Abstract
Introduction
Coronary artery fistula (CAF) is a rare congenital anomalous connection between the coronary arteries (CA) and a cardiac chamber or great vessel. Treatment options of symptomatic CAF consist of transcatheter or surgical closure.
Methods
Retrospective analysis of all patients with CAF diagnosed between 1993 and 2014 concerning treatment approaches and follow-up after closure.
Results
In a cohort of more than 25000 patients, 194 (<0.01 %) were diagnosed to have CAF. Median age at diagnosis was 6 months (0 day–18 years). Treatment was indicated in ten patients (5.2 %). Six patients (60 %) were treated by catheter interventional approach using Coils (three patients), Amplatzer Vascular Plugs (two patients) and Amplatzer Duct Occluder (one patient). One of these patients showed a significant residual shunt through the fistula 5 days after interventional closure, necessitating surgical removal of the device and closure of CAF. At a median follow-up of 7 (range 2–12) years, the remaining five patients showed successfully closed CAF without causing thrombosis of the CA. Control angiography in three patients showed persistent dilated CA. Surgical closure of CAF was performed in four (40 %) patients; in two as an isolated procedure and in the remaining two as a part of another congenital cardiac corrective procedure.
Conclusions
CAF in paediatric cardiology patients is a very rare finding. Intervention in childhood is rarely needed; nevertheless, it is known that small fistulas may become relevant in adulthood. Transcatheter closure techniques are effective and are considered the treatment of choice, especially in isolated CAF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
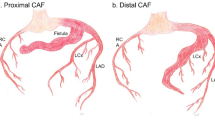
Coronary artery fistulas (CAF) are defined as congenital anomalous vascular connections between branches of the left (LCA) or right (RCA) coronary artery and any of the left or right cardiac chambers, the aorta or the pulmonary artery [1, 2]. Bypassing the myocardial capillary network, CAF may result in coronary steal and myocardial ischemia. The resultant left-to-right shunt may lead to volume overload and clinical signs of heart failure [1, 2]. The incidence of CAF in the paediatric population is not exactly known. For the adult population, angiographic investigations show an overall incidence of 0.13–0.6 % [3]. Children with CAF are usually asymptomatic, especially when the CAF are very small. The small CAF in children without hemodynamically significant left-to-right shunt may be incidently detected by echocardiographic evaluation of a typical continuous heart murmur [2]. Thanks to the continuous improvement of transthoracic echocardiography in the last decades, even small CAF without cardiac murmur can be detected [4]. Therefore, most of small CAF have become an incidental finding with rather low clinical or hemodynamic relevance and no need for further treatment. In rare cases, spontaneous closure of CAF has been reported [5]. In contrast, large CAF require further diagnostic work up. For detailed description of the cardiac anatomy of singular or multiple CAF, selective coronary angiography remains the gold standard, which may be complemented by myocardial perfusion study using MR angiography [1, 6, 7]. Treatment options of large CAF include either surgical or catheter interventional approaches depending on size, localization and tortuosity of the CAF as well as on the age and body weight of the patient [8–10]. Indication for treatment of CAF has changed during the last decade. Long-term follow-up of originally small, even asymptomatic CAF has shown that they may cause severe hemodynamic, thrombotic or ischemic complications later in adulthood due to a constant increase in their size [8, 11]. In addition, some authors describe that treatment in adulthood appears to be more difficult due to increased tortuosity of the CAF; thus igniting a debate about earlier treatment of CAF [12]. Indications for treatment of CAF in the paediatric age group include large CAF with clinical signs of heart failure due to significant ventricular volume overload, leading to fatigue, failure to thrive and dyspnoea. Treatment is also indicated in children presenting with arrhythmias or with signs of myocardial ischemia and ventricular dysfunction due to coronary steal [1, 2, 12].
Aim of our evaluation was to describe our experience in closure of CAF in childhood with special focus on transcatheter closure techniques and to provide long-term follow-up data.
Methods
In a retrospective data analysis, we evaluated the medical records and echocardiography reports of all consecutive patients with CAF diagnosed by transthoracic 2D Doppler echocardiography (TTE) between 1993 and 2014 concerning significance of CAF, treatment approaches and follow-up after closure. Reasons for admission were cardiologic evaluation of a heart murmur or suspicion of CHD.
TTE was performed using Sonos 5500 or Philips IE 33 (Philips Co., Amsterdam, Netherlands) or Vivid 7 or Vivid 9 (General Electric Company, Fairfield, Connecticut, US) using different types of echo probes ranging from 2 to 12 MHz.
All descriptive results are expressed as mean and standard deviation (SD) or median and range, as appropriate.
Results
In a cohort of 24316 children examined by echocardiography in the department of paediatric cardiology over a time frame of 21 years, 194 patients were diagnosed to have CAF and were enrolled in the analysis (0.01 %; 122 male, 62.9 %). Median age at diagnosis was 6 months (0 days–18 years). Baseline characteristics of all patients diagnosed to have CAF are presented in Table 1.
In 77 patients (39.7 %), CAF were isolated, whereas in 115 patients (59.3 %), CAF were found in association with congenital heart disease (CHD) (Fig. 1). In two cases, CAF were found in patients with acquired heart disease (1 mitral valve prolapse and 1 dilated cardiomyopathy). Patients in which CAF were only detected secondary to heart surgery were excluded from the analysis.
Associated congenital heart diseases in 115 patients with CAF. VSD ventricular septal defect, TOF tetralogy of fallot, CoA coarctation of the aorta, DORV double outlet right ventricle, AVSD atrioventricular septal defect, AS aortic stenosis, PA + IVS pulmonary atresia with intact ventricular septum, other atrial septum defect, hypoplastic left heart syndrome, hypertrophic cardiomyopathy; persistent ductus arteriosus, truncus arteriosus communis, aortic insufficiency, pulmonary stenosis, mitral stenosis, tricuspid atresia, Bland-White-Garland
Origin and drainage of CAF was not detectable in all cases. Origin was identifiable in 80 patients (41.2 %); in 77.5 % of them was the origin from the left coronary artery. Drainage of the fistula was detectable in 157 patients (80.9 %). They drained to the right side of the heart (the right atrium, the right ventricle or the main pulmonary artery) in 86.6 % cases, to the left side of the heart (left atrium or the left ventricle) in 11.5 % of the cases and into the coronary sinus in 3 % of the cases.
Ten patients (5.2 %) were treated for CAF due to hemodynamic relevance. Median age at diagnosis was 25 months (0 days–5 years). Baseline characteristics of patients treated for CAF are summarized in Table 2.
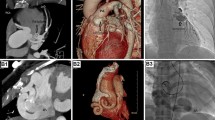
In four of these patients (40 %), CAF was associated with another CHD: atrial septal defect (ASD II, n = 1), Tetralogy of Fallot (TOF, n = 1), Anomalous coronary artery from pulmonary artery (ALCAPA, n = 1) and Pulmonary atresia with ventricular septal defect (PA + VSD, n = 1). In the remaining six cases (60 %), CAF were isolated. Two of these patients showed an aneurysmatically dilated right coronary artery (RCA) (Figs. 3, 4) with signs of volume overload of the right-sided cardiac chambers caused by fistula draining to the right heart. In another two patients (20 %), treatment was indicated due to aneurysmatically dilated coronary artery without signs of ischemia or volume overload of any cardiac chamber (1 dilated RCA and 1 dilated LCA). Two patients (20 %) with CAF draining from the LCA to the coronary sinus showed signs of congestive heart failure (n = 1) and left ventricular dysfunction without electrocardiographic or laboratory findings of myocardial ischemia (n = 1).
In patients treated for CAF, origin of the fistula was equally distributed to the left and right coronary artery (n = 5 each, 50 %). In all cases, CAF drained to the right heart, in eight cases directly to either the right atrium or the right ventricle, and in two cases via the coronary sinus (Table 2).
Interval from diagnosis to intervention in relevant CAF was 1 day to 47 months (median 9 days). In four patients (40 %) CAF were closed surgically, in two patients in combination with the repair of CHD (ALCAPA and PA + VSD), and in two as an isolated procedure. While a simple suture ligature was one of the common techniques used, in a case involving a fistula feeding an aneurysm, the fistulous connection was dissected, divided and suture closed at both the ends. At a median follow-up of 2 years (0–7 years) after surgery, all these patients were free of complications.
In six patients (60 %), CAF were closed with transcatheter techniques: using detachable coils in three (50 %, Fig. 2), Amplatzer Vascular Plugs in two (33.3 %, Fig. 3) and Amplatzer Duct Occluder in one patient (16.7 %, Fig. 4). CAF were primarily entered via the dilated coronary artery and were closed as distally as possible to avoid myocardial ischemia due to closure of more proximal branches of the coronary artery. In three cases (50 %), an arteriovenous wire loop was established by crossing the CAF with a 0.014 inch coronary wire and snaring the tip of the coronary wire from the venous side. With this arterial-venous wire loop a guiding catheter could be directed retrograde into the fistula from the venous side allowing a most distal closure at the opening of the fistula without any impact on coronary perfusion during the procedure. Nevertheless, the establishment of arterial-venous wire loop bears the risk of temporary myocardial ischemia with the risk of ST elevation. The remaining three cases (50 %) were closed antegradely from the arterial side (Table 2). In small tortuous coronary arteries, we used different catheter materials to reach distal parts of coronary arteries including microcatheter such as Cantata® (Cook Comp., Bloomington, IN, USA) to deliver different types of 0.018 embolisation coils (Balt Comp., Montmorency, France).
Median age at transcatheter intervention was 48 months (9 days–14 years) and median weight was 41.5 kg (3.8–68 kg). Median follow-up after transcatheter closure was 7 years (2–12 years). Immediate complete closure was achieved in five out of six patients (83.3 %). Follow-up revealed one significant residual shunt due to device instability 5 days after implantation of an Amplatzer Duct Occluder in a right ventricular CAF opening. Therefore, surgery was needed with removal of the device and closure of the CAF with consequent thrombosis of the CA. Persistent dilated CAs were seen on control angiography in three patients (50 %) and normal CA dimensions in two patients (33.3 %; control angiography in n = 1 and control echocardiography in n = 1). In these five patients, no thrombosis of CA occurred and CAF remained closed. Anticoagulation after interventional closure was performed with phenprocoumon (Marcoumar®, Meda Pharma GmbH) (target INR between 2 and 3) as monotherapy in four patients and phenprocoumon plus aspirin 5 mg/kg once daily (max. 100 mg/day) in two cases. Phenprocoumon was continued till control angiography (1 year post-intervention) in four of the five interventional cases, followed by aspirin only for 1 year. In one case with persistent dilatation, phenprocoumon was continued in combination with aspirin. Anticoagulation was stopped latest 2 years after closure of CAF in all except one patient, where aspirin therapy was stopped after control angiography 9 years following closure even though RCA ostium remained dilated.
Discussion
Incidence
Our data represents a group of patients diagnosed to have CAF in a pre-selected cohort seen in a single paediatric heart centre. The incidence of CAF found in our cohort of less than 0.01 % of children confirms that CAF is a rare finding, especially when it is isolated. Nevertheless, Uysal et al. reported CAF to be the most common coronary artery anomaly in children [13]. As diagnosis of CAF in childhood is predominantly made by echocardiography, compared to adulthood, where the main and more accurate diagnostic tool is coronary angiography, the low incidence of CAF in our paediatric cohort might be underestimated. With better echocardiography techniques, incidence of CAF might rise in the future as even small and irrelevant fistulas might be visualized. Remarkable was the high number of CAF associated with CHD in contrast to a low number of isolated CAF in our population [1, 3]. Older investigations have shown incidences of CAF in association with CHD in 5–30 % [14], whereas recent investigations have described higher percentage values for CAF in combination with CHD [15] of up to 50 %, especially in CAF arising from the right coronary artery. These newer findings go along with our results, where CAF were associated with CHD in 59.3 %, with VSD and Tetralogy of Fallot being the most common associated CHD.
The overall incidence in our data approximately confirms findings from Welisch et al. [15] in a paediatric cohort (0.43 %) where CAF were found in 0.13–0.6 % of patients undergoing angiographic examination with an overall incidence of 0.002 % in the general population [3, 9, 16].
Treatment indication
The majority of paediatric patients with CAF are and remain asymptomatic without cardiac medication, when CAF is an incidental echocardiographic finding [1, 8, 9, 17, 18]. Nevertheless, CAF may become symptomatic in adults presenting with chest pain due to myocardial ischemia or congestive heart failure and clinical signs of continuous heart murmur [8, 9, 11]. Further complications of CAF include coronary artery thrombosis, CAF rupture or infective endocarditis [1, 9]. As documented in the literature, our experience suggests that leading clinical features were congestive heart failure due to volume overload (n = 7, 70 %), without necessarily showing signs of myocardial ischemia [1, 2]. In 2008, guidelines of the American Heart Association (AHA) for adults with congenital heart disease recommended closure of large CAF and closure of symptomatic small and moderate CAF [10, 11, 19]. Guidelines for the paediatric population are not available so far. Therefore, treatment in paediatric patients remains an individual decision, orientating on the size of CAF, clinical signs (heart murmur, congestive heart failure), and complications (myocardial ischemia).
Closure technique
Treatment options for hemodynamically relevant CAF are surgical and transcatheter closure. Transthoracic echocardiography does not always reveal exact anatomy of CAF, therefore, to plan closure, detailed anatomy of CAF should be visualized by selective coronary angiography [1, 2]. Before 2000, surgical closure was the treatment of choice in most centres. Surgical treatment has been reported with excellent short and long-term results, even in childhood, despite complications including myocardial infarction and valve insufficiency, especially of the tricuspid valve, occurring in up to 11 % of patients [20]. In the AHA guidelines [19], surgical closure remains the treatment of choice, whereas transcatheter techniques should only be performed in centres with expertise in such procedures. Nevertheless, successful transcatheter closure of CAF even in small children has been reported, first in 1983 by Reidy et al. [21–23]. Due to its low rate of major complications transcatheter closure has been promoted as treatment of choice, even in neonates and infants [1]. Transcatheter approach is less invasive with no need for cardiopulmonary bypass. Smaller case studies have shown its benefit in the last years [12], even though it must be kept in mind that occlusion devices as seen in our population, bare the risk of dislocation with consecutive need for surgical intervention [24]. In addition, some CAF anatomy is not favourable for transcatheter closure, especially when the drainage site is close to the apex of the ventricle or the fistula has multiple orifices or drainage sites [1, 25]. As seen in our patient population, different transcatheter closing (TCC) techniques and different devices can be used safely with good long-term results, even in small infants. The choices of TCC and device selection vary, and are primarily determined by the heterogeneous anatomic characteristics of the fistulae [26]. The AV loop method which allows a most distal positioning of the implantation sheath from the venous site without compromising CA blood flow during the procedure was used in three of our patients and has become the most common and preferred TCC technique [26]. Other major techniques used are the direct antegrade (from the arterial side) or retrograde (from the venous side) access and sheath placement. Both techniques and several different closure devices (detachable coils, duct occluder, vascular plugs) have been used in children and adults [1, 8, 12, 26–30] since the first description in 1983 [23] (see Figs. 2, 3, 4). Nevertheless, there are other catheter interventional techniques described in the literature [31].
Anticoagulation management
Precise anticoagulation management after CAF closure is important. Gowda et al. [7, 32] have shown that in large distal CAF after surgical closure the risk for coronary artery thrombosis is elevated. They also found symptoms of the metabolic syndrome (e.g. arterial hypertension, obesity, …), older age at closure, dilated coronary arteries proximal to the CAF, and drainage to the coronary sinus as additional risk factors for coronary artery thrombosis in adults. Thakkar et al. [18], reported on a series of children who received post interventional antiplatelet therapy with aspirin and clopidogrel for 6 months, followed by aspirin alone. At a mean follow-up of 31 months, Thakkar et al. showed thrombotic occlusion of the fistula with a patent parent coronary artery in those having branch coronary artery fistula. Five of the seven patients with parent coronary artery fistula had near-complete occlusion of fistula extending into the native coronary artery. In our investigation, only the one patient with dislocation of the occlusion device showed a thrombosis of the RCA after surgical intervention, which also included a surgical size reduction of the aneurysmatically dilated CA as an additional risk factor. In all other cases, even when dilated proximal segments of the coronary arteries were still seen in follow-up, no thrombus formation has been detected. Recommendations for anticoagulation regimes are not available [32]. Taking into account the above-mentioned risk factors and considering our personal experience we suggest the primary use of phenprocoumon until control angiography after approximately 12 months, in which residual shunting, thrombus formation and dilatation of the fistula and the coronary artery should be re-evaluated. If normalization of main CA size can be shown, even when the CA origin remains dilated, it seems justifiable to switch to aspirin. If the CA size has not normalized, phenprocoumon should be continued.
Conclusion
As CAF are a very rare disease and treatment is rarely needed, individualized treatment strategies in paediatric patients are necessary. Treatment options are predetermined by individual anatomy and the experience of the treating centre. In cases where transcatheter experience is available and provided the anatomy is favourable, it should be the treatment of choice in isolated CAF, even in neonates. Application of different closure devices have been shown to be successful with good long-term results.
References
Sievert H (2007) Percutaneous interventions for congenital heart disease [Internet]. London; Boca Raton, FL: Informa Healthcare; Distributed in North and South America by Taylor & Francis [cited 2014 Sep 2]
Qureshi SA (2006) Coronary arterial fistulas. Orphanet J Rare Dis 1:51
Yamanaka O, Hobbs RE (1990) Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 21(1):28–40
Sherwood MC, Rockenmacher S, Colan SD, Geva T (1999) Prognostic significance of clinically silent coronary artery fistulas. Am J Cardiol 83(3):407–411
Schleich JM, Rey C, Gewillig M, Bozio A (2001) Spontaneous closure of congenital coronary artery fistulas. Heart Br Card Soc 85(4):E6
Gowda RM, Vasavada BC, Khan IA (2006) Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol 107(1):7–10
Gowda ST, Forbes TJ, Singh H, Kovach JA, Prieto L, Latson LA et al (2013) Remodeling and thrombosis following closure of coronary artery fistula with review of management: large distal coronary artery fistula—to close or not to close? Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 82(1):132–142
Harikrishnan S, Bimal F, Ajithkumar V, Bhat A, Krishnamoorthy KM, Sivasubramonian S et al (2011) Percutaneous treatment of congenital coronary arteriovenous fistulas. J Intervent Cardiol 24(3):208–215
Jama A, Barsoum M, Bjarnason H, Holmes DR, Rihal CS (2011) Percutaneous closure of congenital coronary artery fistulae: results and angiographic follow-up. JACC Cardiovasc Interv 4(7):814–821
Tirilomis T, Aleksic I, Busch T, Zenker D, Ruschewski W, Dalichau H (2005) Congenital coronary artery fistulas in adults: surgical treatment and outcome. Int J Cardiol 98(1):57–59
Liberthson RR, Sagar K, Berkoben JP, Weintraub RM, Levine FH (1979) Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation 59(5):849–854
Wang S-S, Zhang Z-W, Qian M-Y, Zhuang J, Zeng G-H (2013) Transcatheter closure of coronary arterial fistula in children and adolescents. Pediatr Int Off J Jpn Pediatr Soc
Uysal F, Bostan OM, Semizel E, Signak IS, Asut E, Cil E (2013) Congenital Anomalies of coronary arteries in children: the evaluation of 22 patients. Pediatr Cardiol
Wong KT, Menahem S (2000) Coronary arterial fistulas in childhood. Cardiol Young 10(1):15–20
Welisch E, Norozi K, Burrill L, Rauch R (2015) Small coronary artery fistulae in childhood: a 6-year experience of 31 cases in a tertiary paediatric cardiac centre. Cardiol Young 14:1–5
Luo L, Kebede S, Wu S, Stouffer GA (2006) Coronary artery fistulae. Am J Med Sci 332(2):79–84
Sunder KR, Balakrishnan KG, Tharakan JA, Titus T, Pillai VR, Francis B et al (1997) Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. Int J Cardiol 58(1):47–53
Thakkar B, Patel N, Poptani V, Madan T, Saluja T, Shukla A et al (2015) Clinical and angiographic follow-up of coronary artery fistula interventions in children: techniques and classification revisited. Cardiol Young 25(4):670–680
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA et al (2008) ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 52(23):e143–e263
Said SM, Burkhart HM, Schaff HV, Connolly HM, Phillips SD, Suri RM et al (2013) Late outcome of repair of congenital coronary artery fistulas—a word of caution. J Thorac Cardiovasc Surg 145(2):455–460
Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ (1991) Transcatheter embolization in the treatment of coronary artery fistulas. J Am Coll Cardiol 18(1):187–192
Reidy JF, Jones OD, Tynan MJ, Baker EJ, Joseph MC (1985) Embolisation procedures in congenital heart disease. Br Heart J 54(2):184–192
Reidy JF, Sowton E, Ross DN (1983) Transcatheter occlusion of coronary to bronchial anastomosis by detachable balloon combined with coronary angioplasty at same procedure. Br Heart J 49(3):284–287
Strecker T, Hakami L, Singer H, Weyand M, Cesnjevar R (2006) Congenital coronary artery fistula draining into the right ventricle: successful surgical closure after failed transcatheter coil embolization. Thorac Cardiovasc Surg 54(1):61–63
Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE (2002) Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol 39(6):1026–1032
Xiao Y, Gowda ST, Chen Z, Delaney JW, Amin Z, Latson LA et al (2015) Transcatheter closure of coronary artery fistulae: considerations and approaches based on fistula origin: transcatheter closure of coronary artery Fistulae. J Intervent Cardiol 28(4):380–389
Wang C, Zhou K, Li Y, Qiao L, Wang Y, Shi X et al (2014) Percutaneous transcatheter closure of congenital coronary artery fistulae with patent ductus arteriosus occluder in children: focus on patient selection and intermediate-term follow-up results. J Invasive Cardiol 26(7):339–346
Gribaa R, Slim M, Ouali S, Neffati E, Boughzela E (2014) Transcatheter closure of a congenital coronary artery to right ventricle fistula: a case report. J Med Case Rep 8(1):432
Perry SB, Rome J, Keane JF, Baim DS, Lock JE (1992) Transcatheter closure of coronary artery fistulas. J Am Coll Cardiol 20(1):205–209
Kharouf R, Cao Q-L, Hijazi ZM (2007) Transcatheter closure of coronary artery fistula complicated by myocardial infarction. J Invasive Cardiol 19(5):E146–E149
Dietze T, Paliege R, Berger D, Gradaus R, Neuzner J (2014) Closure of a coronary to pulmonary artery fistula by radiofrequency catheter ablation in the pulmonary artery. Clin Res Cardiol Off J Ger Card Soc 103(1):73–74
Gowda ST, Latson LA, Kutty S, Prieto LR (2011) Intermediate to long-term outcome following congenital coronary artery fistulae closure with focus on thrombus formation. Am J Cardiol 107(2):302–308
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure
All authors have no financial relationships relevant to this article to disclose.
Conflict of interest
All authors have no potential conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Christmann, M., Hoop, R., Dave, H. et al. Closure of coronary artery fistula in childhood: treatment techniques and long-term follow-up. Clin Res Cardiol 106, 211–218 (2017). https://doi.org/10.1007/s00392-016-1041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1041-6