Abstract
Background
Due to lack of high-level evidences, prophylactic subcutaneous drainage has so far not been recommended in relevant guidelines as a countermeasure against incisional infections. This meta-analysis aims to clarify the efficacy of subcutaneous drainage in reducing incisional infections in colorectal surgeries.
Methods
Cochrane Library, Embase, and PubMed were searched for randomized controlled trials comparing the incidence rate of incisional infections between patients receiving prophylactic subcutaneous drainage (interventions) and those not receiving (controls) after digestive surgeries. Results from included RCTs were pooled multiple times according to different surgical types. Heterogeneity, publication bias, and certainty of evidences were estimated.
Results
Eight randomized controlled trials were included. Three RCTs each included patients receiving all sorts of digestive surgeries (gastrointestinal, hepatobiliary, and pancreatic); pooled incisional infection rates between the drainage group and the control group were not significantly different (RR = 0.76, 95%CI: 0.48–1.21, p = 0.25). Four RCTs included patients receiving colorectal surgeries; pooled incisional infection rate in the drainage group was significantly lower than that in the control group (RR = 0.34, 95%CI: 0.19–0.61, p = 0.0004). Four RCTs included patients receiving upper GI and/or HBP surgeries; pooled incisional infection rates in the drainage group and the non-drainage group were not significantly different (RR = 0.85, 95%CI: 0.54–1.34, p = 0.49).
Conclusions
Prophylactic subcutaneous drainage significantly reduces post-operative incisional infections in colorectal surgeries but was not efficacious in digestive surgeries in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical site infection (SSI) is one of the most common complications after digestive (gastrointestinal, hepatobiliary, and pancreatic) surgeries. It refers to infections that influence the incision or deep tissues at the operation site within 30 days after a surgical procedure [1] and was first introduced in 1992 to replace an old term surgical wound infection [2]. SSI can significantly increase the risk of mortality and frequently lead to other post-operative complications, extending hospital stay and increasing medical costs, meanwhile imposing excessive load on healthcare systems [1]. To date, a number of measures have been proposed to reduce the incidence of SSI, some of which, like control of the peri-operative level of blood glucose, application of prophylactic antibiotics, glove change, and wound wash before closing abdomen, have been widely accepted by the general surgery community. In 2016, WHO updated its recommendations on measures to reduce SSIs, in which a novel technique called prophylactic negative pressure wound therapy (NPWT) was conditionally recommended (for high-risk wounds only) [3]. Yet, the cost of NPWT is unusually high (a unit of single-use NPWT system costs up to $500), whereas its efficacy in laparotomy is still controversial [4]. In recent years, the potential efficacy of subcutaneous drainage has drawn quite some attention with a significant advantage in reducing SSIs in colorectal surgeries [5, 6]. The subcutaneous drainage device is mostly disposable, economical, and conveniently applicable, yet so far has not been recommended in any relevant guidelines [3, 7,8,9,10] due to lack of high-level evidence.
In this meta-analysis, we pooled results from randomized controlled trials (RCT) reporting the efficacy of subcutaneous drainage in digestive surgeries, particularly in colorectal surgeries, in an effort to acquire meaningful evidence to facilitate future clinical decision-making.
Methods
Registration and search strategy
The protocol of this meta-analysis was developed by a series of group discussions (participated primarily by K.P., Peilin, S., and J.L.) and registered a priori on PROSPERO (CRD42020196501) [11].
Cochrane Library, Embase, and PubMed were searched for relevant studies with no limitation on publication time. Search results from 3 databases were exported and further handled with Mendeley Desktop software (3 digital files available as appendix material). Keywords in search strategy included: SUBCUTANEOUS DRAINAGE, SUBCUTANEOUS DRAIN, ABDOMINAL, DIGESTIVE, WOUND INFECTION, SURGICAL SITE INFECTION, SSI, RANDOMIZED, though specific combinations of words varied when searching on different databases (detailed search strategy available in Supplementary File). Database searching was conducted by one experienced researcher (Na. Z.). Each of subsequent screening processes (duplicate removing, title screening, abstract screening) was conducted by two researchers independently and simultaneously (X.Y. and Lei, J.), where disputes were settled a senior researcher (Lan, J.). Full-text review was conducted mostly by one senior researcher (K.P.), with the help of a series of group discussions (participated by K.P., Peilin, S., J.L., X.Y., Lei, J., and Lan, J.).
Endpoints
The sole endpoint was post-operative incisional infections, of which accurate definition was not pursued, as certain early publications may not have cohered to the latest CDC criteria for SSI [12] and an exact technical definition might therefore not be practical, nor necessary.
Eligibility criteria
Considering the fact that some researches focusing on digestive surgeries in general rather than specifically on colorectal surgeries might also have independent and extractable colorectal subgroup, we expanded our literature search range to include those focusing on digestive surgeries, rather than solely on colorectal surgeries. Therefore, randomized controlled trials focusing on the efficacy of subcutaneous drainage in reducing incisional infections after digestive surgeries (i.e., gastrointestinal, hepatobiliary, and pancreatic) were included. The intervention was subcutaneous drainage only, with the control group receiving identical treatment except for the absence of subcutaneous drainage. Researches were excluded if they focused on wrong types of drainage (e.g., cavity drainage), included patients receiving wrong types of surgeries (e.g., gynecological, obstetrical, orthopedic), or arranged inappropriate interventions and controls. Publications written in a language other than English or included less than 30 patients in either arm were also excluded.
Evaluation of RCT quality
The risk of bias of included studies was assessed with the Cochrane Collaboration’s tool [13] (by K.P. and Peilin, S. independently, where disagreements were settled by Lan, J.). Direct quotes or supporting points gathered from literature texts that facilitated the assessment were arranged into a table for convenient reviewing (Supplementary File). Registration information of included RCTs was sought and reviewed additionally for potentially useful information.
Data extraction
Items extracted for a table summarization of included RCTs were author, journal name and year of publication, sample size, definition of endpoint, surgical site, immune suppression status, wound classification, drainage type (with or without suction), irrigation through the drain (Y/N), time of removal, and conclusive recommendation of subcutaneous drainage.
Items extracted for pooling were number of incisional infection cases in the drainage group, total number of cases in the drainage group, number of incisional infection cases in the non-drainage group, and total number of cases in the non-drainage group.
Data were extracted by two researchers (Lei, J. and X.Y.) independently. The two versions of results were combined after a consistency check by a third researcher (K.P.).
Statistics
Statistical analyses in this systematic review consist of pooling of outcomes, test of heterogeneity, and assessment of publication bias.
Pooling was conducted a total of 3 times (in each time, different sets of RCTs were pooled) so as to acquire the pooled outcomes for, respectively, digestive surgeries in general (twice) and colorectal surgeries. Data to be pooled were dichotomous; therefore, the relative risk with corresponding 95% confidence interval was calculated with the Mantel-Haenszel (M-H) model [14], as the M-H model has better statistical properties when events are few. Heterogeneity was assessed with chi-square test and I2 test [15], where I2 < 25% [16] was considered insignificant and a fixed-effect model was used, and I2 ≥ 25% [16] or p < 0.1 [15] was considered significant and a random-effect model was adopted [17]. These analyses were performed on Revman software, version 5.4.
Due to the fact that less than 10 studies were included in this research, the funnel plot was not suitable for the assessment of publication bias [18]; instead, we performed Egger’s test that was conducted separately on STATA software, version 15.0. Egger’s test with p < 0.1 [19, 20] was considered significant publication bias.
Evaluation of pooled evidence
Results from each pooling were subjected to an evaluation of certainty according to the criteria of the GRADE system [21, 22] (conducted by K.P. and Peilin, S., where disputes were settled by Z.Z.).
Results
Study selection
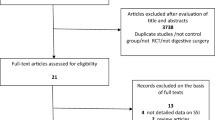
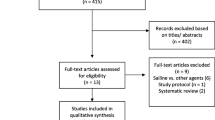
According to the specified search strategy, a total of 14,867 items were identified across 3 databases. After removal of duplicates, the remaining 10,295 items were screened by title for a first round and then by abstract for a second round, where 10,280 items were further discarded for low relevance (e.g., drainage not subcutaneous, not on digestive surgeries, obviously not RCT, plainly not relevant, etc.). The remaining 15 items were subjected to full-text review, where 4 were excluded for being non-RCTs [23,24,25,26], 1 was excluded for being an RCT with less than 30 cases in either arm [27], 1 was excluded for being an RCT written in German (desired information items not extractable due to the language) [28], and 1 was excluded for being an RCT with inappropriate control [29] (Fig. 1).
Characteristics of included studies
A total of 8 randomized controlled trials [5, 6, 30,31,32,33,34,35] were included. Three RCTs [30,31,32] included patients receiving all sorts of digestive surgeries (upper GI, lower GI, and HBP), of which 1 RCT [30] has extractable colorectal subgroup information; 3 RCTs [5, 6, 33] included only patients receiving colorectal surgeries; 1 RCT [34] included patients receiving upper GI surgeries only; and 1 RCT [35] included patients receiving liver surgeries only. Detailed characteristics of respective studies were gathered and displayed in Table 1. Notably, we found no studies reporting specifically patients receiving small intestine surgery or with extractable small intestine subgroup information.
Risk of bias
The risk of bias of included RCTs was rated and summarized in Fig. 2. Additionally, a complete table with quotes and comments facilitating our rating was presented in the Supplementary File. Notably, the performance bias and detection bias were particularly high because surgical interventions are generally difficult to be blinded to as compared to drug interventions [38].
Efficacy of subcutaneous drainage in digestive surgeries in general
All 8 included RCTs [5, 6, 30,31,32,33,34,35] compared the incidence rate of SSI between the drainage group and the non-drainage group. Seven RCTs focused on drainage with suction and the other 1 focused on drainage without suction. Pooled results of all 8 RCTs indicated that the SSI rate in the drainage group was significantly lower than the non-drainage group (RR = 0.66, 95%CI: 0.48–0.89, p = 0.007) (Fig. 3a). A fixed-effect model was adopted for the analysis as heterogeneity in this pooling was not significant (p = 0.45, I2 = 0%). Egger’s test indicated no significant publication bias (p = 0.387). Additionally, pooled results of the 7 RCTs [5, 30,31,32,33,34,35] focusing on drainage WITH SUCTION (subgroup analysis) indicated that the SSI rate in the drainage (with suction) group was significantly lower than the non-drainage group (RR = 0.70, 95%CI: 0.51–0.97, p = 0.03) (Fig. 3a) as well. A fixed-effect model was adopted for the analysis as heterogeneity in this pooling was not significant (p = 0.55, I2 = 0%). Egger’s test indicated no significant publication bias (p = 0.715).
Efficacy of subcutaneous drainage in digestive surgery in general. a Pooling of all included studies, with subgroup analysis (suction subgroup and no suction subgroup); b pooling of studies that included patients receiving all sorts of digestive surgeries rather than a specific type (upper GI, lower GI, HBP) of digestive surgeries
As 3 [30,31,32] out of the 8 RCTs each included patients receiving all sorts of digestive surgeries (upper GI, lower GI, and HBP), a separate pooling of these 3 RCTs (all focused on drainage with suction) was conducted. The result indicated that SSI rates between the drainage (with suction) group and the non-drainage group were not significantly different (RR = 0.76, 95%CI: 0.48–1.21, p = 0.25) (Fig. 3b). A fixed-effect model was adopted for the analysis as heterogeneity in this pooling was not significant (p = 0.40, I2 = 0%). Egger’s test indicated no significant publication bias (p = 0.427).
Efficacy of subcutaneous drainage in colorectal surgeries
Four RCTs [5, 6, 30, 33] compared the incidence rate of SSI between the drainage group and the non-drainage group in colorectal surgeries, of which 3 [5, 30, 33] focused on drainage with suction and 1 [6] focused on drainage without suction. Pooled results of all 4 RCTs indicated that the SSI rate in the drainage group was significantly lower than the non-drainage group (RR = 0.34, 95%CI: 0.19–0.61, p = 0.0004) (Fig. 4). A fixed-effect model was adopted for the analysis as heterogeneity in this pooling was not significant (p = 0.69, I2 = 0%). Egger’s test indicated no significant publication bias (p = 0.412). Additionally, pooled results of the 3 RCTs focusing on drainage with suction (subgroup analysis) indicated that the SSI rate in the drainage (with suction) group was significantly lower than the non-drainage group as well (RR = 0.34, 95%CI: 0.17–0.70, p = 0.003) (Fig. 4). A fixed-effect model was adopted for the analysis as heterogeneity in this pooling was not significant (p = 0.49, I2 = 0%). Egger’s test was not viable due to the insufficiency of studies.
GRADE score of certainty and importance
For a convenient overview, the GRADE score of certainty and importance of the evidence that have had all pooled results (overall significance, heterogeneity, Egger’s test) considered were summarized in the 3rd section of Supplementary File.
Discussion
The RCTs included in this meta-analysis can be conveniently tagged as all-digestive, upper GI, HBP, and colorectal, according to the surgical type of their included patients. Therefore, we pooled outcomes based on categorization and explored the efficacy of subcutaneous drainage in digestive surgeries in general and in colorectal surgeries, respectively. Indicated by the results displayed in Fig. 4, subcutaneous drainage can reduce incisional infections significantly in colorectal surgeries. Interestingly, as pooling was conducted two separate times to check out subcutaneous drainage’s efficacy in digestive surgeries in general (one time all included studies were pooled, the other time only studies each including all sorts of digestive surgeries was pooled), conflicting results (Fig. 3a and b) were acquired. A subsequent thorough inspection on Fig. 3 suggested that results in Fig. 3a were obviously influenced and deviated to a heavy extent by the 3 colorectal studies, whereas results in Fig. 3b can more objectively reflect the true efficacy of subcutaneous drainage in digestive surgeries in general. Notably though, no RCTs, or even cohort studies, specifically focused on the efficacy of subcutaneous drainage in surgeries on small intestines to date, which is worthy of conducting an RCT to fill up the missing piece of the puzzle.
Subcutaneous drainage devices currently in the application can generally be categorized into active/suction drainage (Blake drain [33], Jackson-Pratt drain [35], Redon drain [31], etc.) and passive drainage (Penrose drain [6]), depending on the involvement of negative pressure. In this meta-analysis, all included RCTs focused on drainage with suction except for Numata’s research [6]. Therefore, whenever a pooling involved Numata’s RCT (i.e., in Figs. 3a and 4), subgroup analysis was conducted specifically for the suction drainage subgroup. As results on digestive surgeries in general in Fig. 3a were heavily deviated by the 3 colorectal RCTs and therefore cannot reflect the true efficacy of subcutaneous drainage in digestive surgeries in general, it will not be subjected to further discussion. In Fig. 4 though, subcutaneous drainage with suction is efficacious in colorectal surgeries (test for overall effect: p = 0.003; heterogeneity: p = 0.49, I2 = 0%), which was in line with the overall efficacy of subcutaneous drainage (with and without suction combined) in colorectal surgeries (test for overall effect: p = 0.0004; heterogeneity: p = 0.69, I2 = 0%), but the absence of Egger’s test for publication bias reduced its strength of evidence.
In 2019, a meta-analysis including 8 RCTs concluded that “the presence of prophylactic subcutaneous suction drain does not impact significantly on the incidence of SSI in clean-contaminated abdominal surgery” [39]. However, it is apparently flawed research for it included an RCT that arranged inappropriate controls [29], where patients in the intervention groups received irrigation with saline (after fascia closure) and placement of subcutaneous drainage, and patients in the control group received no subcutaneous drainage, but received irrigation with ANTIBIOTICS after fascia closure and prior to skin closure, resulting in wound infection rates of 4.4% in the intervention/drainage group and 4.1% in the control/no-drainage group. Notably though, this particular RCT included 659 patients respectively in both intervention and control group and surely deviated to a heavy extent the overall outcome, as well as the original purpose of that meta-analysis. Also, it failed to include two RCTs focusing on colorectal surgeries and did not attempt to perform analysis regarding subtypes of digestive surgeries.
In conclusion, prophylactic subcutaneous drainage significantly reduced post-operative incisional infections in colorectal surgeries but was not efficacious in digestive surgeries in general.
References
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Horan TC et al (1999) Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 20(4):250–278. https://doi.org/10.1086/501620
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13(10):606–608. https://doi.org/10.2307/30148464
Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, Gomes SM, Gans S, Wallert ED, Wu X, Abbas M, Boermeester MA, Dellinger EP, Egger M, Gastmeier P, Guirao X, Ren J, Pittet D, Solomkin JS, WHO Guidelines Development Group (2016) WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 16(12):e288–e303. https://doi.org/10.1016/S1473-3099(16)30402-9
Kuper TM, Murphy PB, Kaur B, Ott MC (2020 Jan) Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta-analysis of randomized controlled trials. Ann Surg 271(1):67–74. https://doi.org/10.1097/SLA.0000000000003435
Lauscher JC, Schneider V, Lee LD, Stroux A, Buhr HJ, Kreis ME, Ritz JP (2016) Necessity of subcutaneous suction drains in ileostomy reversal (DRASTAR)-a randomized, controlled bi-centered trial. Langenbecks Arch Surg 401(4):409–418. https://doi.org/10.1007/s00423-016-1436-x
Numata M, Godai T, Shirai J, Watanabe K, Inagaki D, Hasegawa S, Sato T, Oshima T, Fujii S, Kunisaki C, Yukawa N, Rino Y, Taguri M, Morita S, Masuda M (2014) A prospective randomized controlled trial of subcutaneous passive drainage for the prevention of superficial surgical site infections in open and laparoscopic colorectal surgery. Int J Colorectal Dis 29(3):353–358. https://doi.org/10.1007/s00384-013-1810-x
Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, Abbas M, Atema JJ, Gans S, van Rijen M, Boermeester MA, Egger M, Kluytmans J, Pittet D, Solomkin JS, WHO Guidelines Development Group (2016) WHO Guidelines Development Group. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 16(12):e276–e287. https://doi.org/10.1016/S1473-3099(16)30398-X
Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KMF, Dellinger EP, Ko CY, Duane TM (2017) American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 224(1):59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP, for the Healthcare Infection Control Practices Advisory Committee (2017 Aug) Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 152(8):784–791. https://doi.org/10.1001/jamasurg.2017.0904
World Health Organization. Global guidelines on the prevention of surgical site infection. https://www.who.int/gpsc/ssi-prevention-guidelines/en/. Published Nov, 2016. Accessed Jun, 2020
International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/
National Healthcare Safety Network. Centers for Disease Control and Prevention. Surgical site infection (SSI) event. http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Published Jan, 2017. Accessed Apr 26, 2020
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ:343 oct18 2–343 ocd5928. https://doi.org/10.1136/bmj.d5928
Jonathan J. Deeks, Julian PT Higgins, Douglas G Altman, on behalf of the Cochrane Statistical Methods Group Section 10-4-1 (2019) Mantel-Haenszel methods, Cochrane Handb Syst Rev Interv Vers 6. https://training.cochrane.org/handbook/current/chapter-10#section-10-4-1. Accessed 2 May 2020
Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group Section 10-10-2 (2019) Identifying and measuring heterogeneity. Cochrane Handb Syst Rev Interv Vers 6. https://training.cochrane.org/handbook/current/chapter-10#section-10-10-2. Accessed 2 May 2020
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003 Sep) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Deeks JJ (2019) Julian PT Higgins, Douglas G Altman; on behalf of the Cochrane Statistical Methods Group, Section 10-10-4-1 Fixed or random effects? Cochrane Handbook Syst Rev Interv, Vers 6. https://training.cochrane.org/handbook/current/chapter-10#section-10-10-4-1. Accessed 2 May 2020
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333(7568):597–600. https://doi.org/10.1136/bmj.333.7568.597
Cappelleri JC, Ioannidis JP, Schmid CH, de Ferranti SD, Aubert M, Chalmers TC, Lau J (1996) Large trials vs meta-analysis of smaller trials: how do their results compare? JAMA 276(16):1332–1338. https://doi.org/10.1001/jama.1996.03540160054033
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://doi.org/10.1136/bmj.39489.470347.AD
GRADEpro. https://gradepro.org/. Accessed 11 May 2020
Tochika N, Namikawa T, Kamiji I, Kitamura M, Okamoto K, Hanazaki K (2011) Subcutaneous continuous suction drainage for prevention of surgical site infection. J Hosp Infect 78(1):67–68. https://doi.org/10.1016/j.jhin.2011.01.022
Pan HD, Wang L, Peng YF, Li M, Yao YF, Zhao J, Zhan TC, du CZ, Gu J (2015) Subcutaneous vacuum drains reduce surgical site infection after primary closure of defunctioning ileostomy. Int J Colorectal Dis 30(7):977–982. https://doi.org/10.1007/s00384-015-2168-z
Fujii T, Tabe Y, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H (2011) Effects of subcutaneous drain for the prevention of incisional SSI in high-risk patients undergoing colorectal surgery. Int J Colorectal Dis 26(9):1151–1155. https://doi.org/10.1007/s00384-011-1228-2
Imada S, Noura S, Ohue M, Shingai T, Sueda T, Kishi K, Yamada T, Ohigashi H, Yano M, Ishikawa O (2013) Efficacy of subcutaneous penrose drains for surgical site infections in colorectal surgery. World J Gastrointest Surg 5(4):110–114. https://doi.org/10.4240/wjgs.v5.i4.110
Arer IM, Yabanoglu H, Aytac HO, Ezer A (2016) The effect of subcutaneous suction drains on surgical site infection in open abdominal surgery A prospective randomized study. Ann Ital Chir 87:49–55
Peiper C, Conze J, Ponschek N, Schumpelick V (1997) Stellenwert der subcutanen Drainage bei der Reparation primärer Leistenhernien. Eine prospektive randomisierte Studie an 100 Fällen. Chirurg 68(1):63–67. https://doi.org/10.1007/s001040050151
Farnell MB, Worthington-Self S, Mucha P Jr, Ilstrup DM, McIlrath DC (1986) Closure of abdominal incisions with subcutaneous catheters. A prospective randomized trial. Arch Surg 121(6):641–648. https://doi.org/10.1001/archsurg.1986.01400060035003
Kaya E, Paksoy E, Ozturk E, Sigirli D, Bilgel H (2010) Subcutaneous closed-suction drainage does not affect surgical site infection rate following elective abdominal operations: a prospective randomized clinical trial. Acta Chir Belg 110(4):457–462. https://doi.org/10.1080/00015458.2010.11680655
Baier PK, Glück NC, Baumgartner U, Adam U, Fischer A, Hopt UT (2010) Subcutaneous Redon drains do not reduce the incidence of surgical site infections after laparotomy. A randomized controlled trial on 200 patients. Int J Colorectal Dis 25(5):639–643. https://doi.org/10.1007/s00384-010-0884-y
Lubowski D, Hunt DR (1987) Abdominal wound drainage—a prospective, randomized trial. Med J Aust 146(3):133–135. https://doi.org/10.5694/j.1326-5377.1987.tb120154.x
Watanabe J, Ota M, Kawamoto M, Akikazu Y, Suwa Y, Suwa H, Momiyama M, Ishibe A, Watanabe K, Masui H, Nagahori K (2017) A randomized controlled trial of subcutaneous closed-suction Blake drains for the prevention of incisional surgical site infection after colorectal surgery. Int J Colorectal Dis 32(3):391–398. https://doi.org/10.1007/s00384-016-2687-2
Shaffer D, Benotti PN, Bothe A Jr, Jenkins RL, Blackburn GL (1987) A prospective, randomized trial of abdominal wound drainage in gastric bypass surgery. Ann Surg 206(2):134–137. https://doi.org/10.1097/00000658-198708000-00003
Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S (2014) Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepatobiliary Pancreat Sci 21(7):509–517. https://doi.org/10.1002/jhbp.93
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27:97–132 quiz 133–134; discussion 196
Stübs P, Schmidt C, Lippert H, Tautenhahn J (2004) Inzidenz und Einteilung postoperativer Wundinfektionen in der Viszeralchirurgie. Viszeralchirurgie 39(3):166–169. https://doi.org/10.1055/s-2004-822728
Probst P, Grummich K, Heger P et al (2016) Blinding in randomized controlled trials in general and abdominal surgery: protocol for a systematic review and empirical study. Syst Rev 5:48. https://doi.org/10.1186/s13643-016-0226-4
Coletta D, Del Basso C, Giuliani G, Guerra F (2019) Subcutaneous suction drains do not prevent surgical site infections in clean-contaminated abdominal surgery-results of a systematic review and meta-analysis. Langenbeck’s Arch Surg 404(6):663–668. https://doi.org/10.1007/s00423-019-01813-x
Acknowledgements
Gratitude to all authors of the original researches that constitutes this systematic review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pang, K., Sun, P., Li, J. et al. Prophylactic subcutaneous drainage reduces post-operative incisional infections in colorectal surgeries: a meta-analysis of randomized controlled trials. Int J Colorectal Dis 36, 1633–1642 (2021). https://doi.org/10.1007/s00384-021-03908-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-03908-8