Abstract
Introduction
Currently, cold snare polypectomy (CSP) without submucosal injection is recommended for removing polyps < 10 mm. Use of viscous submucosal agents has not been previously evaluated in CSP. We investigate the potential role of EverLift™ (GI Supply, Pennsylvania) in CSP.
Methods
The study is a single-center prospective randomized non-inferiority clinical trial evaluating CSP of non-pedunculated 4–9 mm polyps, with or without submucosal injection of EverLift™. Patients 18–80 years of age presenting for colonoscopy were recruited. Eligible polyps underwent block randomization to CSP with or without EverLift™. Following CSP, two biopsies were performed at the CSP site margin. The primary non-inferiority outcome was complete resection rate, defined by absence of residual polyp in the margin biopsies (non-inferiority margin −10%).
Results
A total of 291 eligible polyps underwent CSP, with 142 removed using EverLift™. There was similar polyp size and distribution of pathology between the two groups. Overall, there was a low rate of positive margins with (1.4%) or without submucosal injection (2.8%), with no significant difference in complete resection (difference 1.28%, 95% CI: −2.66 to 5.42%), demonstrating non-inferiority of EverLift™ injection. Use of EverLift™ significantly increased CSP time (109.8 vs 38.8 s, p < 0.0001) and frequency of use of hemostatic clips (13.4 vs 3.6%, p = 0.002).
Conclusion
Submucosal injection of EverLift™ was non-inferior to CSP of 4–9 mm polyps without injection and increased time for resection as well as use of hemostatic clips to control acute bleeding. Our results suggest that polypectomy of 4–9 mm polyps can be safely performed without submucosal injection of EverLift™.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over time, different solutions have been utilized during polypectomy, including normal saline, fibrinogen, succinylated gelatin, hydroxyethyl starch, glycerol, glucose solution, sodium hyaluronate, and hydroxypropyl methylcellulose. These solutions vary in histopathological integrity, ability to maintain a submucosal cushion, complete resection rate, and cost [1]. In a meta-analysis reviewing different submucosal injection solutions used in endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), compared with normal saline, other submucosal injection solutions (group comprising of fibrinogen mixture, dextrose, sodium hyaluronic acid, mesna, hydroxyethyl starch, and succinylated gelatin) had a higher rate of complete resection [2].
Currently, guidelines recommend removal of colon polyps < 10 mm with cold snare, without need for submucosal injection [1, 3]. However, there are few studies evaluating the use of submucosal injection in polyps < 10 mm. In 2018, Zhang et al. [4] compared CSP with or without submucosal injection of normal saline/epinephrine/indigo carmine in the removal of polyps 6–9 mm. Incomplete resection rate was significantly lower in the group with compared to the group without submucosal injection (1.5 vs 8.5%, p= 0.001) [4]. In contrast, in 2020, Shimodate compared cold snare polypectomy (CSP) with submucosal injection of normal saline/indigo carmine/epinephrine to without submucosal injection among polyps 3–10 mm, and found no difference in rates of complete muscularis mucosal resection (43.9% [submucosal injection] vs 53.3% [no submucosal injection], p= 0.22). Interestingly, negative lateral and vertical margins were significantly lower in the saline submucosal injection group [5]. The differences in resultsmay be related to the sizes of the polyps evaluated and the methodology to determine complete resection, but suggest that the benefit of submucosal injection is unclear. Another question is whether the specific submucosal injection agent used impacts the complete resection rate. While the two studies used normal saline/indigo carmine/epinephrine, to date, there has been no evaluation performed of viscous submucosal lifting agents [5].
In the past few years, multiple viscous lifting agents have been developed [6, 7]. In March 2017, the FDA approved Eleview™, which has been found to be useful in assisting with ESD and EMR for polyps ≥ 10 mm [6, 7]. ORISE™ has also been approved, with similar composition to Eleview [8]. However, these solutions have not been studied in polyps < 10 mm. In June 2020, EverLift™ submucosal lifting agent (GI Supply, Mechanicsburg, Pennsylvania) was approved by the United States Food and Drug Administration (FDA). EverLift™ is composed of water, glycerin, hydroxyethyl cellulose, benzyl alcohol, sodium and potassium phosphate, and methylene blue [9]. Currently, there have been no published studies evaluating the use of EverLift™.
Methods
Study design
The study is a single-center prospective randomized non-inferiority clinical trial, evaluating the use of submucosal injection of EverLift™ in cold snare polypectomy for polyps 4–9 mm. The study protocol was performed in accordance to the Declaration of Helsinki and approved by the institutional review board of Stanford University. The study is registered at ClinicalTrials.gov (NCT04551014). There were no funding provided for this study. The full trial protocol can be made available on request to the corresponding author.
Patient selection
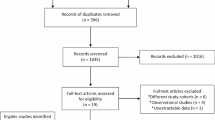
Adults (age 18–80) who presented to Veterans Affairs Palo Alto for outpatient screening, surveillance, or diagnostic colonoscopy between September 16, 2020, and May 31, 2021, were invited to participate. We included patients on anticoagulation therapy, provided that the anticoagulant agent(s) were held consistent with ASGE guidelines prior to the procedure [10]. Exclusion criteria were age < 18, age > 80, patients who did not provide informed consent, who were pregnant, as well as patients with inflammatory bowel disease or polyposis syndromes. Inclusion criteria for analysis were patients who had one or more polyps 4–9 mm removed during the colonoscopy. Colonoscopies without removal of polyps 4–9 mm were excluded from analysis.
Endoscopists
All procedures were performed by staff endoscopists at VA Palo Alto hospital, with or without assistance of fellows. Each of the staff (SF, JYP, SYQ, RW) has at least 4 years of experience after completion of fellowship and has performed > 2,500 colonoscopies. If colonoscopies were performed with fellow assistance, the staff endoscopist was present to supervise throughout the procedure.
Randomization and concealment
Each polyp sized 4–9 mm underwent block randomization to being removed by cold snare polypectomy with or without submucosal injection of EverLift™. The assigned technique was concealed in opaque, sealed, sequentially numbered envelopes. When a polyp was encountered, the envelope was opened to reveal the technique the endoscopist would be performing polypectomy by cold snare, either with or without submucosal injection [11]. Randomization was performed using random block sizes of 4, 6, and 8, following lists created for each endoscopist. Block randomization was performed using a computer-generated randomization sequence [12].
Polypectomy and randomization
The endoscopist measured and recorded the polyp size using the open Exacto® cold snare (Steris, California, USA) as reference. Description and location of the polyp were also recorded at time of the procedure. Polyps were classified based on the Paris classification [13]. Removal of the polyp en bloc or piecemeal was recorded. All polyps were removed and placed in individual containers. Each colonoscopy withdrawal video was recorded. One author (MTW) reviewed the videos and measured the time between when the snare was introduced into the field until completion of polypectomy. The number of snare passes to remove all polyp tissue was recorded. Use of hemostasis clips was documented as well.
Once the endoscopist felt they had adequately removed all polyp tissue from the polypectomy site using the cold snare, two cold biopsies were performed at the circular margins of the polypectomy site and placed in a separate jar [14]. Polyps were excluded from analysis if the polyp or if the margin biopsies were not retrieved for analysis.
Histopathology examination
An expert gastrointestinal pathologist and her team (CL, RB, MC, KJ, HL) reviewed and classified all polyps and evaluated each polyp for residual polyp tissue at the margins. The pathologist was blinded to the method of polypectomy. The pathology requisition forms were redacted to eliminate details of the polypectomy. Neoplastic polyps included conventional adenoma (tubular, tubulovillous, or villous), traditional serrated adenomas, sessile serrated adenomas/polyps, high-grade dysplastic lesions, and cancer. Non-neoplastic polyps included hyperplastic polyps [15]. Review of completeness of polyp resection was based on the two biopsy specimens of the polypectomy site margins. Complete resection was defined by absence of polyp tissue in both of the two biopsies.
Study outcomes
The primary outcome measured was comparison of completeness of resection between cold snare with or without EverLift™ injection groups. The primary outcome was tested for non-inferiority. Secondary outcomes included time to resection completion, number of snare attempts needed to remove the tissue adequately to the endoscopist’s judgment. Additional outcomes included rates of complications such as perforation, early post-polypectomy bleed (within 24 h) and delayed post-polypectomy bleed (between 24 h and 30 days). Early post-polypectomy bleed and delayed post-polypectomy bleed were determined based on emergency room (ER) visit, inpatient stay, transfusion needed, repeat colonoscopy required, surgical intervention required, and mortality. Secondary outcomes were tested for superiority.
Sample size calculation
In a study by Papastergiou et al. [16] cold snare EMR of non-pedunculated polyps 6–10 mm achieved complete resection 92.8% of polypectomies. Utilizing this data, to perform a non-inferiority trial, we hypothesized that polypectomy following submucosal injection of EverLift™ would achieve 92.8% complete resection rate. For polypectomy without submucosal injection of EverLift™ to be considered non-inferior, we calculated that 115 polyps were needed in each group to achieve an alpha value of 0.05, power of 90%, and non-inferiority margin of −10% [17]. Specifically, if the lower bound of the 95% confidence interval was greater than −10%, non-inferiority between polypectomy with or without EverLift™ could be concluded [16]. Patients were enrolled sequentially, and the results tabulated monthly until the minimal enrollment was met. The trial ended upon reaching the required number of patients.
Statistical analysis
In all analyses, P < 0.05 was considered significant. All tests were two-tailed. Student’s t test was performed to evaluate the average of normally distributed continuous variables, and χ [6] test was performed to evaluate frequencies of categorical outcomes. Multivariate logistic regression was used to assess factors affecting complete resection of polyps, including use of EverLift™, polyp size, Paris classification, time of polypectomy, attending performance of polypectomy, piecemeal resection, and polyp pathology. Analyses were performed using R Studio (RStudio, Inc.).
Results
Patient and procedure characteristics
A total of 159 patients were included, 105 of whom had polyps removed with EverLift™ submucosal injection, and 109 patients who had polyps removed without EverLift™ (Table 1). There were 55 patients who had polyps removed both with and without EverLift™. Patients who had polyps removed with EverLift™ had similar age, proportion of males, race/ethnicity compared to patients who had polyps removed without EverLift™. In addition, patients in the two groups had similar height, weight and body mass index (BMI). There was similar proportion of patients on anticoagulation (13.3 vs 8.3%, p = 0.231) or who had cirrhosis (1.9 vs 2.8%, p = 0.682). There were similar proportions of procedure indications: 69.5% of patients who had polyps removed with EverLift™ underwent colonoscopy for polyp surveillance compared to 67.0% of patients who had polyps removed without EverLift™. There were similar proportion of patients undergoing moderate sedation (89.5 vs 89.9%, p = 0.672). There was similar mean Boston Bowel Prep Score (7.6 vs 7.7, p = 0.734) as well as mean withdrawal time (31.1 vs 30.3 min, p = 0.670) between the two cohorts.
Polyp characteristics
A total of 291 non-pedunculated polyps ranging from 4 to 9 mm in size were identified, with 142 polyps removed using submucosal injection of EverLift™ (Table 2). The polyp sizes were similar between the with and without EverLift™ cohorts (5.3 vs 5.3 mm, p = 0.949). Polyps were identified in similar proportions of locations between the two cohorts (p = 0.892). There were similar proportions of tubular adenomas identified, with 87.3% of polyps removed with compared to 84.6% without EverLift™ submucosal injection (p = 0.189). Polyps removed were predominantly Paris classification Is (85.9 vs 86.6%) and IIa (13.4 vs 12.8%) [p = 0.567].
Outcomes
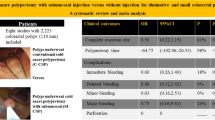
There was no significant difference in complete resection between the with (98.6%) and without EverLift™ injection (97.2%) [p= 0.424] cohorts (difference 1.28%, 95% CI: −2.66 to 5.42%), demonstrating non-inferiority (non-inferiority margin −10% < −2.66%) of with compared to without EverLift™ submucosal injection [16, 18]. Use of EverLift™ significantly increased polypectomy time (109.8 vs 38.8 s, p < 0.0001). Hemostatic clips were used more frequently for polyps receiving EverLift™ (13.4 vs 3.6%) [p = 0.002]. On multivariate logistic regression, use of EverLift™, polyp size, histology (TA versus SSP), Paris classification (Is vs IIa), and presence of fellow were not found to be statistically significant factors for incomplete resection. As there were no cases of incomplete resection for polyps that were IIb or IsP, polyps classified as hyperplastic polyps, located in the left colon, had no clip performed, or removed piecemeal, these variables were not included in the multivariate regression (Supplemental Table 1).
Complications
There were two patients (1.3%) who suffered 30-day complications. One patient had history of atrial fibrillation on rivaroxaban and was admitted five days following procedure due to splenic artery thrombosis, thought to be related to being off rivaroxaban for the procedure. The second patient presented with blood per rectum to an outside hospital, requiring two units of blood transfusion. Computed tomography angiography identified bleeding at the proximal ascending colon. Colonoscopy found that the blood loss was most likely due to post-polypectomy bleeding at the site of EMR for a separate 12 mm polyp. None of the patients had complications related to polypectomies performed for the study.
Discussion
In the Complete Adenoma Resection (CARE) Study, Pohl et al. [15] evaluated incomplete resection rates for non-pedunculated polyps 5–20 mm in size, in which 10.1% were found to have incomplete resection, including 6.8% for 5–9 mm polyps. This study emphasized the importance of optimizing polypectomy technique to achieve complete resection of polyps, in particular given risk of interval cancer growth related to incomplete polyp resection [19]. Pohl et al.’s 2013 study utilized hot snare for its polyp removals and has since raised questions whether different polypectomy techniques including type of snare, cold versus hot, and use of submucosal injection may improve polyp removal for patients and decrease risk of interval cancer growth [15].
There are limited studies available evaluating the use of submucosal injection in subcentimeter polyps. However, the available studies have only evaluated non-viscous agents [4, 5]. While there are few studies evaluating endoscopic mucosal resection [20,21,22,23] of larger polyps, an even more limited subgroup has looked at completeness of polyp resection [24,25,26,27]. Given the lack of data available, we performed the first randomized non-inferiority clinical trial evaluating the resection rate of viscous submucosal injection in subcentimeter polyps. In this study, we found that use of submucosal injection of EverLift™ was not inferior to CSP without injection, but submucosal injection did not offer any clinical benefits. Specifically, injection did not increase the complete resection rate of non-pedunculated polyps sized 4–9 mm compared to without use of EverLift™ (98.6 vs 97.2%, p = 0.424). However, use of EverLift™ was associated with increased polypectomy time (109.8 vs 38.8 s, p < 0.0001) as well as use of hemostatic clips (13.4 vs 3.6%, p = 0.002). Based on the results, the additional cost and time associated with use of EverLift™ are not justified.
In a randomized clinical trial by Shioji et al. [28] evaluating hemostatic clip application in the EMR of polyps ≥ 5 mm, there was no difference in clipping compared to no clipping in delayed bleeding. In our study, we allowed our physicians to use their clinical judgment to clip polypectomy sites. From that perspective, this study suggests viscous submucosal injection in polyps 4–9 mm may result in more acute bleeding at the polypectomy site. However, it is unclear whether this tendency is specific to EverLift™ or could have occurred with other submucosal injection materials.
There were several limitations to our study. Firstly, the study was performed at a single center. While performing a multi-center trial may have added more diversity to our patient population, which was primarily male, by utilizing a randomized controlled trial, we sought to mitigate patient differences. Additionally, the proceduralists and pathologists were not fully blinded to the techniques used. As the endoscopist had to inject the submucosal solution and as the pathologist can see discoloration from the EverLift™, complete blinding is not feasible. Nevertheless, we attempted to provide as much blinding as feasible for the study. The endoscopist only knew what technique to perform when an envelope was opened by our research coordinator after encountering a polyp of the correct size. The pathologist’s requisition form was censored of information regarding whether submucosal injection was performed to limit bias. Another limitation is that sizing of the polyp may potentially be inaccurate [29, 30]. However, we attempt to limit this issue by utilizing the open Exacto® cold snare as a ruler to compare the size of the polyp.
A further potential limitation is the method used for establishing complete resection. In our study, we utilized the double-biopsy technique, in which two biopsies at the edges of the polypectomy site were evaluated for residual polyp tissue. In the study by Shimodate et al. [5] complete resection was defined by presence of muscularis mucosae in > 80% of the horizontal dimension of the polyp. Zhang et al. [4] performed four quadrant biopsies at the edges of the polypectomy margin, though this is significantly more labor intensive, especially for smaller polyps. However, the double-biopsy technique has been well-validated in other studies [14, 15, 31]. Finally, the study may be underpowered. However, given the high percentage of complete resection achieved (> 97%) in each arm, we felt that extending the trial would not change the overall conclusion.
While submucosal injection is widely practiced for resection of larger polyps, it is worth noting that the submucosal injection technique has been adopted without establishment of a benefit by randomized trials. Indeed, randomized trials of EMR have compared saline injection with non-viscous agents, or underwater EMR without injection to conventional EMR with injection, but not standard polypectomy (EMR without injection) to EMR with injection. Based on the results of our study, it would be reasonable to conduct a study comparing resection of polyps ≥ 10 mm with injection of a viscous solution to resection without injection.
Conclusion
In the first randomized clinical trial evaluating viscous submucosal injection in cold snare removal of polyps 4–9 mm, we find that submucosal injection of EverLift™ met the non-inferiority criteria for complete resection but did not offer any clinical benefits. Injection was found to increase the time needed to perform polypectomy as well as frequency of use of hemostatic clips following polypectomy. There was no significant difference in complete resection between polypectomy with or without EverLift™ submucosal injection. Our results suggest that polypectomy of 4–9 mm polyps can be safely performed without submucosal injection of EverLift™. Further studies are needed to better evaluate the utility of EverLift™ and other viscous submucosal injections in polyps 10 mm or greater.
References
Castro R, Libânio D, Pita I, Dinis-Ribeiro M (2019) Solutions for submucosal injection: what to choose and how to do it. World J Gastroenterol 25(7):777–788. https://doi.org/10.3748/wjg.v25.i7.777
Huai ZY, Feng Xian W, Chang Jiang L, Xi CW (2015) Submucosal injection solution for endoscopic resection in gastrointestinal tract: a traditional and network meta-analysis. Gastroenterol Res Pract 2015:702768. https://doi.org/10.1155/2015/702768
Kaltenbach T, Anderson JC, Burke CA et al (2020) Endoscopic removal of colorectal lesions-recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 158(4):1095–1129. https://doi.org/10.1053/j.gastro.2019.12.018
Zhang Q, Gao P, Han B, Xu J, Shen Y (2018) Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc 87(3):733–740. https://doi.org/10.1016/j.gie.2017.06.010
Shimodate Y, Itakura J, Takayama H et al (2020) Impact of submucosal saline solution injection for cold snare polypectomy of small colorectal polyps: a randomized controlled study. Gastrointest Endosc 92(3):715-722.e1. https://doi.org/10.1016/j.gie.2020.05.039
Repici A, Wallace M, Sharma P et al (2018) A novel submucosal injection solution for endoscopic resection of large colorectal lesions: a randomized, double-blind trial. Gastrointest Endosc 88(3):527-535.e5. https://doi.org/10.1016/j.gie.2018.04.2363
Girotra M, Triadafilopoulos G, Friedland S (2018) Utility and performance characteristics of a novel submucosal injection agent (Eleview. Transl Gastroenterol Hepatol 3:32. https://doi.org/10.21037/tgh.2018.06.01
Westbrook LM, Henn PA, Cornish TC (2020) Lifting agent granuloma. Am J Clin Pathol 153(5):630–638. https://doi.org/10.1093/ajcp/aqz204
Supply GI (2020) EverLift Submucosal Lifting Agent 510(k) Premarket Notification
Acosta RD, Abraham NS, Chandrasekhara V et al (2016) The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 83(1):3–16. https://doi.org/10.1016/j.gie.2015.09.035
Kim J, Shin W (2014) How to do random allocation (randomization). Clin Orthop Surg 6(1):103–109. https://doi.org/10.4055/cios.2014.6.1.103
Sealed Envelope. Randomisation and online databases for clinical trials. https://www.sealedenvelope.com
The Paris endoscopic classification of superficial neoplastic lesions (2003) esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 58(6 Suppl):S3-43. https://doi.org/10.1016/s0016-5107(03)02159-x
Huh CW, Kim JS, Choi HH, Maeng IS, Jun SY, Kim BW (2019) Jumbo biopsy forceps versus cold snares for removing diminutive colorectal polyps: a prospective randomized controlled trial. Gastrointest Endosc 90(1):105–111. https://doi.org/10.1016/j.gie.2019.01.016
Pohl H, Srivastava A, Bensen SP et al (2013) Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 144(1):74-80.e1. https://doi.org/10.1053/j.gastro.2012.09.043
Papastergiou V, Paraskeva KD, Fragaki M et al (2018) Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6–10 mm: a randomized trial. Endoscopy 50(4):403–411. https://doi.org/10.1055/s-0043-118594
Sealed Envelope. Power (Sample Size) calculators. Binary outcome non-inferiority trial. https://www.sealedenvelope.com/power/binary-noninferior/
MedCalc. Comparison of proportions calculator. https://www.medcalc.org/calc/comparison_of_proportions.php
Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE (2010) Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc 71(1):111–117. https://doi.org/10.1016/j.gie.2009.05.010
Rex DK, Broadley HM, Garcia JR et al (2019) SIC-8000 versus hetastarch as a submucosal injection fluid for EMR: a randomized controlled trial. Gastrointest Endosc 90(5):807–812. https://doi.org/10.1016/j.gie.2019.06.040
Kishihara T, Chino A, Uragami N et al (2012) Usefulness of sodium hyaluronate solution in colorectal endoscopic mucosal resection. Dig Endosc 24(5):348–352. https://doi.org/10.1111/j.1443-1661.2012.01244.x
Fasoulas K, Lazaraki G, Chatzimavroudis G et al (2012) Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg Laparosc Endosc Percutan Tech 22(3):272–278. https://doi.org/10.1097/SLE.0b013e318251553c
Katsinelos P, Kountouras J, Paroutoglou G et al (2008) A comparative study of 50% dextrose and normal saline solution on their ability to create submucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest Endosc 68(4):692–698. https://doi.org/10.1016/j.gie.2008.02.063
Han SJ, Jung Y, Cho YS et al (2018) Clinical effectiveness of submucosal injection with indigo carmine mixed solution for colon endoscopic mucosal resection. Dig Dis Sci 63(3):775–780. https://doi.org/10.1007/s10620-018-4918-6
Horiuchi A, Makino T, Kajiyama M, Tanaka N, Sano K, Graham DY (2016) Comparison between endoscopic mucosal resection and hot snare resection of large nonpedunculated colorectal polyps: a randomized trial. Endoscopy 48(7):646–651. https://doi.org/10.1055/s-0042-105557
Yoshida N, Naito Y, Inada Y et al (2012) Endoscopic mucosal resection with 0.13% hyaluronic acid solution for colorectal polyps less than 20 mm: a randomized controlled trial. J Gastroenterol Hepatol 27(8):1377–83. https://doi.org/10.1111/j.1440-1746.2012.07166.x
Hurlstone DP, Fu KI, Brown SR et al (2008) EMR using dextrose solution versus sodium hyaluronate for colorectal Paris type I and 0-II lesions: a randomized endoscopist-blinded study. Endoscopy 40(2):110–114. https://doi.org/10.1055/s-2007-966987
Shioji K, Suzuki Y, Kobayashi M et al (2003) Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc 57(6):691–694. https://doi.org/10.1067/mge.2003.193
Anderson BW, Smyrk TC, Anderson KS et al (2016) Endoscopic overestimation of colorectal polyp size. Gastrointest Endosc 83(1):201–208. https://doi.org/10.1016/j.gie.2015.06.058
Utsumi T, Horimatsu T, Nishikawa Y et al (2021) Factors associated with inaccurate size estimation of colorectal polyps: a multicenter cross-sectional study. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.15464
Lee CK, Shim JJ, Jang JY (2013) Cold snare polypectomy vs. Cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol 108(10):1593–600. https://doi.org/10.1038/ajg.2013.302
Acknowledgements
We would like to acknowledge all our reviewers for their contribution towards this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shai Friedland: consultant to Capsovision and Intuitive Surgical. Mike Wei: consultant to Neptune Medical and AgilTx. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, M.T., Louie, C.Y., Chen, Y. et al. Randomized controlled trial investigating use of submucosal injection of EverLift™ in rates of complete resection of non-pedunculated 4–9 mm polyps. Int J Colorectal Dis 37, 1273–1279 (2022). https://doi.org/10.1007/s00384-022-04136-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04136-4