Abstract
Background
Heart failure (HF) with reduced ejection fraction (HFrEF) has a worse prognosis than HF with preserved EF (HFpEF). The study aimed to evaluate whether different comorbidity profiles of HFrEF- and HFpEF-patients or HF-specific mechanisms contribute to a greater extent to this difference.
Methods
We linked data from two health insurances to data from a cardiology clinic hospital information system. Patients with a hospitalization with HF in 2005–2011, categorized as HFrEF (EF < 45%) or HFpEF (EF ≥ 45%), were propensity score (PS) matched to controls without HF on comorbidites and medication to assure similar comorbidity profiles of patients and their respective controls. The balance of the covariates in patients and controls was compared via the standardized difference (SDiff). Age-standardized 1-year mortality rates (MR) with 95% confidence intervals (CI) were calculated.
Results
777 HFrEF-patients (1135 HFpEF-patients) were PS-matched to 3446 (4832) controls. Balance between patients and controls was largely achieved with a SDiff < 0.1 on most variables considered. The age-standardized 1-year MRs per 1000 persons in HFrEF-patients and controls were 267.8 (95% CI 175.9–359.8) and 86.1 (95% CI 70.0–102.3). MRs in HFpEF-patients and controls were 166.2 (95% CI 101.5–230.9) and 61.5 (95% CI 52.9–70.1). Thus, differences in MRs between patients and their controls were higher for HFrEF (181.7) than for HFpEF (104.7).
Conclusions
Given the similar comorbidity profiles between HF-patients and controls, the higher difference in mortality rates between HFrEF-patients and controls points more to HF-specific mechanisms for these patients, whereas for HFpEF-patients a higher contribution of comorbidity is suggested by our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) ranks among the most common and severe diseases in industrialized countries [1]. It affects 2–3% of the adult population in the Western world, with an increase expected during the next years due to aging [2]. A recently published epidemiological study which was based on German healthcare claims data estimated an overall prevalence of 4% and an incidence of 655/100.000 (corresponding to 524.000 cases each year in Germany) [3]. Results from the same study also indicated, that despite the potential fatality of HF, less than half of all patients are treated according to recommended guidelines [4].
Based on their ejection fraction (EF) and other echocardiographic findings, patients with HF are classified as having either preserved EF (HFpEF) or reduced EF (HFrEF). Since 2016, the European guidelines introduced also a third group with mid-range left-ventricular ejection fraction (LVEF 40–49%). However, studies conducted in the last years used varying thresholds to define HFpEF, with cut-off values ranging from > 40 to > 55%.
The epidemiology of HFpEF and HFrEF is well described. Risk factors for HF in general include age, male sex, obesity, hypertension and ischemic heart disease [5,6,7]. Heart failure is associated with high rates of mortality, ranging between 10% 1 month after incident diagnosis and up to 60% 5 years later [1, 8]. The proportion of HFpEF among HF-patients is frequently estimated at around 50%, although it varies between 33% and up to 84% across community-based studies [9]. There is evidence for significant higher all-cause mortality rates in HFrEF-patients with as compared to HFpEF-patients [5, 10]. However, varying mortality rates have been reported for these subgroups, possibly also due to methodological differences between studies [11, 12]. Comorbidities are very common in HF [13, 14], including arterial hypertension, which is present in 42–64% of HF-patients. Non-cardiovascular comorbidities such as chronic obstructive pulmonary disease [15, 16], anemia [17], and mental disorders are also common [18]. While recent studies have provided evidence of different underlying pathophysiological processes in patients with HFrEF and HFpEF [19,20,21], it remains unclear, whether differences in mortality can be attributed to HF-specific mechanisms or to different comorbidity profiles in both subtypes of HF.
We aimed to compare mortality rates between patients with HFrEF or HFpEF and propensity score matched controls without HF but with a similar comorbidity profile to each HF subgroup, as well as similar age, gender and overall health. In addition, we directly compared the mortality rate between propensity score matched HFrEF-patients and HFpEF-patients with similar comorbidities, age and gender.
Methods
Study design
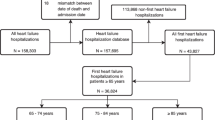
We conducted a retrospective cohort study of HF-patients hospitalized during the period 2005–2011 in the Clinic of Cardiology of the Oldenburg Hospital and followed them up to death, loss-to-follow-up or end of the study period. For comparison purposes, we identified patients hospitalized without HF during the same period in the region of the hospital (Lower Saxony and Bremen).
We used a deterministically linked data set comprising claims data from two German statutory health insurances (SHIs: AOK Bremen/Bremerhaven, AOK Niedersachsen) and the hospital information system (HIS) from the Clinic of Cardiology of the Oldenburg Hospital, mentioned above.
All analyses were based on the years 2004–2010 (with data from the AOK Niedersachsen), and from 2004 to 2011 (data from the AOK Bremen/Bremerhaven), respectively.
Study population
HF patients
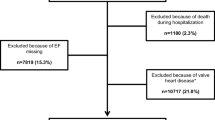
All insurants with a hospitalization during 2005–2011 with a main or secondary HF discharge diagnosis as well as data available on EF as documented in the HIS were eligible for the study. HF was based on the following codes of the German Modification of the International Classification of Diseases 10th Revision (ICD-10-GM): (a) heart failure (I50.-), (b) hypertensive heart disease with (congestive) HF (I11.0-), (c) hypertensive heart and renal disease with (congestive) HF (I13.0-), (d) hypertensive heart and renal disease with both (congestive) HF and renal disease (I13.2-), or (e) other congestive HF codes from the Elixhauser comorbidity measure (I09.9-, I25.5-, I42.0-, I42.5-, I42.6-, I42.7-, I42.8-, I42.9-, I43-, P29.0-). The first hospitalization with a diagnosis of HF had to be preceded by a continuous 365-day insurance period (baseline period) without any HF-related hospitalization as documented in the SHI data. Cohort entry was defined as the date of the first hospital admission during which a HF diagnosis was documented. Cohort exit was defined either as the end of the insurance period, the date of death or the end of the follow up (31.12.2011). Depending on their EF rate, patients with HF were categorized as having HFpEF (EF ≥ 45%) or HFrEF (EF < 45%). An EF of 45% was chosen as cut-off point, since this was the cut-off used in recent large international RCTs in HFpEF patients (e.g. TOPCAT, I-Preserve [22, 23]) and in lack of a commonly accepted standardized definition when our study protocol was developed. Overall, 2473 patients with HF diagnosis were identified in the HIS database, of whom 1986 had data on the EF available and were included in the final analyses.
Controls
Up to five controls were identified in the SHI data and PS-matched to each HFrEF/HFpEF patient. Controls had to meet the same inclusion and exclusion criteria as HF cases yet without any HF diagnosis during the first hospitalization, and during the 365-day baseline period preceding the hospitalization. Cohort entry for controls was defined as the date of the first hospital admission during the study period. Cohort exit for controls was defined either as the end of the insurance period, the date of death, an inpatient main or secondary discharge diagnosis of HF, a confirmed outpatient diagnosis of HF or the end of the follow up (31.12.2011).
Patient characteristics
Patient characteristics at baseline period for HF-cases and potential controls were ascertained preceding cohort entry. Comorbidities were identified using confirmed outpatient diagnoses as well as hospital main and secondary discharge diagnoses. Coding of cardiovascular and non-cardiovascular comorbidities of HF, diagnostic and therapeutic procedures as well as of medications used in the therapy of HF, are shown in supplemental Table S1.
Propensity score matching
Two PS matched cohorts were built for the comparison between HF patients and matched controls. The PS was defined as the estimated probability of being HFpEF (or HFrEF) vs. not having HF, calculated for all HF patients and their potential controls by logistic regression analyses. The following independent variables were included in the model: sex, age at and year of cohort entry, all diseases of the Elixhauser comorbidity measure (excluding congestive heart failure), sleep apnea, stable angina pectoris, acute coronary syndrome, ischemic stroke, prior (12 months before cohort entry) percutaneous coronary intervention or coronary artery bypass graft, number of hospitalizations during the 365-day period, number of office visits to specialists for internal medicine, and the current medication (see Suppl. Table 1 for more details). A greedy matching strategy with 100,000 strata at the initial step was used to match HF patients to their potential controls [24].
The balance of the covariates in patients with HF and their controls prior and after the matching was compared via the standardized difference (SDiff). The SDiff compares the difference in means in units of the pooled standard deviation and it is not influenced by sample size, which allows the comparison of the relative balance of variables measured in different units. A SDiff > 0.1 after the matching was considered imbalanced.
The PS matching was repeated for direct comparisons between patients with HFpEF and HFrEF. The PS was defined as the estimated probability of being HFrEF vs. being HFpEF. The same variables as for the other two PS matched cohorts plus the number of hospitalizations due to HF were used to calculate the PS. Since the populations of patients with HFpEF and HFrEF were of similar size, a 1:1 greedy matching was performed.
Statistical analyses
The mortality risk of HFrEF-/HFpEF-patients and controls were calculated by dividing the number of deaths during the study period by the sum of the person-times in each cohort. Confidence intervals (CIs) were estimated using the substitution method, assuming a Poisson distribution of the number of events. Age-standardized 1-year mortality rates were calculated separately for men and women, based on the German population as of 1st of January, 2010. CIs for the standardized mortality estimates were calculated according to the method proposed by Chiang [25]. As the main analysis of the study was the calculation of mortality rates in HFrEF and HFpEF patients and their controls in propensity score matched cohorts, the Cox regression mainly served as an exploratory analysis to quantify the influence of the single comorbidities on mortality and was therefore not adjusted for multiple testing. To determine factors associated with mortality, a Cox regression model of mortality was performed, including the following independent variables in each model: presence of HFpEF (or HFrEF), sex, age at and year of cohort entry, all diseases of the Elixhauser comorbidity measure (excluding congestive heart failure), sleep apnea, stable angina pectoris, acute coronary syndrome, ischemic stroke, hyperlipidemia, prior percutaneous coronary intervention, prior coronary artery bypass graft.
The Cox regression analysis was repeated for the PS matched cohort of patients with HFrEF and HFpEF. Further, Kaplan–Meier curves were generated to compare the survival rates of patients with HFpEF and HFrEF. A log-rank test (p < 0.05) was used to test for differences in the survival curves. All analyses were conducted with SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Ethics
The approval of the study was based on the endorsement of the project by the SHIs and their responsible superior authorities based on § 75 of volume 10 of the German Social Code. Informed patient consent was not required. Two authors (OR, DE) had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Results
Main analysis
Patient characteristics
For 1986 patients, data on the EF was available, with 811 (40.8%) patients having HFrEF and 1175 (59.2%) having HFpEF (for excluded patients due to missing data on the EF please see supplemental Table S2). Patients with HFpEF were older than those with HFrEF (73.8 ± 11.7 vs. 69.3 ± 12.7 years) and fewer were below 50 years among HFpEF patients compared to HFrEF (4.6 vs. 8.8%). Both subgroups had similar heart rates (HFpEF patients: 69.1 ± 16.9 beats per minute (bpm) vs. HFrEF patients: 64.7 ± 12.5 bpm) and body mass indices (HFpEF patients: 28.1 ± 7.8 vs. HFrEF patients: 29.0 ± 14.2). Mean EF in HFrEF patients was 32.1 ± 7.2 vs. 58.0 ± 9.4 in HFpEF patients. The distribution of the Elixhauser comorbidity measure was similar for patients with HFrEF (4.2 ± 2.5) and for patients with HFpEF (4.4 ± 2.4). For a full characterization of the study population please see supplemental Table S3. In total, 37 patients (1.9%) were lost to follow-up due to other reasons than death. Hereof, 20 patients suffered from HFpEF (mean age 60.1 ± 17.7 years) and 17 were patients with HFrEF (mean age 55.4 ± 18.4 years.)
In the PS matched cohorts, 777 HFrEF-patients were matched to 3446 controls and 1135 HFpEF-patients were matched to 4832 controls. As shown in Table 1, patients and controls were balanced in regard to demographics and comorbidities except for valvular heart diseases (both cohorts) and cardiac arrhythmias (HFpEF matched cohort) with SDiffs > 0.1. (The medication status of patients and matched controls can be seen in Supplemental Table 4).
Mortality rates
In the overall study population, mortality rates were 228.7 (95% CI 205.6–253.7) per 1000 person years (pys) among HFrEF patients and 186.5 (95% CI 169.6–204.6) among HFpEF patients. In both patient groups, mortality rates increased steadily with advancing age and reached a peak for patients aged 90 years and older (HFrEF: 1119.2 per 1000 pys, 95% CI 558.7–2002.5; HFpEF: 697.7, 95% CI 516.2–922.5). Among HFrEF patients, females had a higher overall mortality rate than males (253.3, 95% CI 210.0–302.9 vs. 218.0, 95% CI 191.2–247.6). Similarly, among HFpEF patients, females had a higher overall mortality rate than males (215.9, 95% CI 189.9–244.5 vs. 159.4, 95% CI 138.1–183.1).
Table 2 shows the age-stratified mortality rates in the PS-matched cohorts. HFrEF patients and HFpEF patients featured substantially higher mortality rates than their corresponding matched controls.
The age-standardized 1-year mortality rate per 1000 persons was 218.8 (95% CI 160.2–277.3) across all HF-cases in the PS matched cohorts. Patients with HFrEF had a higher rate than patients with HFpEF (267.8, 95% CI 175.9–359.8 vs. 166.2, 95% CI 101.5–230.9). The rates in their corresponding controls were substantially lower (controls for HFrEF patients: 86.1, 95% CI 70.0–102.3; controls for HFpEF patients: 61.5, 95% CI 52.9–70.1). Substantial differences between males and females were neither found among patients nor among controls.
Mortality risks
The adjusted risks for mortality in patients and their respective matched controls are depicted in Table 3.
Direct comparison between both HF groups
Patient characteristics
For direct comparisons between both HF groups, 680 HFrEF-patients with were PS-matched with 680 HFpEF-patients with (see Table 4). Both subgroups were well balanced with no SDiffs > 0.1 on any variable considered for PS matching.
Mortality risks
Table 5 shows the adjusted mortality risks of HFpEF-patients compared with PS matched HFrEF-patients. Higher age, the presence of chronic pulmonary disease other than COPD or asthma, diabetes, renal failure and peptic ulcer were associated with an increased mortality.
Figure 1 shows the Kaplan–Meier curves for both heart failure disease entities. Among HFrEF-patients, the estimated survival probability dropped to 0.5 after 40 months, whereas for HFpEF-patients, it dropped to a minimum level of 0.52 after 64 months. The log-rank test statistic revealed significant differences between both entities (p < 0.001).
Discussion
While it is well known that HF is associated with higher mortality rates, it remains unclear, whether patients with HF die due to the underlying HF condition or due to other contributing factors (e.g. comorbidities). Especially HFpEF is still a somewhat enigmatic syndrome, where, despite several clinical trials conducted, heart failure therapies as yet failed in showing convincing evidence to alter the natural history of disease. In fact, due to the high burden of comorbidities, treatment strategies, where clinicians target comorbidities, in addition to the underlying cardiac dysfunction, have been advocated as being particularly relevant for patients with HFpEF [26]. Thus, in the present study, we compared mortality rates among hospitalized patients with HFrEF, hospitalized patients with HFpEF and patients with similar comorbidity profiles but without HF diagnosis. In both HF groups we found substantially decreased survival rates compared to controls. This is in accordance with previous findings of higher mortality rates in HFpEF patients as compared to age- and sex-matched controls without HF in the community [8]. However, our analyses extend these findings since our comparisons were not only based on similar distributions of age and sex, but were also made between patients who additionally had similar comorbidity profiles, treatment regimens and parameters of general health. As the main finding of our study, the PS-adjusted mortality differences suggest that comorbidities have a higher impact in the mortality of patients with HFpEF as compared to patients with HFpEF (as further explained below).
Our findings have several implications. First of all, the results underscore that HFrEF and HFpEF are severe conditions which are associated with substantial mortality. Second, all-cause mortality was higher in patients with HFrEF than in those with HFpEF. Thus our results confirm previously reported high mortality rates among patients with HFrEF and among patients with HFpEF [5, 10]. HFrEF patients revealed a substantially decreased life expectancy with 50% of patients having deceased after 40 months while in HFpEF patients 52% of patients were still alive after 64 months of observation.
Comparing the survival of HFrEF and HFpEF patients with their respective controls, who were similar with respect to sociodemographic characteristics as well as patterns of comorbidities and medications, the difference in mortality was more pronounced in HFrEF. In reverse, this suggests that comorbidity had a higher contribution to mortality in HFpEF patients than in those with HFrEF. This was also supported by the comparisons of the age-standardized 1-year mortality rates in HFpEF and HFrEF patients compared to their comorbidity-matched controls without HF. While the difference in the 1-year mortality rate between HFrEF patients and their controls was 182/1000 persons, it was only 105/1000 persons between HFpEF patients and their controls. This suggests a higher contribution of comorbidity to mortality in HFpEF patients as compared with HFrEF patients.
Several parameters confirmed our study sample to be representative for the population of HF patients attending to the hospital care sector. HFpEF occurred more frequently than HFrEF with proportions of 59.2% vs. 40.8%. This is in accordance with occurrence rates that have been previously described [10, 27]. Similarly, patients with HFrEF were more frequently male and of younger age than patients with HFpEF [10]. Also, our results are in agreement with previous findings regarding clinical characteristics of the included patients. Both subgroups of patients were highly comorbid, and the frequency of comorbidities were comparable to those reported earlier [28,29,30], with arterial hypertension representing the most common one in patients with HF, being more frequent among patients with HFpEF than among patients with HFrEF. Moreover, the comorbidity profile of the patients in this study also fit well with figures obtained from previous epidemiological studies on HF that were based on healthcare claims data [3]. We also found high proportions of diabetes and elevated BMI in both subgroups of HF patients who had similar BMIs and with similar BMI [5]. Thus, regarding the sociodemographic and clinical characteristics, the study sample seems representative of the HF patient population in this care sector. Also, the results of a recently published study that was based on The Health Improvement Network (THIN) primary care database are comparable to our findings, with similar effects of hyperlipidemia, diabetes and renal impairment on mortality in patients with incident HF [31].
So far, only few studies have investigated the differential associations between comorbidity patterns and outcomes in patients with HFrEF or HRpEF and were mostly restricted to one of both subtypes [32,33,34,35]. The present study provides new insights by the inclusion of both HF subtypes and the direct and indirect comparison of the associated comorbidities. In addition, our study was based on a linked dataset combining detailed clinical data from the HIS with long follow up information and a comprehensive patient history from the health insurance data, which combines the advantages of using claims databases and hospital information for conducting epidemiological research. For the evaluation of the role of comorbidities in mortality among HF patients, we used a sophisticated PS matching approach that allowed us to generate comparison groups largely similar to the HF populations under study. In fact, we think that the novelty of our study is not inherent in the results—some of which were already known—but rather in the methodological approach that was developed to obtain them, and which has not been chosen before.
Yet some limitations should be kept in mind when interpreting our results. First, approximately 20% of patients were excluded due to lack of information on the EF, which might bear the risk of bias or non-random drop-outs. As information on the EF was lacking for these patients, the underlying form of HF remains unknown. Based on the age and gender distribution, the age might suggest a preponderance of HFrEF patients while the proportion of males would rather point to a preponderance of HFpEF cases. Importantly, however, taking the total Elixhauser score as well as the rates of its single entities among excluded patients into account, our data do not provide an indication that excluded patients had more or less comorbidities than included patients. Therefore a systematic sampling error due to the exclusion of these patients is unlikely. Also concerning the EF, it should be noted that our study was conceptualized before the EF boundaries for a third group with mid-range left-ventricular EF (40–49%) was established. Thus, this third group was not excluded in our analyses. When designing the study, we have deliberately chosen the presented cutoff of 45% as it had been used in various clinical trials then [9, 22, 23], and even a recently published study relied on the same cutoff [36]. However, future studies that are based on health claims data should certainly consider this third group as well.
Further limitations refer to the nature of claims data as stored in the SHI data. Data on lifestyle-related factors, that might have an impact on the morbidity and mortality of the patients (e.g. smoking habits) are not stored in SHI data and thus could not be taken into account. The comorbidity profiles of our study population were ascertained by the presence of ICD-10-GM diagnoses, and the severity of comorbid diseases could not be disentangled, potentially leading to residual confounding. In this study, however, we tried to compensate this flaw by the comprehensive PS matching approach and by factoring in the medications of the patients under study. In this context, one could also object that putting all comorbidities into one single PS model does not reflect clinical reality by leading to a potential underestimation of the pathophysiological contribution of each single comorbidity to mortality. However, it should be noted that, although the comorbidities were all grouped together in the PS model, the single comorbidities were well balanced after matching with standardized differences equal or below 0.1 on all comorbidities considered, except for valvular heart diseases and cardiac arrhythmias, as was shown in the results. Thus, the possibly differing pathophysiological weights of the comorbidities can be regarded as being accounted for in this study, diminishing the clinical contribution of our data only marginally. Similarly, a further potential concern relates to the question of the external validity of the Elixhauser measure, since it rather factors in the mere presence of a comorbid disease but not its severity, which might of course vary among patients. While the Elixhauser measure has been repeatedly proven to be valid in the analyses of administrative data, especially when investigating mortality [37,38,39], this still is a potential flaw. We have tried to compensate for this by not only including the ICD-10 codes but also medications into the model, which should have further decreased the risk that the considered groups in our study differed substantially regarding the severity of the comorbidities.
Also, with reference to the limitations of our study sample, in an optimal study design control patients without HF would have been chosen among those, who were hospitalized in the very same hospital, to enable comparability regarding further parameters that are not explicitly stored in our database (e.g. socio-economic status). Yet since we are not able to identify unique hospitals in our database for data protection reasons, this factor could not be considered for the selection of our controls. However, we do not expect substantial biases given that the catchment area of the Oldenburg Hospital is very large and covers many regions in Lower Saxony and Bremen. To sum up, given the similar comorbidity profiles between HF-patients and controls, the higher difference in mortality rates between HFrEF-patients and controls points more to HF-specific mechanisms for these patients, whereas for HFpEF-patients a higher contribution of comorbidity is suggested by our results. Further studies are needed, especially taking further aspects (e.g. lab parameters, life-style related behaviour) into account.
References
Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8:30–41
Zarrinkoub R, Wettermark B, Wandell P et al (2013) The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 15:995–1002
Stork S, Handrock R, Jacob J et al (2017) Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 106:913–922
Stork S, Handrock R, Jacob J et al (2017) Treatment of chronic heart failure in Germany: a retrospective database study. Clin Res Cardiol 106:923–932
Brouwers FP, de Boer RA, van der Harst P et al (2013) Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013 34:1424–1431
Kannel W (2000) Incidence and epidemiology of heart failure. Heart Fail Rev 5:167–173
Lee GKM, Lee LC, Liu CWY et al (2010) Framingham risk score inadequately predicts cardiac risk in young patients presenting with a first myocardial infarction. Ann Acad Med Singap 39:163–167
Lam CSP, Donal E, Kraigher-Krainer E, Vasan RS (2011) Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 13:18–28
in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail 18: 54–65
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259
Paulus WJ, van Ballegoij JJM (2010) Treatment of heart failure with normal ejection fraction an inconvenient truth! J Am Coll Cardiol 55:526–537
Somaratne JB, Berry C, McMurray JJV, Poppe KK, Doughty RN, Whalley GA (2009) The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail 11:855–862
Fonarow GC, Stough WG, Abraham WT et al (2007) Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure—a report from the OPTIMIZE-HF registry. J Am Coll Cardiol 50:768–777
Yancy CW, Lopatin M, Stevenson LW et al (2006) Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function—a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 47:76–84
Dahlstrom U (2005) Frequent non-cardiac comorbidities in patients with chronic heart failure. Eur J Heart Fail 7:309–316
Rutten FH, Cramer MJM, Lammers JWJ, Grobbee DE, Hoes AW (2006) Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J Heart Fail 8:706–711
Hsich EM, Grau-Sepulveda MV, Hernandez AF et al (2012) Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J 163:430-U380
Jiang W, Alexander J, Christopher E et al (2001) Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 161:1849–1856
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. New Engl J Med 350:1953–1959
Zile MR, Gottdiener JS, Hetzel SJ et al (2011) Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 124:2491–2501
Massie BM, Carson PE, McMurray JJ et al (2008) Irbesartan in patients with heart failure and preserved ejection fraction. New Engl J Med 359:2456–2467
Solomon SD, Claggett B, Lewis EF et al (2016) Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 37:455–462
Parsons L (2001) Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SUGI26. Paper 214–26
Chiang C (1967) Standard error of the age-adjusted death rate. Vital Statistics—Special Reports, Selected Studies 47
Mentz RJ, Kelly JP, von Lueder TG et al (2014) Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 64:2281–2293
Hogg K, Swedberg K, McMurray J (2004) Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 43:317–327
Centres for Medicare & Medicaid Services (2012) Chronic conditions among medicare benificiaries—Chartboo, 2012 Edition. Centes for Medicare & Medicaid Services, Baltimore
Tschope C, Birner C, Bohm M et al (2018) Heart failure with preserved ejection fraction: current management and future strategies. Clin Res Cardiol 107:1–19
van Deursen VM, Urso R, Laroche C et al (2014) Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 16:103–111
Ruigomez A, Michel A, Martin-Perez M, Rodriguez LAG (2016) Heart failure hospitalization: an important prognostic factor for heart failure re-admission and mortality. Int J Cardiol 220:855–861
Ather S, Chan W, Bozkurt B et al (2012) Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 59:998–1005
Felker GM, Shaw LK, Stough WG, O’Connor CM (2006) Anemia in patients with heart failure and preserved systolic function. Am Heart J 151:457–462
MacDonald MR, Petrie MC, Varyani F et al (2008) Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure. Eur Heart J 29:1377–1385
Smith DH, Thorp ML, Gurwitz JH et al (2013) Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction the cardiovascular research network PRESERVE study. Circ Cardiovasc Qual Outcomes 6:333–342
Lupon J, Diez-Lopez C, de Antonio M et al (2017) Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail 19:1615–1623
Chang HJ, Chen PC, Yang CC, Su YC, Lee CC (2016) Comparison of Elixhauser and Charlson methods for predicting oral cancer survival. Medicine 95
Li B, Evans D, Faris P, Dean S, Quan H (2008) Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res 8
Sharabiani MTA, Aylin P, Bottle A (2012) Systematic review of comorbidity indices for administrative data. Med Care 50:1109–1118
Acknowledgements
The authors are grateful to the statutory health insurances AOK Bremen/Bremerhaven and AOK Niedersachsen for contributing data to the study. This study was supported by Bayer AG, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A Michel and D Vizcaya are full-time employees of Bayer AG. J. Berg is a full-time employee of the statutory health insurance AOK Bremen/Bremerhaven. S. Eberhard is full-time employee of the statutory health insurance AOK Niedersachsen. O. Riedel, D. Enders, N. Schlothauer, A. Elsässer and C. Ohlmeier have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riedel, O., Ohlmeier, C., Enders, D. et al. The contribution of comorbidities to mortality in hospitalized patients with heart failure. Clin Res Cardiol 107, 487–497 (2018). https://doi.org/10.1007/s00392-018-1210-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1210-x