Abstract
About 50% of all patients suffering from heart failure (HF) exhibit a reduced ejection fraction (EF ≤ 40%), termed HFrEF. The others may be classified into HF with midrange EF (HFmrEF 40–50%) or preserved ejection fraction (HFpEF, EF ≥ 50%). Presentation and pathophysiology of HFpEF is heterogeneous and its management remains a challenge since evidence of therapeutic benefits on outcome is scarce. Up to now, there are no therapies improving survival in patients with HFpEF. Thus, the treatment targets symptom relief, quality of life and reduction of cardiac decompensations by controlling fluid retention and managing risk factors and comorbidities. As such, renin–angiotensin–aldosterone inhibitors, diuretics, calcium channel blockers (CBB) and beta-blockers, diet and exercise recommendations are still important in HFpEF, although these interventions are not proven to reduce mortality in large randomized controlled trials. Recently, numerous new treatment targets have been identified, which are further investigated in studies using, e.g. soluble guanylate cyclase stimulators, inorganic nitrates, the angiotensin receptor neprilysin inhibitor LCZ 696, and SGLT2 inhibitors. In addition, several devices such as the CardioMEMS, interatrial septal devices (IASD), cardiac contractility modulation (CCM), renal denervation, and baroreflex activation therapy (BAT) were investigated in different forms of HFpEF populations and some of them have the potency to offer new hopes for patients suffering from HFpEF. On the basic research field side, lot of new disease-modifying strategies are under development including anti-inflammatory drugs, mitochondrial-targeted antioxidants, new anti-fibrotic and microRNA-guided interventions are under investigation and showed already promising results. This review addresses available data of current best clinical practice and management approaches based on expert experiences and summarizes novel approaches towards HFpEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
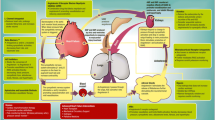
In the US, with 5.7 million adults suffering from heart failure (HF) in 2012 [1], total annual costs for HF-related therapy were estimated amounting to 60 billion dollars [2]. About a half of patients exhibit a reduced ejection fraction (i.e. EF < 40%; the so-called HFrEF), whereas the other half experiences symptoms of heart failure despite a preserved EF (i.e. EF ≥ 50%, the so-called HFpEF). For Europe, an estimated prevalence of 1% of HFpEF in the general population accounts for 3–4 millions of affected patients. In these patients, symptoms primarily originate from impaired filling of the left ventricle (LV). However, as some patients exhibit concomitant systolic impairment, an isolated diastolic dysfunction does not sufficiently characterize this syndrome. Isolated LV diastolic dysfunction is rare and most likely not the only problem, and can include over the time left atrial enlargement and dysfunction, pulmonary hypertension as well as right ventricular impairment. According to European recommendations, the following triad establishes the presence of HFpEF: (1) clinical symptoms of heart failure; (2) EF ≥ 50%; (3) diastolic dysfunction [3]. However, application of this “simple definition” both in clinical research and practice remains difficult [4]. We thus need more appropriate criteria to identify those patients, e.g. including information on stroke volume and its change during exercise [5] or abnormal echocardiographic strain values [6]. An important aspect in the management of patients with suspected HFpEF is the identification of common treatable aetiologies including risk factors, comorbidities and specific cardiomyopathies (Fig. 1).
HFpEF predominantly concerns older patients whose left ventricular diameter is small. However, numerous other cardiac (e.g. disturbed chronotropy, impaired ventricular coupling, and right ventricular impairment) and non-cardiac abnormalities (pulmonary, renal and metabolic comorbidities) may contribute to this syndrome, which is very heterogeneous with regard to its aetiology and phenotypes. Despite a “normal” EF, patients with HFpEF exhibit an impaired prognosis [7,8,9]. There are differences between the epidemiology and the aetiology of HFpEF and HFrEF. Patients with HFpEF are older, more often female, show less myocardial ischemic events, but display risk factors such as obesity, hypertension and diabetes mellitus [7,8,9,10]. Although there is hardly any difference between mortality and hospitalisation rates of HFpEF and HFrEF, both types of heart failure differ regarding to treatment response to neuroendocrine antagonists. While there is solid evidence on the prognostic effects of treatment with renin–angiotensin–aldosterone system (RAAS) blockers and beta-blockers in HFrEF, no such effects were yet demonstrated for these substance classes in patients with HFpEF. The reasons may lie in the incomplete understanding of its pathophysiology, and different definitions and classifications. The latest recommendation in this respect is to define HFpEF not as a solely cardiac disease, but as a systemic heterogeneous syndrome.
While there have been no breakthrough clinical trials showing a reduction in mortality, it is still important to develop management strategies for these patients to reduce risk factors, cardiac decompensations advents and to improve quality of life. This is in general agreement with the current ESC-heart failure guidelines, which lack of practical details [3, 4]. We aim to propose management strategies, helpful for decisions in the daily routine based on expert experiences. Thus, in this review paper, we (a) summarize the current recommendations for the management of HFpEF (Fig. 1), (b) discuss the implications on best clinical practice of studies investigating therapeutic approaches towards HFpEF (Fig. 2), and (c) present an overview on potential new innovative and disease-modifying treatment options for the future (Fig. 3).
Current management of risk factors, comorbidities and symptoms in HFpEF. ARB angiotensin receptor blocker, ACEi angiotensin-converting enzyme inhibitor, MRA mineralocorticoid receptor antagonist, HFpEF heart failure with preserved ejection fraction, CCB calcium channel blocker, RF risk factor; modified from [22]
Development of new medical and non-medical treatment strategies in HFpEF. ARB angiotensin receptor blocker, ACEI angiotensin-converting enzyme inhibitor, MRA mineralocorticoid receptor antagonist, ARNI angiotensin receptor and neprilysin inhibitor, HFpEF heart failure with preserved ejection fraction, AGEs advanced glycation end products, SGLT2 sodium–glucose cotransporter-2, sGC soluble guanylate cyclase, LOXl2 lysyl oxidase-like 2 antibody, RyR2 ryanodine receptor 2, IASD interatrial septal device, eNOS endothelial NO-synthase, NCX: CRT cardiac resynchronization therapy, iv intravenously, CCM cardiac contractility modulation, RDT renal denervation therapy, BAT baroreflex activation therapy
Current management of risk factors, comorbidities and symptoms in HFpEF
HFpEF is accompanied by a large variety of risk factors and comorbidities (Fig. 1). Behind aging and female gender hypertension, obesity and diabetes mellitus belongs to the most important risk factors and comorbidities in HFpEF. A successful treatment of HFpEF requires a thorough understanding of these contributing entities, including also conditions such as cardiac ischemia, sleep apnoea, renal dysfunction, deconditioning, anaemia and iron deficiency, and sarcopenia. Older patients (> 67 years), with chronic kidney disease, pulmonary hypertension, right ventricular dysfunction and high filling indices are high-risk patients [11]. Death in HFpEF is attributable to non-cardiac causes in more than 60%, but only in less than 35% of patients with HFrEF. Hence, the current guidelines emphasize stringent cardiovascular risk management as the principal approach to improved symptoms and possibly prognosis, although hard evidence for this perspective is scarce [3, 4]. Despite the common mantra that HFpEF has no effective treatments, closer inspection of HFpEF clinical trials reveals that several of the drugs tested are associated with benefits in exercise capacity and quality of life, and reduction in heart failure hospitalization.
Comorbid conditions impose serious threats by triggering rehospitalisation in HFpEF [12]. This is particularly important for uncontrolled systolic and diastolic blood pressure [13], which explains the importance of angiotensin-converting enzyme inhibitors (ACEi), angiotensin-2 type-1 receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA), diuretics, CCB and beta-blockers as pivotal preventive strategies. These substance classes can induce a regression of left ventricular hypertrophy, which in turn may ameliorate diastolic heart failure [14,15,16]. The selection of the anti-hypertensive drugs complies with the recommendations of the current hypertension guidelines [17]. However, it has not been shown that regression of a left ventricular hypertrophy improves long-term prognosis. Since myocardial ischemia can further worsen HFpEF, appropriate diagnostic measures and revascularization is needed. Obesity, diabetes mellitus, anaemia, sleep apnoea, pulmonary hypertension, obstructive lung disease, deconditioning, depression and kidney failure constitute important comorbidities disturbing the ventricular coupling with the vessel system. With respect to iron deficiency intravenously (CONFIRM-HF trial; [18]) but not orally (IRONOUT-HF study; [19]) administered iron to supplement reduced transferrin saturation improves symptoms and quality of life of patient with HFrEF [20]. Smaller studies in HFpEF populations showed that iron deficiency was prevalent in half of the patients, even in the absence of anaemia [21]. It is conceivable that patients with HFpEF who are treated for iron deficiency might also experience symptom improvement. Iron supplementation has been suggested to improve mitochondrial energy supply that is impaired in HFpEF.
In conclusion, the conditions listed may be causally involved in the development and/or accelerated progression of HFpEF [22]. Therefore, the comprehensive treatment approach to HFpEF should systematically screen for these conditions and include their optimization and—if appropriate—repeated monitoring into the management strategy (Fig. 1).
Diuretics
In HFrEF, fluid retention can be treated with diuretics. Mechanistically, patients with HFrEF and HFpEF differ regarding changes in total blood volume (TBV). TBV expansion in HFpEF is predominantly characterized by a red cell mass deficit, indicating that true anaemia (i.e. haemoglobin concentration < 12 mg/day) and a compensatory plasma volume expansion reflect the qualitative changes of TBV in most of the decompensated HFpEF patients [23]. Loop diuretics, thiazide and thiazide-like drugs are necessary to overcome TBV expansion and congestion in both forms of HF [24].In the Hong Kong Diastolic Heart Failure Study [25], diuretic therapy significantly improved symptoms, and neither the ARB irbesartan nor the ACEi ramipril had a significant additional effect. However, diuretics in combination with RAAS (renin–angiotensin–aldosterone system) inhibitors marginally improved LV systolic and diastolic longitudinal LV function, and lowered NT-proBNP over a period of 1 year. Thus, diuretics appear indispensable for the improvement of symptom relief and belong to the current ESC guideline recommendations under these conditions, especially when post-capillary pulmonary hypertension occurs [24]. However, an excessive preload reduction by diuretics can lead to an under-filling of the left ventricle, and therefore to a reduction of stroke volume and cardiac output. This is particularly a problem in HFpEF patients with pronounced left ventricular hypertrophy and small ventricles including patients with cardiomyopathies such as hypertrophic cardiomyopathies (H(O)CM), storage diseases (amyloidosis) or cardiac inflammation (myocarditis) [26] (Fig. 1).
ACE inhibitor and AT1 receptor antagonists
Inhibition of neurohumoral activation by RAAS inhibitors is key principles in the treatment of dilated ventricles (with predominantly eccentric remodelling) in patients with HFrEF. In contrast, HFpEF is characterized by concentric remodelling, left ventricular hypertrophy and small ventricles [27]. However, treatment with RAAS inhibitors in HFpEF might improve diastolic function by counteracting left ventricular hypertrophy and fibrotic processes [28]. Three large studies investigated this pathophysiologic concept. In the PEP-CHF study [29], the ACEi perindopril showed no benefit on hospitalization or all-cause death. Uncertainty remains about the effects of perindopril on long-term morbidity and mortality in this clinical setting since this study had insufficient power for its primary endpoint. However, improved symptoms and exercise capacity and fewer hospitalizations for heart failure in the first year were observed in patients on perindopril, suggesting that it may benefit this patient population. In the CHARM Preserved study [30], the ARB candesartan reduced the hospitalisation risk but did not alter mortality. In the PRESERVE trial, the ARB irbesartan [31] did not improve mortality or hospitalisation risks after 50 months in HFpEF patients. However, in real life scenarios reflected by large registries, which include also more morbid HFpEF patients, the use of ACEI and ARBs was found to be effective [32, 33].
Since none of these agents improved hard clinical endpoints in HFpEF trials, as a consequence, current guidelines do not recommend the use of ACEi or ARB for the direct treatment of HFpEF [24], as long as they are not explicitly part of the regimen for their acknowledged effect on comorbidities such as hypertension [16] (Fig. 2).
Mineralocorticoid receptor antagonists (MRA)
Animal experiments showed that aldosterone is involved in the pathology of cardiac hypertrophy and fibrosis [34]. In the ALDO-HF study 25 mg spironolactone showed a slight reduction of blood pressure, significant improvements in diastolic LV function such as an E/eʹ reduction, decrease of left ventricular mass, and reduction of the NT-proBNP level, but no effects on exercise capacity as assessed by cardiopulmonary exercise testing (CPET) [35].
The investigators of the TOPCAT trial found that the incidence of the primary composite end point of death from cardiovascular causes, hospitalization for heart failure, or resuscitated cardiac arrest was not significantly lower in the spironolactone group than in the placebo group, but the incidence of hospitalization for heart failure was significantly lower in the spironolactone group [36]. However, significant differences in the clinical profiles, event rates, and responses to spironolactone were identified between the patients who were enrolled in the trial in the Americas (United States, Canada, Brazil, and Argentina) and the patients who were enrolled in Russia and Georgia, and these differences have aroused concerns about study conduct at the Russian and Georgian sites [37]. To further explore potential regional disparities in medication use, concentrations of canrenone (an active metabolite of spironolactone) were measured of 366 patients in the TOPCAT trial (206 patients from the United States and Canada and 160 patients from Russia). Analyses showed that canrenone concentrations were undetectable in a higher percentage of participants from Russia than from the United States and Canada (30 vs. 3%, P < 0.001) [38]. The findings suggest that the trial results obtained in Russia do not reflect the true therapeutic response to spironolactone, which is important since patients from countries such as the United States, Canada, Brazil, and Argentina met the primary endpoint of the study.
Based on these data, aldosterone antagonists are not generally recommended for patients with HFpEF today. Thereby, careful monitoring of potassium levels and renal markers is required to further clarify the role of aldosterone antagonism in HFpEF, the SPIRIT-HF phase III trial has been initiated by the German Centre for Cardiovascular Research (DZHK), to investigate the effects of spironolactone in well-characterized HFpEF patients. In addition, safety and efficacy of new aldosterone antagonists including dihydropyridine-like CCB, non-steroidal aldosterone antagonists, and aldosterone synthase inhibitors are under investigation [39,40,41].
Beta-blockers
High resting heart rate is a risk factor for adverse outcomes in patients with HFpEF [42]. The reduction of heart rate may improve the left ventricular filling time in abnormally stiff left ventricles and thus contribute to improved coronary perfusion. Therefore, heart rate control and maintenance of sinus rhythm with beta-blockers is considered favourable for patients prone to atrial fibrillation [43,44,45,46]. However, during exercise an adequate increase in heart rate is necessary to maintain cardiac output, especially when stroke volume is low, as shown as for HFpEF patients [47].
However, evidence for a general use of beta-blockers in patients with HFpEF is incoherent. In the SENIORS study [48], a pre-specified post hoc analysis showed that nebivolol improved the outcome of HFpEF patients to a similar degree as in HFrEF [49], although the echocardiographically measured diastolic function remained unaltered. In the ELLANDD study, the decrease of heart rate by nebivolol improved oxygen consumption but not load reserve or NYHA status [50]. Similar, registry data reported controversial results. In the OPTIMIZE registry [51], no benefits were seen with regard to mortality and rehospitalisation rates in patients having an EF > 40%. But the COHERE registry [52] as well as a recent meta-analysis found that beta-blockers reduced the mortality risk by 21% in HFpEF populations [53]. A report from the nation-wide Swedish registry showed that HFpEF patients treated with beta-blockers had a reduced total mortality, although no effect was observed regarding the combined endpoint of total mortality and heart failure-related hospitalisation [54]. Thus, studies are required to examine the role of beta-blockers in HFpEF more explicitly.
Current recommendations do not favour a general treatment recommendation for beta-blockers in HFpEF, as long as they are not indicated to optimise the therapy of comorbidities and symptoms including the control of ventricle frequency, angina pectoris or hypertension [55, 56]. If necessary, vasodilatory beta-blockers might be preferred agents. Chronotropic incompetence must be excluded before beta-blocker therapy is initiated.
If-channel inhibitor
Aside of its anti-ischemic properties, If channel inhibition using ivabradine may improve diastolic function by lowering sinus heart rate [57] and/or improving the function of the intracellular calcium ATPase SERCA2a [58]. Animal data suggested that the latter effect may occur independent of heart rate regulation [59]. Small studies in patients led to inconsistent results with respect to changes in E/e´ or peak VO2 [60, 61]. However, the recently finalized EDIFY study showed no influence on E/e´, exercise intolerance or NT-proBNP levels despite a heart rate reduction of 13% (mean HF about 63 bpm) after treatment with ivabradine [62]. Thus, ivabradine cannot be recommended for the primarily treatment of HFpEF. However, according to guidelines, ivabradine is useful in patients with angina pectoris and high heart rates despite ß-blocker therapy, independent of EF.
Calcium channel blockers
No large prospective trials investigated the effects of CCB in HFpEF [63]. In a recent retrospective analysis in hospitalized older patients with HFpEF, initiation of CCB had no effect on mortality or heart failure-related hospitalization, regardless of the class of CCB [64]. Thus, CCB for HFpEF cannot be generally recommended, but may be used for the optimisation of hypertension and angina symptoms [24].
Late sodium current inhibitors
Ischemia-triggered symptoms due to microvessel disease and vascular rarefication are considered pivotal pathophysiological principles of HFpEF [65, 66]. Preclinical data have shown that under ischemic conditions, an upregulation of the late sodium channels of myocytes may occur. This can lead to an increase of the intracellular calcium concentration, and therefore to a deceleration of diastolic relaxation [67, 68]. An inhibition of the late sodium current with drugs such as ranolazine or eleclazine might, therefore, improve diastolic function. In a first proof-of-concept study (RALI-DHF), intravenous administration of ranolazine in patients with HFpEF resulted in a reduction of the end-diastolic pressure [69]. However, a 14-day oral continuation of the therapy neither led to a continuous improvement of diastolic function nor to a decline of NT-proBNP levels or improvement of the load capacity. Further studies are necessary to investigate the role of ranolazine in HFpEF. Ranolazine is available for the treatment of patients with angina pectoris and may thus be tested for symptom relief in patients with HFpEF and typical or atypical angina pectoris.
Cardiac glycosides
The DIG study analysed treatment with digoxin or placebo in 980 patients with an EF of just over 45% [70]. After a median of 37 months, a trend towards the reduction of the hospitalisation rate was found in the digoxin group without any reduction in total or cardiovascular mortality. In patients suffering also from coronary artery disease, the mortality was increased.
However, patients with HFpEF often develop atrial fibrillation [71]. Cessation of the atrial contraction diminishes the left ventricular filling, and along with that decreases cardiac output and increases the risk for decompensation. Hence, restoration of sinus rhythm including ablation strategies and pharmacologic interventions including class I, II or III antiarrhythmic drugs may improve clinical symptoms. If this is not possible, ventricular heart rate should be controlled using beta-blockers, heart rate lowering CCBantagonists or glycosides [55].
In conclusion, cardiac glycosides should not generally be used in HFpEF, but they may have a place in the control of atrial fibrillation [24]. Digitoxin, the agent preferably used in Germany, has not been systematically investigated in this regard.
Statins
The large GISSI-HF trial did not show a positive effect of rosuvastatin in HFrEF [72]. Statins can positively affect left ventricular hypertrophy and the development of fibrosis under experimental conditions [73]. A small study suggested that statins may improve mortality risk in HFpEF patients [74]. Similar, the results of a recent meta-analysis suggest a potential mortality benefit of statins in HFpEF. Further prospective and randomized controlled trials should be planned to confirm this observations [75]. While the ACCF/AHA HF guidelines support the use of statin therapy for patients with known atherosclerotic disease, statins are not recommended for the treatment of HFpEF alone, i.e. in the absence of other indications for their use.
Non-pharmacological therapy approaches in HFpEF
Exercise in HFpEF
Higher levels of physical activity (PA) in healthy subjects have been associated with a lower risk of adverse cardiovascular (CV) outcomes, including lower incident heart failure, with a well-described dose–response relationship. In a recent post hoc analysis of the TOPCAT study was shown that in patients with HFpEF, poor and intermediate baseline PA compared to ideal PA were associated with a twofold higher risk of HF hospitalization and mortality [76]. In the Ex-DHF pilot trial [77], 64 patients with HFpEF were treated either according to the current recommendations or were exposed to an additional dedicated training programme. After 3 months, patients in the intervention group exhibited an improved peak VO2 and improved physical fitness. This was accompanied by an improvement of both diastolic and atrial function. Mechanistically, exercising can lead to an improvement of neurohormonal activation, including anti-inflammatory and anti-oxidative stress properties [78, 79]. The benefit of exercising was corroborated by a recent meta-analysis by Pandey et al. [80] and showed how safe and important training programs are for HFpEF patients.
Diet in HFpEF
In a very small study, 3 weeks of treatment with a salt-restricted DASH diet improved diastolic function, arterial stiffness, and ventricular–arterial coupling in 13 subjects with HFpEF [81]. Further, a 20-week caloric-restriction diet was feasible in obese HFpEF patients, and improved symptom burden, peak oxygen consumption, and quality of life. Quantitatively, the improvement in quality of life was greater with diet than exercise. The combination of diet with endurance exercise training appeared additive [82]. However, much larger studies are needed for any clinical recommendations.
The hope for new disease-modifying strategies
Novel strategies for the treatment of HFrEF are under investigation to prove whether they are sufficient to control disease progression and influence outcome rather than to control primarily risk factors comorbidities or symptoms. They include the regulation of energy and calcium homoeostasis, including anti-diabetic drugs, may be useful also in non-diabetic patients, matrix regulation, inflammation, angiogenesis, oxidative stress, and the cGMP axis (Fig. 3).
Targeting the NO-cGMP-PK-axis
In HFpEF, the intracellular nitrogen monoxide-cGMP-protein kinase (NO-cGMP-PK) signal cascade is disturbed [66]. The myocyte decline of cGMP appears to be a specific mechanism during HFpEF and differs from HFrEF [83]. A disorder in this signal cascade contributes to the development of concentric remodelling, increased cardiomyocyte stiffness by disturbances of regulation of titin and to an increase of fibrosis [3, 66]. This new concept has consequences for the development of new therapeutic options because it may be possible to mechanistically intervene with inorganic nitrates, phosphodiesterase-5 (PDE5) inhibitors, orally available soluble guanylate cyclase stimulators, or by angiotensin receptor neprilysin inhibitors (ARNI).
Organic nitrates and endothelial NO-synthase (eNOS) activators
Currently, direct NO-donators such as organic nitrates (isosorbide-nitrate) are not discussed favourably in the treatment of HFpEF due to their risk of a strong preload reduction and the possibility of tachyphylaxis. In a multicentre, double blind, crossover study, 110 patients with HFpEF were randomly assigned to a 6-week dose-escalation regimen of isosorbide mononitrate. Patients on isosorbide mononitrate were less active and did not have better quality of life or submaximal exercise capacity compared to the placebo group [84]. Therefore, only short-acting nitrates are recommended to overcome angina symptoms in HFpEF. By contrast, eNOS activators like the eNOS transcription amplifier AVE3085 have been promisingly investigated in animal experiments [85] and await clinical testing.
Inorganic nitrates (nitrites)
In contrast to organic nitrates, the inorganic nitrate–nitrite pathway represents an important alternative route to restore NO signalling in HFpEF by increasing myocardial nitric oxide bioavailability [86]. Acute infusion of sodium nitrite reduced diastolic LV pressures and pulmonary artery pressures during exercise while restoring cardiac output reserve towards normal levels, without reducing systemic blood pressure. Part of this benefit was mediated by vasodilation, but evidence for a direct myocardial benefit, such as increased stroke work, was also observed [87]. Similar effects were seen by inhaled sodium nitrate [88]. Another recent study found that inorganic nitrate (precursor to nitrite), delivered as on a week of once-daily beetroot juice drink, improved submaximal exercise endurance [89]. INDIE and KNO3CKOUT-HFpEF are phase II trials testing inhaled or oral nitrites, respectively, in HFpEF.
Phosphodiesterase-5 inhibitors
PDE5-inhibition belongs to another strategy to simulate the cGMP system, which could lead to an improvement of cardiac relaxation and diastolic performance in HFpEF. This concept had been investigated in the RELAX-Trial [90], investigating elderly HFpEF patients without pulmonary hypertension. But none of the investigated endpoints of the study reached significance. A similar negative result was shown by the study from Hoendernis et al. investing patients with HFpEF and post-capillary hypertension [91]. However, sildenafil was effective in patients with severe combined post- and precapillary pulmonary hypertension (CpC-PH; diastolic pressure gradient > 7 mmHg) and preserved EF [92], a form of pulmonary hypertension detectable also at least in some forms of HFpEF [93, 94]. Whether this observed benefit is reproducible and drug specific is yet still to be determined. Registry data indicate predominantly PDE5 inhibitors are used in Cpc-PH-HFpEF but this do not represent a robust scientific evidence [95, 96].
Thus, sildenafil cannot be recommended in HFpEF patients without or post-capillary hypertension. The use of sildenafil in other forms of pulmonary hypertension, characterized by increased pulmonary vascular resistance, and HFpEF needs further investigations.
Angiotensin receptor neprilysin inhibitor
LCZ696 (sacubitril/valsartan), a water–salt complex consisting of the ARB valsartan and a neprilysin inhibitor is able to stimulate in the NO-cGMP-PK signal cascade. Inhibition of neprilysin prevents the degradation of numerous vasoactive peptides, including biologically active natriuretic peptides such as ANP, BNP and CNP. These peptides stimulate the formation of cGMP via specific receptors, and are therefore thought to be directly involved into the pathomechanisms of HFpEF. Natriuretic peptides exert anti-fibrotic, vasodilatory and natriuretic effects. Besides blood pressure reduction, the induction of diuresis by BNP can reduce volume overload and pulmonary pressure. In addition, neprilysin inhibition includes the prevention of glucagon degradation, which has a beneficial impact of the diabetic status and could offer an additional support for diabetic HFpEF patients [97]. This concept was investigated in the phase II PARAMOUNT trial [98]. A decline in NT-proBNP levels after 12 weeks, the primary endpoint, was observed in the LCZ696 group, and atrial volumes were reduced and NYHA functional class improved after 36 weeks. Currently, the PARAGON-HF trial further explores these encouraging findings investigating the effect of LCZ696 on mortality risk among patients with HFpEF.
Soluble guanylate cyclase (sGC) stimulators and activators
Stimulators and activators of sGC increase the enzymatic activity of sGC to generate cGMP independently of NO. This property might be relevant under conditions of diminished NO bioavailability. Clinical data on vericiguat and riociguat, direct sGC stimulators, appear promising as treatment strategies in heart failure [99, 100]. The DILATE-1 trial examined the use of riociguat in patients with post-capillary pulmonary hypertension (PH-HFpEF). While there was no effect on peak decrease in mean pulmonary artery pressure, riociguat increased stroke volume and cardiac index [100]. Recently, the results of SOCRATES-PRESERVED have been reported, showing no effect of vericiguat on a change of NT-proBNP and LAV at 12 weeks compared with placebo. However, the quality of life improved [101]. Further studies are on-going.
Cytokine inhibitors
Patients with HFpEF exhibit signs of chronic myocardial inflammation [102]. Endothelial activation enables immigration of activated inflammation cells that can activate the local cytokine cascade. Increased cardiac expression of TGFß stimulated formation of pro-inflammatory myofibroblasts, which release collagens and chemokines [103,104,105]. In the small D-HARD study, the effect of the interleukin-1 inhibitor anakinra was examined over a period of 14 days in 12 HFpEF patients, who had increased plasma C-reactive protein levels (> 2 mg/dl). In this study, load capacity and C-reactive protein levels improved compared to placebo [106]. Whether HFpEF patients without signs of systemic inflammation may benefit from such intervention remains to be shown. Similarly, new adhesion molecule antagonists targeting integrins (ICAM or VCAM) and colchicine are under investigation to prevent myocardial invasion of inflammatory cells.
Anti-diabetic drugs
In some HFpEF patients, chronically increased β-adrenergic stimulation and insulin resistance is present, leading to an unfavourably altered cardiac metabolism along with a compromised energy production [107]. A large proportion of patients with HFpEF suffer from diabetes mellitus [108]. Thiazolidines, incretins, and inhibitors of the sodium–glucose cotransporter-2 (SGLT2) may possibly represent an additional therapy option in the future for diabetic and non-diabetic HFpEF patients.
Thiazolidines
Pioglitazone, an agonist for the PPAR-y receptor, is able to improve myocardial energy production and glycolysis [109]. The PIRAMID study showed that in patients with uncomplicated type-2 diabetes myocardial glucose assimilation and diastolic function were improved after 24 weeks of therapy [110]. Further studies will be necessary to evaluate thiazolidine as therapeutic option also for HFpEF patients without diabetes mellitus. Currently, thiazolidine is contraindicated in patients with HFrEF and NYHA functional class II–IV.
Incretins
The glucagon-like peptide 1 (GLP-1) is a hormone of the incretin family that is released from the gastrointestinal tract after food intake [111]. GLP receptors have also been found in the heart [112]. Stimulation of myocardial GLP receptors leads to an increased cardiac glucose assimilation, thus activating myocyte glycolysis [113]. Two pharmacological strategies stimulate this metabolic pathway: (a) GLP-1 analogues such as exenatide, semaglutide, liraglutide; and (b) DPP-IV inhibitors such as sitagliptin, saxagliptin, or linagliptin. The latter are able to additionally stimulate the cGMP axis [114]. Exenatide improved cardiac diastolic function in diabetic patients [115, 116]. Two large phase-III trials showed a mortality risk reduction in diabetic patients with cardiovascular risks with semaglutide [117] and liraglutide [118]. Similarly, DPP-IV inhibitors are under investigation with respect to their effect on left ventricular diastolic function. In a small study focussing on non-diabetic patients with non-ischaemic cardiomyopathy, sitagliptin improved myocardial glucose utilization [119]. Linagliptin and sitagliptin improved diastolic function in diabetic HFpEF patients with chronic kidney disease [120]. However, in patients with reduced EF, increased basal BNP levels and renal dysfunction, DPP-IV inhibitors such as saxagliptin increased the rehospitalisation risk due to adverse effects on heart failure [121]. Further studies will show whether an incretin-based therapy approach with diabetic and/or non-diabetic patients and HFpEF can lead to improvements in symptom burden or mortality risk.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors
This drug class currently includes approved antihyperglycemic agents such as empagliflozin, dapagliflozin, and canagliflozin. Renal SGLT2 inhibition prevents glucose reabsorption and induces a diuretic effect by glycosuria. The EMPA-REG OUTCOME trial investigated the effects of empagliflozin in patients with type 2 diabetes and found an unexpected but strikingly consistent relative risk reduction in cardiovascular mortality (38%), hospitalization for heart failure (35%), and death from any cause (32%). Accordingly, it was hypothesized that mechanisms other than those observed in the trial, i.e. modest improvements in glycaemic control, glycosuria-induced diuresis, body weight, blood pressure and uric acid level, may play a role [122]. One possible explanation might be that under conditions of mild, persistent hyperketonaemia, such as those prevailing during treatment with SGLT2 inhibitors, β-hydroxybutyrate is freely taken up by the heart and oxidized in preference to fatty acids [123]. Such fuel selection may improve the transduction of oxygen consumption into work efficiency at the mitochondrial level. In addition, the haemoconcentration that typically follows SGLT2 inhibition enhances oxygen release to the tissues, thereby establishing a powerful synergy with the metabolic substrate shift. Empagliflozin is now recommended in diabetic heart failure patients by the ESC in combination with metformin (IIA recommendation; [24]). Studies are on-going in non-diabetic HFrEF (EMPEROR-HFrEF-Trial) and HFpEF (EMPEROR-HFpEF-Trial) patients.
Szeto–Schiller peptides
Heart failure represents a mismatch between ATP supply and demand. This mismatch may result from damaged mitochondria, decreased mitochondrial production of ATP including increased workload to the myocardium following ischemia, hypertension and diastolic dysfunction [124,125,126]. Current HF treatments rely on “energy sparing” by decreasing workload. Targeting mitochondrial plasticity to improve ATP supply may provide an alternative approach. New mitochondria-targeted antioxidant peptides were developed that are able to restore the mitochondrial electron transport chain to optimize efficiency of electron transport and restore cellular bioenergetics [127, 128]. Modulation of myocardial energy balance and restoration of mitochondrial bioenergetics is possible by the so-called Szeto–Schiller (SS) peptides such as elamipretide (MTP-131, SS31), that binds the phospholipid cardiolipin and stabilizes the components of electron transport and ATP generation. Elamipretide is the first of these compounds that has entered clinical development and is studied in phase-II trials for HFrEF and HFpEF. However, elamipretide was not able to reduce infarct size in a phase-II trial in patients with acute ST-elevation myocardial infarction (EMBRACE STEMI study, [126]).
Cross-link breakers
Oxidative stress can lead to a formation of advanced glycation products (AEGs), whereby proteins and carbohydrates form a compound which leads to “cross-linking” with the extracellular matrix [129] and contributes to LV stiffness in HFpEF [130]. The so-called cross-link breaker alagebrium chloride was examined in a small study involving 23 older patients with HFpEF [131]. After 16 weeks, an improvement of the diastolic function was observed. However, due to drug toxicity, this approach is currently not further investigated.
Lysyl oxidase-like 2 (Loxl2) is an enzyme that cross-links collagen and has been shown to be essential for interstitial fibrosis and mechanical dysfunction of stressed hearts. Antibody-mediated inhibition of Loxl2 in mice has been shown to greatly reduce stress-induced cardiac fibrosis and chamber dilatation, improving systolic and diastolic functions [132]. Further studies are in preparation to prove different concept strategies of cross-link breaking in HFpEF.
Modulators of intracellular calcium homoeostasis
Disorders of the intracellular calcium homoeostasis contribute to diastolic dysfunction [133] via interference with the ryanodine receptor (RyR2) [134], the SERCA2a pathway, and the sodium–potassium pump [135]. The so-called RyR2 stabilizers like K201 improved diastolic function in experimental models [136] as did substances that are capable of inhibiting the Na+/Ca2+ exchanger (NCX), e.g. SES0400 [137]. Strategies targeting SERCA2A are thought not only to improve diastolic function but also decrease myocardial hypertrophy [138]. Although these are sound pathophysiological concepts, their value in the clinical setting has not been explored.
MicroRNAs
MicroRNAs (miRNAs) are small non-coding RNA involved in RNA silencing and post-transcriptional regulation of gene expression. miRNA have been reported to influence genes that are important for HF [139] and different miRNA profiles were reported for patients with HFpEF compared to HFrEF (miR-30c, -146a, -221, -328, and -375; miR-125a-5p, -190a, -550a-5p, and -638) [140, 141]. However, the role of miRNAs as biomarkers in HFpEF is still not clear. Currently, the importance of miRNAs and/or certain inhibitors (antagomirs) are investigated as inducers of angioneogenesis or modifiers of fibrosis, e.g. inhibition of miRNA 21. For this molecule, anti-apoptotic and anti-fibrotic effects were shown in an animal experiment related to diastolic heart failure [142]. Further, miRNAs are presently being discussed as possible therapeutic targets for the treatment of HFpEF [143, 144].
Device therapy in HFpEF
Several device studies are on-going in HFpEF populations (Fig. 3)
Online monitoring
Increased pressures in the left atrium and the small circulation frequently cause the symptoms of HFpEF. Importantly, the rise in atrial and right-sided pressures can be detected prior to symptom deterioration or overt decompensation. Online haemodynamic monitoring could detect such early indicators of incipient decompensation.
The CardioMEMS device is a small pressure sensor and monitor, which is implanted into the pulmonary artery and calibrated in the course of a right-heart catheter procedure. After discharge, the patients record their pulmonary artery pressure via a cushion-based wireless radiofrequency transmitter. These values are online monitored by dedicated staff and may be used to adjust medication. In the CHAMPION trial, the use of CardioMEMS transmitted information lowered hospitalisation rates of NYHA III patients with either HFrEF or HFpEF [145]. In addition, data from a recent large real-world study reported that pulmonary pressure could be very effective targeted with this system by adapting especially the diuretic-based therapy [146,147,148].
Atrial shunt device
The reduction of increased left atrial pressure belongs to the principal haemodynamic objectives of treating HFpEF [149]. The hypothesis that a small, artificially induced left–right shunt might function as an overflow valve is based on historical observations, showing that patients with an untreated mitral stenosis and concomitant atrial defect had better survival (Lutembacher syndrome [150]). In a small study of 11 HFpEF patients (EF ≥ 45%, PCP > 15 mmHg at rest, or PCP ≥ 25 mmHg during exercise), an interatrial septal device (IASD) was implanted in the septum using a catheter-based technique enabling a small shunt [151]. After 30 days, the filling pressure had fallen and mean NYHA classification improved. No patient developed pulmonary hypertension over this time. More recently, the REDUCE LAP-HF study analysed 68 HFpEF patients who underwent IASD implantation and showed that more than 50% of patients showed a reduction in wedge pressure at rest or during exertion [152]. These results need to be repeated in long-term trials.
Cardiac resynchronisation therapy (CRT)
Approximately 20% of HFpEF patients exhibit left ventricular asynchrony, which is associated with about 15% myocardial energy and contractility loss [153]. Unpublished results of an Asian study investigating about 130 HFpEF patients with mechanical asynchrony (regional level mechanical delay ≥ 65 ms) suggest that temporary stimulation with a CRT system may improve diastolic parameters. However, up to now patients with a narrowed QRS complex are not eligible for CRT.
Cardiac contractility modulation (CCM)
This device delivers a strong electrical current in the refractory period into the septum, thus triggering molecular remodelling which is thought to improve EF and optimise symptoms of symptomatic HFrEF patients. The effect seems to be more pronounced in patients with better EF (i.e. ≥ 35%) [154]. A recent case series found that early after initiating CCM treatment patients improved in NYHA classification, 6 min walking distance, quality of life, also exhibiting a significant reduction of the diastolic filling index (E/eʹ) and an improved EF reserve [155]. A clinical exploratory study, the CCM-HFpEF-Trial, has been initiated to further investigate the role of CCM in HFpEF.
Renal denervation
HFpEF is associated with an increased tone of the sympathetic nervous system (SNS). Reduction of blood pressure by renal denervation therapy (RDT) improved left ventricular hypertrophy and diastolic left ventricular function in a small series of patients with refractory hypertension [156]. This effect was prospectively investigated in the RDT-PEF study [157]. In this single-centre open trial, 25 patients with HFpEF were randomized (2:1) to RDT with the Simplicity™ catheter or continuing medical therapy. The primary endpoint was not met in that there were no differences between groups at 12 months for quality of life and markers for diastolic function. Some patients improved with respect to peak VO2 although markers of macro- and microvascular function such as augmentation index or endothelial function, respectively, did not improve [158]. Up to now, the sustained clinical value of RDT remains unclear.
Baroreflex activation therapy (BAT)
BAT electrically stimulates the carotid sinus via an implanted electrode in close vicinity to the glomus caroticus. The device has originally been studied for the treatment of hypertension. Potential (long-term) benefits of such an approach include regression of left ventricular hypertrophy, normalization of the sympathovagal balance, and inhibition of the RAAS, arterio- and venodilation, and preservation of renal function. BAT had been successfully investigated in HFrEF showing an improvement of functional status, quality of life, exercise capacity, and BNP reduction [159]. The clinical utility of BAT in treatment of HFpEF needs further investigation [160].
Summary, expert opinion and conclusion
The management of and clinical research in patients with HFpEF remains an on-going challenge. Especially, since HFpEF is a heterogeneous syndrome. Therefore, clinical management and future clinical trials mandate an individualized, phenotype-specific approach instead of a “one-size-fits-all” strategy. Options to improve patients’ symptoms and quality of life include control of fluid overload, heart rate, risk factors, and comorbidities (Figs. 1, 2). Comorbidities such as coronary artery disease, hypertension and diabetes mellitus must be stringently treated. Maintaining mobility and regular exercise should be implemented in the treatment plan and is the most effective treatment strategy in HFpEF. Diuretics and/or mineralocorticoid receptor antagonists can be used to stabilize the optimal volume status. RAAS inhibitors and (vasodilatory) beta-blockers are preferred to control blood pressure in euvolaemic patients. Beta-blockers should be avoided in patients with chronotropic incompetence [161].
Devices such as the CardioMEMS system seem to be extremely helpful for critical HFpEF patients with several decompensation episodes. But guiding patients by a telemedical approach asks for a specific organization structure of the clinic, which needs a significant reimbursement backup. Similarly, an atrial shunt device will be in our opinion a strategy for a limit group of severe HFpEF patients, only.
Recently, our understanding of the pathological processes involved in HFpEF has led to the discovery of new treatment targets and holds promise for more specific treatment options of HFpEF in the future (Fig. 3). Some strategies, such as LCZ696, have already been successfully studied in a phase-II trial [98]; other potential treatment targets, e.g. involving cGMP stimulation, mitochondria-targeted antioxidant peptides, new devices or the role of anti-diabetic drugs such as empagliflozin in normoglycaemic patients are currently investigated. The later offer the opportunity to develop more specific disease-modifying treatment strategies and to be able not only to treat comorbidities and symptoms. The future will show whether new treatment strategies including disease-modifying approaches are sufficient to improve outcome in HFpEF.
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2014) Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129 (3):e28-e292. doi:10.1161/01.cir.0000441139.02102.80
Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C (2014) A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol 37(5):312–321
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550. doi:10.1093/eurheartj/ehm037
Tschope C, Lam CS (2012) Diastolic heart failure: What we still don’t know. Looking for new concepts, diagnostic approaches, and the role of comorbidities. Herz 37(8):875–879. doi:10.1007/s00059-012-3719-5
Kasner M, Sinning D, Lober J, Post H, Fraser AG, Pieske B, Burkhoff D, Tschöpe C (2015) Heterogeneous responses of systolic and diastolic left ventricular function to exercise in patients with heart failure and preserved ejection fraction. ESC Heart Failure 2(3):121–132. doi:10.1002/ehf2.12049
Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD (2015) Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 132(5):402–414. doi:10.1161/CIRCULATIONAHA.115.015884
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355(3):260–269. doi:10.1056/NEJMoa051530
Lam CS, Donal E, Kraigher-Krainer E, Vasan RS (2011) Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 13(1):18–28. doi:10.1093/eurjhf/hfq121
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259. doi:10.1056/NEJMoa052256
Lam CS, Teng TH (2016) Understanding heart failure with mid-range ejection fraction. JACC Heart failure 4(6):473–476. doi:10.1016/j.jchf.2016.03.025
Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC (2015) Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 131(3):269–279. doi:10.1161/CIRCULATIONAHA.114.010637
Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A (2012) Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 59(11):998–1005. doi:10.1016/j.jacc.2011.11.040
Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, Group HS (2008) Treatment of hypertension in patients 80 years of age or older. N Engl J Med 358(18):1887–1898. doi:10.1056/NEJMoa0801369
Wachtell K, Bella JN, Rokkedal J, Palmieri V, Papademetriou V, Dahlof B, Aalto T, Gerdts E, Devereux RB (2002) Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation 105(9):1071–1076
Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE (2003) A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 115(1):41–46
Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP, Valsartan In Diastolic Dysfunction I (2007) Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 369 (9579):2079–2087. doi:10.1016/S0140-6736(07)60980-5
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5):507–520. doi:10.1001/jama.2013.284427
Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, Investigators C-H (2015) Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 36(11):657–668. doi:10.1093/eurheartj/ehu385
Lewis GD, Semigran MJ, Givertz MM, Malhotra R, Anstrom KJ, Hernandez AF, Shah MR, Braunwald E (2016) Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure. Circ Heart Fail 9 (5). doi:10.1161/CIRCHEARTFAILURE.115.000345
Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P, Investigators F-HT (2009) Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 361(25):2436–2448. doi:10.1056/NEJMoa0908355
Kasner M, Aleksandrov AS, Westermann D, Lassner D, Gross M, von Haehling S, Anker SD, Schultheiss HP, Tschope C (2013) Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int J Cardiol 168(5):4652–4657. doi:10.1016/j.ijcard.2013.07.185
Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP, Tschope C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM (2014) New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 35(40):2797–2815. doi:10.1093/eurheartj/ehu204
Tschope C, Pieske B (2016) “One size does not fit all”: how to individualize decongestive therapy strategies in heart failure. JACC Heart Failure 4(6):460–463. doi:10.1016/j.jchf.2016.03.013
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18(8):891–975. doi:10.1002/ejhf.592
Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE (2008) The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 94(5):573–580. doi:10.1136/hrt.2007.117978
Kasner M, Aleksandrov A, Escher F, Al-Saadi N, Makowski M, Spillmann F, Genger M, Schultheiss HP, Kuhl U, Pieske B, Morris DA, Noutsias M, Tschope C (2017) Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): The role of 2D speckle-tracking echocardiography. Int J Cardiol. doi:10.1016/j.ijcard.2017.05.038
Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C (2008) Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 117(16):2051–2060. doi:10.1161/CIRCULATIONAHA.107.716886
Benedict CR, Johnstone DE, Weiner DH, Bourassa MG, Bittner V, Kay R, Kirlin P, Greenberg B, Kohn RM, Nicklas JM et al (1994) Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. SOLVD Investig J Am College Cardiol 23(6):1410–1420
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, Investigators P-C (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27(19):2338–2345. doi:10.1093/eurheartj/ehl250
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C, Committees (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467. doi:10.1056/NEJMoa0805450
Lund LH, Benson L, Dahlstrom U, Edner M (2012) Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 308(20):2108–2117. doi:10.1001/jama.2012.14785
Fukuta H, Goto T, Wakami K, Ohte N (2017) Effect of renin-angiotensin system inhibitors on mortality in heart failure with preserved ejection fraction: a meta-analysis of observational cohort and randomized controlled studies. Heart Fail Rev. doi:10.1007/s10741-017-9637-0
Lijnen P, Petrov V (2000) Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol 32(6):865–879. doi:10.1006/jmcc.2000.1129
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo DHFI (2013) Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 309(8):781–791. doi:10.1001/jama.2013.905
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370(15):1383–1392. doi:10.1056/NEJMoa1313731
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B (2015) Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 131(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL (2017) Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 376(17):1690–1692. doi:10.1056/NEJMc1612601
Martin-Martinez M, Perez-Gordillo FL, Alvarez de la Rosa D, Rodriguez Y, Gerona-Navarro G, Gonzalez-Muniz R, Zhou MM (2017) Modulating mineralocorticoid receptor with non-steroidal antagonists. New opportunities for the development of potent and selective ligands without off-target side effects. J Med Chem. doi:10.1021/acs.jmedchem.6b01065
Furuzono S, Meguro M, Miyauchi S, Inoue S, Homma T, Yamada K, Tagawa YI, Nara F, Nagayama T (2017) A novel aldosterone synthase inhibitor ameliorates mortality in pressure-overload mice with heart failure. Eur J Pharmacol 795:58–65. doi:10.1016/j.ejphar.2016.11.049
Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE (2016) Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail 18(1):28–37. doi:10.1002/ejhf.444
O’Neal WT, Sandesara PB, Samman-Tahhan A, Kelli HM, Hammadah M, Soliman EZ (2017) Heart rate and the risk of adverse outcomes in patients with heart failure with preserved ejection fraction. Eur J Prevent Cardiol 24(11):1212–1219. doi:10.1177/2047487317708676
Bonow RO, Udelson JE (1992) Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management. Ann Intern Med 117(6):502–510
Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM (2010) Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56(11):845–854. doi:10.1016/j.jacc.2010.03.077
Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M (2009) Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54(5):402–409. doi:10.1016/j.jacc.2009.05.012
Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M (2010) Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail 3(1):29–34. doi:10.1161/CIRCHEARTFAILURE.109.877720
Borlaug BA, Kane GC, Melenovsky V, Olson TP (2016) Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 37(43):3293–3302. doi:10.1093/eurheartj/ehw241
Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA, Investigators S (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26(3):215–225. doi:10.1093/eurheartj/ehi115
van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD, Investigators S (2009) Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure). J Am Coll Cardiol 53(23):2150–2158. doi:10.1016/j.jacc.2009.02.046
Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L (2012) Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 14(2):219–225. doi:10.1093/eurjhf/hfr161
Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC (2009) Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (organized program to initiate lifesaving treatment in hospitalized patients with heart failure) Registry. J Am Coll Cardiol 53(2):184–192. doi:10.1016/j.jacc.2008.09.031
Massie BM, Nelson JJ, Lukas MA, Greenberg B, Fowler MB, Gilbert EM, Abraham WT, Lottes SR, Franciosa JA, Physicians CP (2007) Comparison of outcomes and usefulness of carvedilol across a spectrum of left ventricular ejection fractions in patients with heart failure in clinical practice. Am J Cardiol 99(9):1263–1268. doi:10.1016/j.amjcard.2006.12.056
Fukuta H, Goto T, Wakami K, Ohte N (2017) The effect of beta-blockers on mortality in heart failure with preserved ejection fraction: a meta-analysis of observational cohort and randomized controlled studies. Int J Cardiol 228:4–10. doi:10.1016/j.ijcard.2016.11.239
Lund LH, Benson L, Dahlstrom U, Edner M, Friberg L (2014) Association between use of beta-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 312(19):2008–2018. doi:10.1001/jama.2014.15241
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33(14):1787–1847. doi:10.1093/eurheartj/ehs104
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F, American Heart Association Task Force on Practice G (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62 (16):e147–e239. doi:10.1016/j.jacc.2013.05.019
Colin P, Ghaleh B, Hittinger L, Monnet X, Slama M, Giudicelli JF, Berdeaux A (2002) Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol 282(2):H672-679. doi:10.1152/ajpheart.00547.2001
Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, Fries P, Muller A, Lenski M, Custodis F, Graber S, Frohlig G, Steendijk P, Neuberger HR, Bohm M (2013) Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J 34(36):2839–2849. doi:10.1093/eurheartj/ehs218
Becher PM, Lindner D, Miteva K, Savvatis K, Zietsch C, Schmack B, Van Linthout S, Westermann D, Schultheiss HP, Tschope C (2012) Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and beta-receptor blockade. Hypertension 59(5):949–957. doi:10.1161/HYPERTENSIONAHA.111.183913
Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH (2013) Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol 62(15):1330–1338. doi:10.1016/j.jacc.2013.06.043
Pal N, Sivaswamy N, Mahmod M, Yavari A, Rudd A, Singh S, Dawson DK, Francis JM, Dwight JS, Watkins H, Neubauer S, Frenneaux M, Ashrafian H (2015) Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 132(18):1719–1725. doi:10.1161/CIRCULATIONAHA.115.017119
Komajda M, Isnard R, Cohen-Solal A, Metra M, Pieske B, Ponikowski P, Voors AA, Dominjon F, Henon-Goburdhun C, Pannaux M, Bohm M, prEserve DlvefchFwisI (2017) Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur J Heart Fail. doi:10.1002/ejhf.876
Setaro JF, Zaret BL, Schulman DS, Black HR, Soufer R (1990) Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol 66(12):981–986
Patel K, Fonarow GC, Ahmed M, Morgan C, Kilgore M, Love TE, Deedwania P, Aronow WS, Anker SD, Ahmed A (2014) Calcium channel blockers and outcomes in older patients with heart failure and preserved ejection fraction. Circ Heart Fail 7(6):945–952. doi:10.1161/CIRCHEARTFAILURE.114.001301
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM (2015) Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131(6):550–559. doi:10.1161/CIRCULATIONAHA.114.009625
Tschope C, Van Linthout S (2014) New insights in (inter)cellular mechanisms by heart failure with preserved ejection fraction. Curr Heart Fail Rep 11(4):436–444. doi:10.1007/s11897-014-0219-3
Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L (2006) Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318(1):214–222. doi:10.1124/jpet.106.101832
Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS (2011) Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res Cardiol 106(2):263–272. doi:10.1007/s00395-010-0136-x
Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C (2013) RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail 1(2):115–122. doi:10.1016/j.jchf.2012.12.002
Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF Jr, Gheorghiade M (2006) Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 114(5):397–403. doi:10.1161/CIRCULATIONAHA.106.628347
Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M (2016) Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 68(20):2217–2228. doi:10.1016/j.jacc.2016.08.048
Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi HFI (2008) Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372(9645):1231–1239. doi:10.1016/S0140-6736(08)61240-4
Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A (2004) Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation 109(18):2234–2239. doi:10.1161/01.CIR.0000127939.16111.58
Fukuta H, Sane DC, Brucks S, Little WC (2005) Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation 112(3):357–363. doi:10.1161/CIRCULATIONAHA.104.519876
Fukuta H, Goto T, Wakami K, Ohte N (2016) The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol 214:301–306. doi:10.1016/j.ijcard.2016.03.186
Hegde S, Claggett B, Shah AM, Lewis EF, Anand IS, Shah SJ, Sweitzer NK, Fang JC, Pitt B, Pfeffer MA, Solomon SD (2017) Physical activity and prognosis in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) Trial. Circulation. doi:10.1161/CIRCULATIONAHA.117.028002
Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B (2011) Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 58(17):1780–1791. doi:10.1016/j.jacc.2011.06.054
Seiler M, Bowen TS, Rolim N, Dieterlen MT, Werner S, Hoshi T, Fischer T, Mangner N, Linke A, Schuler G, Halle M, Wisloff U, Adams V (2016) Skeletal muscle alterations are exacerbated in heart failure with reduced compared with preserved ejection fraction: mediated by circulating cytokines?. Circ Heart Fail. doi:10.1161/CIRCHEARTFAILURE.116.003027
Trippel TD, Holzendorf V, Halle M, Gelbrich G, Nolte K, Duvinage A, Schwarz S, Rutscher T, Wiora J, Wachter R, Herrmann-Lingen C, Duengen HD, Hasenfuss G, Pieske B, Edelmann F (2017) Ghrelin and hormonal markers under exercise training in patients with heart failure with preserved ejection fraction: results from the Ex-DHF pilot study. ESC Heart Fail 4(1):56–65. doi:10.1002/ehf2.12109
Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8(1):33–40. doi:10.1161/CIRCHEARTFAILURE.114.001615
Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ, Kolias TJ (2013) Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 6(6):1165–1171. doi:10.1161/CIRCHEARTFAILURE.113.000481
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ (2016) Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 315(1):36–46. doi:10.1001/jama.2015.17346
van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ (2012) Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126(7):830–839. doi:10.1161/CIRCULATIONAHA.111.076075
Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, Network NHFCR (2015) Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 373(24):2314–2324. doi:10.1056/NEJMoa1510774
Westermann D, Riad A, Richter U, Jager S, Savvatis K, Schuchardt M, Bergmann N, Tolle M, Nagorsen D, Gotthardt M, Schultheiss HP, Tschope C (2009) Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol 104(5):499–509. doi:10.1007/s00395-009-0014-6
Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety SH, Kucharska-Newton A, Sueta CA, Mosley TH Jr, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD (2016) Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation. doi:10.1161/CIRCULATIONAHA.116.023361
Borlaug BA, Koepp KE, Melenovsky V (2015) Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 66(15):1672–1682. doi:10.1016/j.jacc.2015.07.067
Simon MA, Vanderpool RR, Nouraie M, Bachman TN, White PM, Sugahara M, Gorcsan J, 3rd, Parsley EL, Gladwin MT (2016) Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight 1(18):e89620. doi:10.1172/jci.insight.89620
Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW (2016) One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 4(6):428–437. doi:10.1016/j.jchf.2015.12.013
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309(12):1268–1277. doi:10.1001/jama.2013.2024
Liu LC, Hummel YM, van der Meer P, Berger RM, Damman K, van Veldhuisen DJ, Voors AA, Hoendermis ES (2017) Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur J Heart Fail 19(1):116–125. doi:10.1002/ejhf.662
Guazzi M, Vicenzi M, Arena R, Guazzi MD (2011) PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53(13):1119–1126. doi:10.1016/j.jacc.2008.11.051
Olson TP, Johnson BD, Borlaug BA (2016) Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 4(6):490–498. doi:10.1016/j.jchf.2016.03.001
Hoeper MM, Lam CS, Vachiery JL, Bauersachs J, Gerges C, Lang IM, Bonderman D, Olsson KM, Gibbs JS, Dorfmuller P, Guazzi M, Galie N, Manes A, Handoko ML, Vonk-Noordegraaf A, Lankeit M, Konstantinides S, Wachter R, Opitz C, Rosenkranz S (2016) Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further researchdagger. Eur Heart J. doi:10.1093/eurheartj/ehw597
Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL (2016) Left ventricular heart failure and pulmonary hypertension. Eur Heart J 37(12):942–954. doi:10.1093/eurheartj/ehv512
Seferovic JP, Claggett B, Seidelmann SB, Seely EW, Packer M, Zile MR, Rouleau JL, Swedberg K, Lefkowitz M, Shi VC, Desai AS, McMurray JJV, Solomon SD (2017) Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 5(5):333–340. doi:10.1016/S2213-8587(17)30087-6
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ, Prospective comparison of AwARBoMOhfwpefI (2012) The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 380(9851):1387–1395. doi:10.1016/S0140-6736(12)61227-6
Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Muller K, Roessig L, Pieske B, Investigators S-R, Coordinators (2015) Effect of Vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED randomized trial. JAMA 314(21):2251–2262. doi:10.1001/jama.2015.15734
Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CS, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM (2014) Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 146(5):1274–1285. doi:10.1378/chest.14-0106
Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M (2017) Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 38(15):1119–1127. doi:10.1093/eurheartj/ehw593
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4(1):44–52. doi:10.1161/CIRCHEARTFAILURE.109.931451
Lindner D, Zietsch C, Tank J, Sossalla S, Fluschnik N, Hinrichs S, Maier L, Poller W, Blankenberg S, Schultheiss HP, Tschope C, Westermann D (2014) Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res Cardiol 109(5):428. doi:10.1007/s00395-014-0428-7
Van Linthout S, Miteva K, Tschope C (2014) Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 102(2):258–269. doi:10.1093/cvr/cvu062
Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E, Abbate A (2013) Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflammation Res Off J Eur Histamine Res Soc [et al]. 62 (7):637–640. doi:10.1007/s00011-013-0625-0
Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A (2014) Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 113(2):321–327. doi:10.1016/j.amjcard.2013.08.047
Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic mechanisms in heart failure. Circulation 116(4):434–448. doi:10.1161/CIRCULATIONAHA.107.702795
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. doi:10.1016/j.jacc.2013.02.092
Reasner CA, 2nd (1999) Promising new approaches. Diabetes, obesity and metabolism. 1(Suppl 1):S41–48
van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M (2009) Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 119(15):2069–2077. doi:10.1161/CIRCULATIONAHA.108.803916
Drucker DJ (1998) Glucagon-like peptides. Diabetes 47(2):159–169
Wei Y, Mojsov S (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358(3):219–224
Inzucchi SE, McGuire DK (2008) New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation 117(4):574–584. doi:10.1161/CIRCULATIONAHA.107.735795
Hamdani N, Hervent AS, Vandekerckhove L, Matheeussen V, Demolder M, Baerts L, De Meester I, Linke WA, Paulus WJ, De Keulenaer GW (2014) Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc Res 104(3):423–431. doi:10.1093/cvr/cvu223
Wang XH, Han LN, Yu YR, Wang C, Wang B, Wen XR, Huang H, Jing XC (2015) Effects of GLP-1 agonist exenatide on cardiac diastolic function and vascular endothelial function in diabetic patients. J Sichuan Univ Med. science edition 46(4):586–590
Scalzo RL, Moreau KL, Ozemek C, Herlache L, McMillin S, Gilligan S, Huebschmann AG, Bauer TA, Dorosz J, Reusch JE, Regensteiner JG (2016) Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complicat. doi:10.1016/j.jdiacomp.2016.10.003
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T, Investigators S- (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19):1834–1844. doi:10.1056/NEJMoa1607141
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS, Investigators LT (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. doi:10.1056/NEJMoa1603827
Witteles RM, Keu KV, Quon A, Tavana H, Fowler MB (2012) Dipeptidyl peptidase 4 inhibition increases myocardial glucose uptake in nonischemic cardiomyopathy. J Card Fail 18(10):804–809. doi:10.1016/j.cardfail.2012.07.009
Connelly KA, Bowskill BB, Advani SL, Thai K, Chen LH, Kabir MG, Gilbert RE, Advani A (2014) Dipeptidyl peptidase-4 inhibition improves left ventricular function in chronic kidney disease. Clin Investig Med 37(3):E172
Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL, Committee S-TS, Investigators* (2014) Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 130(18):1579–1588. doi:10.1161/CIRCULATIONAHA.114.010389
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. doi:10.1056/NEJMoa1504720
Ferrannini E, Mark M, Mayoux E (2016) CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care 39(7):1108–1114. doi:10.2337/dc16-0330
Shi J, Dai W, Hale SL, Brown DA, Wang M, Han X, Kloner RA (2015) Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci 141:170–178. doi:10.1016/j.lfs.2015.09.022
Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS (2011) Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58(1):73–82. doi:10.1016/j.jacc.2010.12.044
Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Janosi A, Merkely B, Godlewski J, Halaby R, Korjian S, Daaboul Y, Chakrabarti AK, Spielman K, Neal BJ, Weaver WD (2016) EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J 37(16):1296–1303. doi:10.1093/eurheartj/ehv597
Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K (2016) Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail 9(2):e002206. doi:10.1161/CIRCHEARTFAILURE.115.002206
Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kastner L, Lipp P, Zeller T, Muller C, Knopp A, Laufs U, Bohm M, Hoth M, Maack C (2015) Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab 22(3):472–484. doi:10.1016/j.cmet.2015.07.008
Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ (2007) Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail 9(12):1146–1155. doi:10.1016/j.ejheart.2007.09.009
Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C (2011) Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol 57(8):977–985. doi:10.1016/j.jacc.2010.10.024
Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, Degroof RC (2005) The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 11(3):191–195
Yang J, Savvatis K, Kang JS, Fan P, Zhong H, Schwartz K, Barry V, Mikels-Vigdal A, Karpinski S, Kornyeyev D, Adamkewicz J, Feng X, Zhou Q, Shang C, Kumar P, Phan D, Kasner M, Lopez B, Diez J, Wright KC, Kovacs RL, Chen PS, Quertermous T, Smith V, Yao L, Tschope C, Chang CP (2016) Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat Commun 7:13710. doi:10.1038/ncomms13710
Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR (2010) Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121(6):822–830. doi:10.1161/CIRCULATIONAHA.109.890954
Currie S, Elliott EB, Smith GL, Loughrey CM (2011) Two candidates at the heart of dysfunction: The ryanodine receptor and calcium/calmodulin protein kinase II as potential targets for therapeutic intervention-An in vivo perspective. Pharmacol Ther 131(2):204–220. doi:10.1016/j.pharmthera.2011.02.006
Hasenfuss G, Pieske B (2002) Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34(8):951–969
Sacherer M, Sedej S, Wakula P, Wallner M, Vos MA, Kockskamper J, Stiegler P, Sereinigg M, von Lewinski D, Antoons G, Pieske BM, Heinzel FR, investigators C (2012) JTV519 (K201) reduces sarcoplasmic reticulum Ca(2)(+) leak and improves diastolic function in vitro in murine and human non-failing myocardium. Br J Pharmacol 167(3):493–504. doi:10.1111/j.1476-5381.2012.01995.x
Primessnig U, Schonleitner P, Holl A, Pfeiffer S, Bracic T, Rau T, Kapl M, Stojakovic T, Glasnov T, Leineweber K, Wakula P, Antoons G, Pieske B, Heinzel FR (2016) Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 18(8):987–997. doi:10.1002/ejhf.524
Sedej S, Schmidt A, Denegri M, Walther S, Matovina M, Arnstein G, Gutschi EM, Windhager I, Ljubojevic S, Negri S, Heinzel FR, Bisping E, Vos MA, Napolitano C, Priori SG, Kockskamper J, Pieske B (2014) Subclinical abnormalities in sarcoplasmic reticulum Ca(2+) release promote eccentric myocardial remodeling and pump failure death in response to pressure overload. J Am Coll Cardiol 63(15):1569–1579. doi:10.1016/j.jacc.2013.11.010
Ohtani K, Dimmeler S (2011) Control of cardiovascular differentiation by microRNAs. Basic Res Cardiol 106(1):5–11. doi:10.1007/s00395-010-0139-7
Watson CJ, Gupta SK, O’Connell E, Thum S, Glezeva N, Fendrich J, Gallagher J, Ledwidge M, Grote-Levi L, McDonald K, Thum T (2015) MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur J Heart Fail 17(4):405–415. doi:10.1002/ejhf.244
Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, Chong JP, Ng JY, Chen YT, Chan MM, Chen Z, Yeo PS, Ng TP, Ling LH, Sim D, Leong KT, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Lam CS, Jeyaseelan K, Richards AM (2015) Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 17(4):393–404. doi:10.1002/ejhf.223
Dong S, Ma W, Hao B, Hu F, Yan L, Yan X, Wang Y, Chen Z, Wang Z (2014) microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol 7(2):565–574
Nair N, Gupta S, Collier IX, Gongora E, Vijayaraghavan K (2014) Can microRNAs emerge as biomarkers in distinguishing HFpEF versus HFrEF? Int J Cardiol 175(3):395–399. doi:10.1016/j.ijcard.2014.06.027
Tschope C, Van Linthout S, Kherad B (2017) Heart failure with preserved ejection fraction and future pharmacological strategies: a glance in the crystal ball. Curr Cardiol Rep 19(8):70. doi:10.1007/s11886-017-0874-6
Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC (2008) Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 118(14):1433–1441. doi:10.1161/CIRCULATIONAHA.108.783910
Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, Sheikh F, Eichorn E, Lamba S, Bharmi R, Agarwal R, Kumar C, Stevenson LW (2017) Impact of practice based management of PA pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. doi:10.1161/CIRCULATIONAHA.116.026184
Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner Stevenson L (2017) Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail. doi:10.1161/CIRCHEARTFAILURE.116.003594
Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, Group CTS (2016) Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 387(10017):453–461. doi:10.1016/S0140-6736(15)00723-0
Sinning D, Kasner M, Westermann D, Schulze K, Schultheiss HP, Tschope C (2011) Increased left ventricular stiffness impairs exercise capacity in patients with heart failure symptoms despite normal left ventricular ejection fraction. Cardiol Res Pract 2011:692862. doi:10.4061/2011/692862
Lutembacher R (1916) De la stenose mitrale avec communication interauirulaier. Arch Mal Coeur Vaiss 9:237–260
Sondergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F (2014) Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail 16(7):796–801. doi:10.1002/ejhf.111
Hasenfuss G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, Malek F, Van der Heyden J, Lang I, Petrie MC, Cleland JG, Leon M, Kaye DM, investigators RL-Hs (2016) A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 387 (10025):1298–1304. doi:10.1016/S0140-6736(16)00704-2
Kasner M, Gaub R, Westermann D, Kaplan H, Akpulat S, Steendijk P, Schultheiss HP, Tschope C (2011) Simultaneous estimation of NT-proBNP on top to mitral flow Doppler echocardiography as an accurate strategy to diagnose diastolic dysfunction in HFNEF. Int J Cardiol 149(1):23–29. doi:10.1016/j.ijcard.2009.11.035
Borggrefe M, Burkhoff D (2012) Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur J Heart Fail 14(7):703–712. doi:10.1093/eurjhf/hfs078
Tschope C, Van Linthout S, Spillmann F, Klein O, Biewener S, Remppis A, Gutterman D, Linke WA, Pieske B, Hamdani N, Roser M (2016) Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function in HFpEF. Int J Cardiol 203:1061–1066. doi:10.1016/j.ijcard.2015.10.208
Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, Hoppe UC (2012) Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 59(10):901–909. doi:10.1016/j.jacc.2011.11.034
Patel HC, Rosen SD, Hayward C, Vassiliou V, Smith GC, Wage RR, Bailey J, Rajani R, Lindsay AC, Pennell DJ, Underwood SR, Prasad SK, Mohiaddin R, Gibbs JS, Lyon AR, Di Mario C (2016) Renal denervation in heart failure with preserved ejection fraction (RDT-PEF): a randomized controlled trial. Eur J Heart Fail 18(6):703–712. doi:10.1002/ejhf.502
Patel HC, Hayward C, Keegan J, Gatehouse PD, Rajani R, Khattar RS, Mohiaddin RH, Rosen SD, Lyon AR, di Mario C (2017) Effects of renal denervation on vascular remodelling in patients with heart failure and preserved ejection fraction: a randomised control trial. JRSM Cardiovasc Dis 6:2048004017690988. doi:10.1177/2048004017690988
Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, Klug D, Lovett EG, Muller-Ehmsen J, Schafer JE, Senni M, Swarup V, Wachter R, Little WC (2015) Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail 3(6):487–496. doi:10.1016/j.jchf.2015.02.006
Georgakopoulos D, Little WC, Abraham WT, Weaver FA, Zile MR (2011) Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail 17(2):167–178. doi:10.1016/j.cardfail.2010.09.004
Polsinelli VB, Shah SJ (2017) Advances in the pharmacotherapy of chronic heart failure with preserved ejection fraction: an ideal opportunity for precision medicine. Expert Opin Pharmacother. doi:10.1080/14656566.2017.1288717
Acknowledgements
European 7th Framework Consortium MEDIA (CT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CT: Steering Committee or Speaker honoraria from AstraZeneca, Novartis, Berlin Chemie, Servier, Bristol-Meyers Squibb GmbH, Roche, Boeheringer Ingelheim, Bayer Healthcare, Impulse Dynamics. CB: Speaker honoraria from Novartis, AstraZeneca. SF: Steering Committee or Speaker honoraria from AMGEN, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Bristol-Meyers Squibb GmbH, Daiichi Sankyo, MSD, Novartis, Pfizer, Servier. MB: Steering Committee, Advisory Board and Speaker honoraria from AstraZeneca, Bristol-Meyers Squibb, Boehringer Ingelheim, Medtronic, Novartis, St. Jude, Servier, Vifor. LSM: Steering Committee, Advisory Board and Speaker honoraria Gilead, Berlin-Chemie, MSD, Böhringer, Zoll, Astra-Zeneca, Novartis, Sanofi, Servier, Daiichi-Sankyo, Edwards, Bayer Healthcare, Medtronic, Pfizer, Abbott. SS: Steering committee or advisory board or speaker honoraria for AMGEN, AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Novartis, Pfizer, Servier. UL: Speaker honoraria from Amgen, Boehringer Ingelheim, MSD, Novartis, Sanofi, Servier. AL: none. BK: none. OB: none.
Rights and permissions
About this article
Cite this article
Tschöpe, C., Birner, C., Böhm, M. et al. Heart failure with preserved ejection fraction: current management and future strategies. Clin Res Cardiol 107, 1–19 (2018). https://doi.org/10.1007/s00392-017-1170-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1170-6