Abstract
Background
This observational study was carried out to investigate the effect of intravenous (i.v.) iron administration on the clinical symptoms of restless legs syndrome (RLS) in geriatric outpatients over 65 years old.
Methods
In this study geriatric outpatients (mean 4.5 ± 3.4 comorbidities and 5.7 ± 4.4 drugs taken) were recruited according to the following inclusion criteria: ≥ 65 years, diagnosis of RLS due to iron deficiency, i.e. ferritin < 50 µg/l or transferrin saturation (TFS) < 16 %) as well as no other iron therapy within 2 weeks prior to the study. Of the patients 7 (41 %) received 500 mg ferric carboxymaltose (FCM) and 10 patients (59 %) received iron gluconate (62.5 mg) based on the degree of iron deficiency. As assessed by the international RLS severity scale (IRLS) symptoms were recorded 3 times: at the beginning of iron therapy (t0), after 2 weeks (t1) and after 12 weeks (t2).
Results
A total of 17 patients (13 female, 4 male, mean age 73.2 ± 5.9 years) were included. The IRLS score significantly improved in all patients as shown by an average decrease from 30.2 (± 4.3) to 20.2 (± 4.7) (p < 0.001) after 2 weeks of i.v. iron treatment and to 23.2 ± 6.6 (p < 0.001) after 12 weeks. There was a high correlation between ferritin values and the IRLS score (C 0.729, p < 0.001). The part of the IRLS referring to activities of daily living (ADL) improved from a median of 3 (scores 3–4) to 2 (scores 2–3, p = 0.001) after 2 weeks (effect size − 0.6).
Conclusion
In this study group of geriatric outpatients i.v. administration of iron was associated with a significant improvement of symptoms in RLS as assessed by the IRLS score 2 weeks after treatment. In geriatric patients with RLS associated with iron deficiency, i.v. iron administration should be considered regarding improvement of RLS symptoms and ADL.
Zusammenfassung
Hintergrund
Es wurde eine Beobachtungstudie zur Untersuchung der Wirkung der intravenösen (i.v.) Eisengabe auf die klinischen Symptome des Restless-legs-Syndroms (RLS) bei geriatrischen ambulanten Patienten über 65 Jahren durchgeführt.
Methoden
Eingeschlossen wurden Patienten (4,5 ± 3,4 Komorbiditäten, 5,7 ± 4,4 eingenommene Wirkstoffe) gemäß folgender Einschlusskriterien: ≥ 65 Jahre, Eisenmangel assoziiertes RLS (Ferritin < 50 µg/l oder Transferrinsättigung (TFS) < 16 %) sowie keine Eisengabe 2 Wochen vor Studieneinschluss. Sieben Patienten (41 %) erhielten 500 mg Eisencarboxymaltose (FCM) und 10 Patienten (59 %) erhielten Eisenglukonat (62,5 mg) basierend auf der Höhe des Eisenmangels. Mittels International RLS Severity Scale (IRLS), wurde der Schweregrad des RLS dreimal bewertet: zu Beginn der Eisengabe (t0), nach 2 Wochen (t1) und nach 12 Wochen (t2).
Ergebnisse
Siebzehn Patienten (13 weiblich, 4 männlich, mittleres Alter 73,2 ± 5,9 Jahre) wurden eingeschlossen. Der IRLS-Score verbesserte sich signifikant bei allen Patienten, basierend auf einer Abnahme des IRLS-Score von 30,2 (± 4,3) auf 20,2 (± 4,7) (p < 0,001) 2 Wochen nach der i.v. Eisenbehandlung und auf 23,2 ± 6,6 (p < 0,001) nach 12 Wochen. Der Schweregrad des RLS zeigte eine hohe Korrelation mit den gemessenen Ferritinwerten (C 0,729, p < 0,001). Der Teil des IRLS, welcher die Aktivitäten des täglichen Lebens (ADL) abbildet verbesserte sich im Median von 3 [3–4] auf 2 [2–3; p = 0,001)] nach 2 Wochen (Effektgröße − 0,6).
Schlussfolgerung
In diesem geriatrischen ambulanten Kollektiv ist eine i.v. Eisengabe mit einer signifikanten Besserung der RLS Symptome nach 2 Wochen verbunden. Bei geriatrischen Patienten mit Eisenmangel assoziiertem RLS sollte immer auch eine i.v. Eisengabe zur Besserung von RLS und ADL erwogen werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Restless legs syndrome (RLS), either idiopathic or secondary, is a common sensorimotor disorder with a reported prevalence of 6–12 % among the population in western countries [4]. It is characterized by strong unpleasant sensations, a nearly irresistible urge to move the legs in the evening or at night and temporary or partial relief of symptoms can be achieved by moving the legs or walking [3]. The association between iron deficiency and the development of a secondary form of RLS is acknowledged [10, 17] and it could be shown that the severity of RLS symptoms correlated with the degree of iron deficiency [10]. It is assumed that iron deficiency impedes the synthesis of dopamine, which is involved in the pathophysiology of RLS [10]. The prevalence of RLS increases with age and is found in 10 % of patients > 65 years old [8]. Furthermore, iron deficiency is more common in geriatric than in younger patients [18].
The current standard treatment of RLS is administration of dopaminergic agents [16] but with the limitation of symptom augmentation [2] due to the therapy. An occurrence of iron deficiency might even enhance symptom augmentation [19]; therefore, further studies should address the optimization of treatment options including the substitution of iron as a causal therapy approach in RLS associated with iron deficiency. Data regarding oral administration of iron are controversial [9, 23] and there are only a few studies on i.v. iron administration [15]. A recent observational study showed an improvement of the mean IRLS score from 30.1 ± 5.9 to 23.1 ± 9.5 (p = 0.001) by i.v. iron administration but not specifically in geriatric patients [15]. At present there is no study known to the authors that addressed the efficacy of i.v. iron therapy in geriatric RLS patients. The findings in terms of an iron substitution are based on few data [21] and make it difficult to issue a general recommendation regarding substitution therapy. According to current data [16] iron substitution is generally recommended in the case of RLS associated with iron deficiency; however, i.v. iron administration is not generally recommended due to a lack of data. In the present prospective non-invasive observational study the effect of i.v. iron administration on the clinical symptoms in geriatric outpatients with RLS was investigated.
Methods
A bicentric observational study of geriatric outpatients (age ≥ 65 years) was performed. The study was approved by the local ethics committees (University Hospital Cologne, reference nr. 12–273 and University of Ulm, reference nr. 309/13). Patients were recruited from members of the German RLS patient support group (PSG) and from patients of an RLS outpatient department. All included patients gave written informed consent to participate. All patients fulfilled the revised essential criteria for the diagnosis of idiopathic RLS defined by the International RLS Study Group (IRLSSG) [3]. In 2011 the IRLSSG revised the essential diagnostic criteria and included a fifth principal criterion requiring the typical RLS symptoms to be without doubt not associated with other medical or behavioral conditions (http://irlssg.org/). In this observational study the inclusion criteria were patients with RLS receiving a medically prescribed i.v. iron therapy due to an iron deficiency, i.e. ferritin < 50 µg/l or transferrin saturation (TFS) < 16 % as well as no other iron therapy within 2 weeks prior to the study. Exclusion criteria were an administration of oral iron therapy or participation in another study.
During the study the IRLS score [22] was assessed three times: immediately after i.v. iron administration (baseline t0), 2 weeks after i.v. iron administration (t1) and 12 weeks after i.v. iron administration (t2). Additionally, blood levels of ferritin and changes in RLS medication were recorded. Details of the RLS medication and on demand medication (fast-acting medication for exacerbation of RLS) are given in Table 1.
The patient characteristics are shown in a Table 2. The impact of RLS on activities of daily living (ADL) was assessed by analysis of the answers to question no. 9 of the IRLS score. Due to the observational study design and for ethical reasons, no placebo control group was included. The statistical analysis was performed using the statistical software SPSS (Statistical Package for the Social Sciences), version 22.
In this study the established IRLS score [22] was applied to assess the severity of RLS as well as to evaluate the effect of the iron therapy within the time frames t0–t1, t1–t2 and t0–t2. The IRLS score consists of 10 questions regarding clinical symptoms within the recent weeks and the impact of RLS on the quality of life and mood state. For every question 0–4 points are given. The RLS is divided into the following severity levels: no RLS = 0, mild RLS = 1–10 points, moderate RLS = 11–20 points, severe RLS = 21–30 points and very severe RLS = 31–40 points [22]. According to the European RLS study group an improvement in the IRLS score of ≥ 6 points in comparison to a control group is considered as being clinically relevant for the patient [20]. The score shows a high inter-examiner reliability, a high test-retest reliability and a high consistency [22]. The answers to question no. 9, which refers to the ability to carry out daily affairs, such as satisfactory home and family life were evaluated separately. All interviews during this study were performed by the same interviewer to avoid methodological errors. None of the patients suffered from clinically relevant cognitive limitations.
Frequencies were used for the description of categorical variables. As the sample size was small both mean ± standard deviation (SD) and median or interquartile range (IQR) were calculated for metric variables and comparisons of change were performed with nonparametric Wilcoxon tests. Effect sizes for IRLS were calculated as Cohen’s d and for ADL by Wilcoxon tests. Correlations were analyzed with Spearman’s rho (ρ).
Results
Out of more than 100 patients screened for the study, only 17 patients met the inclusion criteria, gave informed consent and were included. Of these patients 16 were recruited from the PSG members and 1 patient was recruited from patients of the neurological outpatient department of the University Hospital of Ulm (13 female and 4 male patients). The mean age was 73.2 ± 5.9 years (median 72 years, range 69–75 years). An average of 1.8 ± 1.2 RLS medications were taken per patient (Table 2). The RLS medication did not change over the 12 weeks in the 17 patients. The distribution of the IRLS score and RLS severity of the patients are presented in Fig. 1.
Of the 17 patients 8 (47 %) had very severe RLS at the beginning of the study with 31–40 IRLS points and 1 patient had a very severe relapse of RLS after 12 weeks with 35 IRLS points. No patient showed an IRLS score above 30 points at t1 (2 weeks after i.v. iron administration). Table 3 shows that there was a significant improvement in all 17 patients within the first 2 weeks and which was still remaining 12 weeks after treatment, with effect sizes of − 2.1 and − 1.1 compared to t0, respectively; however, a deterioration of the IRLS was observed between weeks 2 and 12 (effect size 0.6).
The individual profiles of the severity of RLS showed that in 2 of the patients the IRLS score relapsed to the initial level or even higher after 3 months. In 8 patients, the IRLS score was still slightly better but less than 6 points better and in 7 of the patients the improvement remained clinically relevant after 3 months. The ADL was assessed by answers to question no. 9 of the IRLS score. Improvement was evaluated separately for this part of the score which decreased from a median of 3 (range 3–4) to 2(range 2–3) after 2 weeks with an effect size of − 0.6 (p = 0.001).
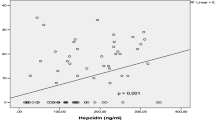
Due to the lack of data only 12 ferritin values were determined at week 0, 4 values at week 2 and 8 values by the end of follow-up. Ferritin values were related to the IRLS score (Fig. 2). The analysis revealed that a lower IRLS score was associated with higher iron blood levels in this study group. No very severe RLS (IRLS score > 30) occurred in patients with a ferritin level higher than 50 µg/l and there was a high correlation (Spearman’s correlation coefficient 0.726, p < 0.001). No correlation was found between improvement of the IRLS score 2 weeks after iron infusion at t0 and the baseline ferritin level (correlation coefficient 0.166, p = 0.627) (Fig. 3).
Discussion
The rationale for this prospective observational study was to analyze the effect of i.v. iron treatment on clinical symptoms in geriatric RLS patients (age ≥ 65 years) as assessed by the IRLS. The results of the present study showed a significant mean improvement of the IRLS score by 10 points within 2 weeks after the first i.v. iron administration and resulted in a clinically relevant improvement of symptoms. The effect size of − 2.1 showed a strong correlation. Only 2 patients had an improvement less than the required 6 points in the IRLS score after 2 weeks; however, these patients had already started with a low IRLS score prior to the i.v. iron administration (i.e. 18 and 27, respectively). The results of this study were similar to those of previous studies also using the IRLS score to objectify symptom improvement after iron application in non-geriatric patients. In these studies different iron formulations and routes of administration were used. Allen et al. applied 500 mg ferric carboxymaltose twice at an interval of 5 days [1], the results were compared with a placebo group and patients with ferritin levels up to 300 µg/l were included. The IRLS showed a significant decrease of 8.9 ± 8.52 versus 4.0 ± 6.11 in the placebo group (p = 0.040). Earley et al. used 1000 mg iron sucrose in a single dose [11] and there was no difference in improvement compared to the placebo group (IRLS decreased by 12.0 ± 11.5 in the placebo group and by 10.1 ± 5.1 in the treated patients). The authors assumed that iron sucrose could be less effective for replenishing iron storage in the brain and bone marrow (center of dopamine synthesis) than newer formulations, such as ferric carboxymaltose. Patients with higher baseline ferritin levels than those of patients in this study were also included. Grote et al. [13] administered 1000 mg iron sucrose in a single dose to patients with lower baseline ferritin levels (≤ 45 µg/l) compared with a placebo group. Improvement of the IRLS score was significant after 11 weeks (IRLS reduced by 12.2 ± 8.3 to 9.9 ± 11.7). Wang et al. [23] included RLS patients with a ferritin value up to 75 µg/l. In this study oral iron (iron sulfate) was administered and showed a significant improvement in the study group of 10.3 ± 7.4 compared with − 1.1 ± 5.6 in the placebo group (p = 0.01). Here again patients were included in the study depending on baseline ferritin values. Hornyak et al. found a higher responder rate in patients with a lower ferritin baseline value [15]. This finding could not be confirmed in our patient group, which might be due to the relatively low number of study patients with only two patients showing a high ferritin baseline value (45–50 µg/l).
Ferritin baseline values seem to be the most important parameter in clinical decision making regarding iron substitution for RLS treatment. Interestingly, previous studies without significant improvement after oral iron administration included patients with ferritin baseline levels > 100 µg/l [9]; however, in geriatric patients higher ferritin levels may be age-associated and might obscure an iron deficiency [6]. Out of 17 patients included in the present study 8 (47 %) were classified with very severe RLS according to the IRLS score at the beginning of treatment. This classification was associated with low ferritin values, which is consistent with the findings of Frauscher et al. [12] who found that a low ferritin value is inversely proportional to the severity of RLS. The concerned patients’ average IRLS score at t0 of 30.2 was significantly higher than the average score of a comparable French geriatric study group (i.e. 16.6) [7], where RLS was present in 24.2 % of the patients, independent of their ferritin values. Our findings further support the assumption of an inverse correlation between RLS symptoms and ferritin values.
In the present study an impact of iron treatment on the performance of ADL could be shown. The mean score for question no. 9 of the IRLS, which refers to the ability to carry out daily affairs, such as satisfactory home and family life improved from a median of 3 (3–4) to 2 (2–3) (p = 0.001) after 2 weeks of treatment (effect size − 0.6). Patients regained independence and control of their private life after treatment. The maintenance of independence and ADL competences in geriatric patients is an important parameter of treatment success.
Patients took on average 1.8 ± 1.2 RLS medications in addition to the standard medication and therefore had an increased risk for interactions and drug-related adverse effects. The number of drugs taken is an independent marker for the appearance of iatrogenic conditions [5] and up to 3 % [14] of hospital admissions of older individuals are caused by drug interactions; therefore, the application of i.v. iron may also be of great interest in connection with a drug reduction of polymedication in geriatric RLS patients.
Finally it has to be underlined, that in this study none of the 17 patients included showed any adverse reactions associated with the i.v. iron administration. Due to a lack of data for the measurable blood parameters, the ferritin-related increase in clinical symptoms 12 weeks after i.v. iron administration remains unknown. Previous studies could show a slight correlation of a worsening of the RLS and a depletion of iron stores [13].
In this study no differences were seen in ferritin baseline values between the patients with durable improvement of RLS and relapsed patients. The study also has some limitations: the observational design partly hinders interpretability and allocation of RLS-related symptom improvement exclusively to iron administration. In addition, the pathophysiological mechanism of the improvement cannot be correlated with ferritin changes due to the lack of data. There was no control group to show the difference to placebo and so the improvement cannot be directly linked to the i.v. iron administration; however, the association is suggested by the correlation of the ferritin values to the improvement. Furthermore, changes in the RLS on demand medication (fast-acting medication in an acute exacerbation of RLS) were not evaluated. This could lead to an even higher difference in the IRLS score. Previous studies with no benefits of iron treatment also included patients with higher ferritin base levels [11].
Conclusion
In summary the results of this small study suggest that in geriatric patients with RLS associated with iron deficiency and no contraindications, i.v. iron administration can be effective in combination with dopamine medication. The IRLS as well as the ability to carry out daily activities can be improved. A subsequent reduction of the standard RLS medication could also improve therapy adherence and reduce drug interactions and hospital admissions. Further research on larger study populations in a randomized controlled setting is needed.
References
Allen RP, Adler CH, Du W et al (2011) Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi-centred, placebo-controlled preliminary clinical trial. Sleep Med 12(9):906–913
Allen RP, Ondo WG, Ball E et al (2011) Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Med 12(5):431–439
Allen RP, Picchietti D, Hening WA et al (2003) Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4(2):101–119
Berger K, Kurth T (2007) RLS epidemiology—frequencies, risk factors and methods in population studies. Mov Disord 22(Suppl 18):S420–S423
Bjerrum L, Gonzalez Lopez-Valcarcel B, Petersen G (2008) Risk factors for potential drug interactions in general practice. Eur J Gen Pract 14(1):23–29
Cankurtaran M, Yavuz BB, Halil M et al (2012) Increased ferritin levels could reflect ongoing aging-associated inflammation and may obscure underlying iron deficiency in the geriatric population. Eur Geriatr Med 3(5):277–280
Celle S, Roche F, Kerleroux J et al (2010) Prevalence and clinical correlates of restless legs syndrome in an elderly French population: the synapse study. J Gerontol A Biol Sci Med Sci 65A(2):167–173
Çurgunlu A, Döventaş A, Karadeniz D et al (2012) Prevalence and characteristics of restless legs syndrome (RLS) in the elderly and the relation of serum ferritin levels with disease severity: hospital-based study from Istanbul, Turkey. Arch Gerontol Geriatr 55(1):73–76
Davis BJ, Rajput A, Rajput ML et al (2000) A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol 43(2):70–75
Earley CJ, Allen RP, Beard JL et al (2000) Insight into the pathophysiology of restless legs syndrome. J Neurosci Res 62(5):623–628
Earley CJ, Horska A, Mohamed MA et al (2009) A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med 10(2):206–211
Frauscher B, Gschliesser V, Brandauer E et al (2009) The severity range of restless legs syndrome (RLS) and augmentation in a prospective patient cohort: association with ferritin levels. Sleep Med 10(6):611–615
Grote L, Leissner L, Hedner J, Ulfberg J. (2009) A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord 24:1445–1452
Hamilton RA, Briceland LL, Andritz MH (1998) Frequency of hospitalization after exposure to known drug-drug interactions in a Medicaid population. Pharmacotherapy 18(5):1112–1120
Hornyak M, Scholz H, Kiemen A et al (2012) Investigating the response to intravenous iron in restless legs syndrome: an observational study. Sleep Med 13(6):732–735
Hornyak M, Scholz H, Kohnen R et al (2014) What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med Rev 18(2):153–164
Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. (2005) CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res 14(1):43–47
Röhrig G, Doehner W, Schaefer RM et al (2012) Anämie und Eisenmangel in der Geriatrie. Prävalenz, Diagnostik und neue Therapieoptionen (Anemia and iron deficiency in the elderly. Prevalence, diagnostics and new therapeutic options). Z Gerontol Geriatr 45(3):191–196
Trenkwalder C, Högl B, Benes H et al (2008) Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med 9(5):572–574
Trenkwalder C, Kohnen R, Allen RP et al (2007) Clinical trials in restless legs syndrome—recommendations of the European RLS Study Group (EURLSSG). Mov Disord 22(Suppl 18):S495–S504
Trotti LM, Bhadriraju S, Becker LA (2012) Iron for restless legs syndrome. Cochrane Database Syst Rev 5:CD007834
Walters AS, LeBrocq C, Dhar A et al (2003) Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4(2):121–132
Wang J, O’Reilly B, Venkataraman R et al (2009) Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: a randomized, double-blind, placebo-controlled study. Sleep Medicine 10(9):973–975
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Roehrig and R. J. Schulz have received honoraria for academic lectures from viforpharma. J. Kassubek has received personal compensation for activities with UCB Pharma, GlaxoSmithKline, Teva, Medtronic and Boehringer Ingelheim Pharmaceuticals as a consultant and honoraria for academic lectures from Merz and Bayer. M. C. Polidori, I. Becker and B. Lieske declare that there are no conflicts of interest.
The study was approved by the local ethics committees of the University Hospital Cologne (reference nr. 12–273) and the University of Ulm (reference nr. 309/13). All included patients gave written informed consent to participate.
Rights and permissions
About this article
Cite this article
Lieske, B., Becker, I., Schulz, R. et al. Intravenous iron administration in restless legs syndrome. Z Gerontol Geriat 49, 626–631 (2016). https://doi.org/10.1007/s00391-015-0984-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-015-0984-y