation CB procedures [10, 11]. The design of the second-generation CB with improved circumferential cooling, results in more extensive circumferential lesions, and might have contributed to improved lesion formation [12]. In addition, previous studies demonstrated that Cryo ablation creates more extensive lesions such as, at least partial, posterior wall ablation or ablation of ganglionated plexi, which might have incremental benefits, resulting in a higher arrhythmia-free survival [13, 14]. Indeed, results of the FIRE&ICE study show a trend of higher success rate after second-generation CB as compared to the first-generation; however, the trial was not powered to test superiority of one of the catheters [6]. Of note, the proportion of patients treated with the nowadays commonly used contact-force RF catheter was small in the Fire&Ice.
In multivariate analysis, persistent AF was an independent predictor of reduced arrhythmia-free survival (hazard ratio 1.667; 95% CI 1.006–2.761; P = 0.047). Catheter ablation of persistent AF remains challenging and is associated with less favorable outcome [7, 15]. There is a need for further improvement of ablation techniques, and knowledge of substrate modification to improve AF ablation outcome in this subgroup of patients. In our present analysis, however, we performed sensitivity analyses in paroxysmal AF patients only, and observed similar results in this subgroup. Moreover, multivariate analysis showed an independent association of cryoablation with arrhythmia-free survival, with the type of AF in the multivariate model, suggesting that possibly also in persistent AF patients, cryoablation could be superior. The number of patients with persistent AF was too little, however, to perform separate analysis. The proportion of patients with persistent AF was not significantly different between treatment groups, although relatively small cohorts are always at risk for selection bias. We realize that this could be a potential limitation.
PV reconnection
Significantly less patients required a repeat ablation after CB as compared to RF (14.7 vs 24.8% P = 0.045), which was in line with the lower recurrence rate. At repeat intervention, all PVs were assessed using a circular mapping catheter. We found a lower number of reconnected PVs after CB ablation as compared to point-by-point RF, as shown in Fig. 2. This finding is in line with previous studies that showed improved lesion durability following PVI with the second-generation cryoballoon [16, 17]. Moreover, the right-sided PVs were more likely to exhibit electrical reconnection after RF ablation as compared to CB ablation. This finding might be explained by a more challenging catheter approach to the right-sided PVs, resulting in reduced durability of the RF ablation lesions. Compared to the RF catheter, the circular design of CB allows a relative, simple positioning and alignment of the catheter, which might lead to improved lesion quality. In contrast to prior reports, we did not find higher reconnection rates of the right inferior PVs and left common PVs after CB ablation [17]. Although a waiting period is constituted before assessing acute PV isolation, when starting ablation in the left pulmonary veins, the waiting period is always shorter for the right-sided PVs. Furthermore, a steerable sheath was not systematically used in the RF-ablation group, but only when deemed necessary in order to reach adequate catheter contact (> 10 g and FTI > 400 g). We did not systematically test for durable PV isolation with adenosine, which might possibly have influenced these results. It could be that cryoablation is less prone to edema-induced acute PV isolation that reconnects in the days to weeks after the procedure. Pre-procedural assessment of PV anatomy may aid in utilization of CB or RF catheter. Limitation of the CB size should be considered in case of increased PV diameter of left common PVs.
Future perspectives
AF recurrences after PVI still remain an important clinical problem. Acute PV isolation does not assure long-term electrical PV isolation, and this might partially explain AF recurrence. Future research is needed to give more insight into mechanisms of conduction recovery. Furthermore, continuous improvement of catheter design and characteristics might improve catheter handling and lesion quality. This might result in better lesion durability and improved clinical outcome.
Limitations
There are several limitations that should be considered for interpretation of the results of this study. This is a single center, prospective, non-randomized study reviewing results of primo pulmonary vein isolation procedures using second-generation CB vs contact-force radiofrequency RF in patients with atrial fibrillation. Unadjusted confounding factors might be present in the study, i.e., no data on PV reconnection in patients without recurrence of arrhythmia are available. Pulmonary vein reconnection was only assessed at repeat ablation procedures, and therefore, by definition in symptomatic patients with AF recurrence.
Conclusions
In this single center, prospective study, improved arrhythmia-free survival and more durable PV isolation is seen after PVI using second-generation CB as compared to contact-force RF, in patients with drug-refractory paroxysmal AF. Complication rates for both ablation techniques are low, and all occurred complications were of a temporary nature.
Change history
06 March 2018
The name of the author Jaap Jan J. Smit was rendered wrongly in the original publication. The original article has been corrected.
References
Haissaguerre M, Jais P, Shah DC et al (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659–666
Calkins H, Kuck KH, Cappato R et al (2012) HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 9:632.e21–696.e21
Packer DL, Kowal RC, Wheelan KR et al (2013) Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 61:1713–1723
Gal P, Buist TJ, Smit JJ et al (2017) Effective contact and outcome after pulmonary vein isolation in novel circular multi-electrode atrial fibrillation ablation. Neth Heart J 25:16–23
Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ (2015) Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 17:370–378
Kuck KH, Brugada J, Furnkranz A et al (2016) Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 374:2235–2245
Verma A, Jiang CY, Betts TR et al (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372:1812–1822
Aryana A, Singh SM, Kowalski M et al (2015) Acute and long-term outcomes of catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: A multicenter experience. J Cardiovasc Electrophysiol 26:832–839
Hunter RJ, Baker V, Finlay MC et al (2015) Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: A randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (the cryo versus RF trial). J Cardiovasc Electrophysiol 26:1307–1314
Furnkranz A, Bordignon S, Dugo D et al (2014) Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 25:840–844
Pandya B, Sheikh A, Spagnola J, Bekheit S, Lafferty J, Kowalski M (2016) Safety and efficacy of second-generation versus first-generation cryoballoons for treatment of atrial fibrillation: a meta-analysis of current evidence. J Interv Card Electrophysiol 45:49–56
Coulombe N, Paulin J, Su W (2013) Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J Cardiovasc Electrophysiol 24:919–925
Kenigsberg DN, Martin N, Lim HW, Kowalski M, Ellenbogen KA (2015) Quantification of the cryoablation zone demarcated by pre- and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28-mm second-generation cryoballoon. Heart Rhythm 12:283–290
Yorgun H, Aytemir K, Canpolat U, Sahiner L, Kaya EB, Oto A (2014) Additional benefit of cryoballoon-based atrial fibrillation ablation beyond pulmonary vein isolation: modification of ganglionated plexi. Europace 16:645–651
Brooks AG, Stiles MK, Laborderie J et al (2010) Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 7:835–846
Reddy VY, Sediva L, Petru J et al (2015) Durability of pulmonary vein isolation with cryoballoon ablation: results from the sustained PV isolation with arctic front advance (SUPIR) study. J Cardiovasc Electrophysiol 26:493–500
Aryana A, Singh SM, Mugnai G et al (2016) Pulmonary vein reconnection following catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: results of a multicenter analysis. J Interv Card Electrophysiol 47:341–348
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
The original version of this article was revised: The name of the author Jaap Jan J. Smit was rendered wrongly.
Rights and permissions
About this article
Cite this article
Buist, T.J., Adiyaman, A., Smit, J.J.J. et al. Arrhythmia-free survival and pulmonary vein reconnection patterns after second-generation cryoballoon and contact-force radiofrequency pulmonary vein isolation. Clin Res Cardiol 107, 498–506 (2018). https://doi.org/10.1007/s00392-018-1211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1211-9