Abstract
Purpose
Enhanced recovery after surgery (ERAS) provides many benefits. However, important knowledge gaps with respect to specific components of enhanced recovery after surgery remain because of limited validation data. The aim of the study was to validate a mature ERAS protocol at a different hospital and in a similar population of patients.
Methods
This is a retrospective analysis of patients undergoing elective colorectal surgery from 2009 through 2016. Patients enrolled in ERAS are compared with those undergoing the standard of care. Patient demographic characteristics, length of stay, pain scores, and perioperative morbidity are described.
Results
Patients (1396) were propensity matched into two equal groups (ERAS vs non-ERAS). No significant difference was observed for age, Charlson Comorbidity Index, American Society of Anesthesiologists score, body mass index, sex, operative approach, and surgery duration. Median length of stay in ERAS and non-ERAS groups was 3 and 5 days (P < .001). Mean pain scores were lower in the ERAS group, measured at discharge from the postanesthesia unit (P < .001), on postoperative day 1 (P = .002) and postoperative day 2 (P = .02) but were identical on discharge.
Conclusions
This ERAS protocol was validated in a similar patient population but at a different hospital. ERAS implementation was associated with an improved length of stay and pain scores similar to the original study. Different than most retrospective studies, propensity score matching ensured that groups were evenly matched. To our knowledge, this study is the only ERAS validation study in a propensity-matched cohort of patients undergoing elective colorectal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protocol of enhanced recovery after surgery (ERAS) has evolved since inception and has been more uniformly implemented by multiple surgical specialties [1,2,3,4]. It is now considered the standard of care for colorectal surgery [5]. The underlying principle of ERAS is to maintain normal physiologic function in the perioperative period through encouragement of early oral intake, ambulation, maintenance of euvolemia, and use of multimodal analgesia. Previously reported benefits include decreased length of stay (LOS), reduced postoperative morbidity, improved postoperative pain control, improved patient satisfaction, and decreased cost of care [6,7,8,9].
Although consensus exists regarding the concept of an ERAS bundle, implementation of its components continues to be variable [10]. These differences in implementation have proven problematic in the design and use of studies to evaluate the influence of ERAS [11,12,13]. Randomized prospective trials are generally small and single-institution assessments, whereas meta-analyses and systematic reviews attempt to merge heterogeneous data with the differing ERAS elements or with different patient populations [14,15,16]. As a result, no validation studies use the exact same protocol in a similar patient population. Herein, we assess the implementation (process and outcomes) at Mayo Clinic in Jacksonville, Florida (MCJ), of a previously described ERAS protocol in its entirety [8, 17]. The primary objective was to determine the effect of ERAS implementation on LOS and pain control. The secondary objective was to establish the effect of ERAS implementation on postoperative complications (i.e., re-admissions, surgical site infection (SSI), anastomotic leaks, and bleeding).
Methods
Patients
Inclusion criteria described adult (age > 18 years) patients who underwent an elective inpatient colorectal operation at MCJ from January 2009 through December 2016. The ERAS protocol was implemented at MCJ in September 2013. The exact pathway described and implemented at Mayo Clinic in Rochester, Minnesota (MCR), was used (Table 1) [8, 17]. Although MCJ and MCR are different sites, they are of the same institution; therefore, patient pathways, services, formulary medications, and staff follow the same guidelines. Patient demographic characteristics included age, sex, body mass index, Charlson Comorbidity Index, and American Society of Anesthesiologists (ASA) classification. Outcome variables included LOS, analog pain score, postoperative complications (e.g., bleeding, anastomotic leak, SSI), and readmissions were obtained from the electronic health record. Compliance with the ERAS protocol was measured reviewing medical records whether protocol requirements were implemented. Total (100%) compliance meant that all ERAS components were followed (Table 1).
To capture all possible SSIs, we used expanded SSI criteria, modified from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), the Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN), and the Centers for Medicare and Medicaid Services Hospital–Acquired Condition (Box). Our definition of postoperative bleeding followed the description of Moriarty et al. [18]. Briefly, bleeding was classified as involving at least 2 of the following 4 criteria: hemoglobin decrease ≥ 3 g/dL, red blood cell transfusion, a bleeding diagnosis, or a return to the operating room for hemorrhage, or a combination. Postoperative pain was measured with the 10-point analog scale at discharge from the postanesthesia care unit and at 4 and 8 h postoperatively. Average pain scores were recorded on postoperative days 1 and 2 and at discharge.
Statistical analysis
Descriptive statistics for categorical variables are reported as frequency and percentage, continuous variables, as mean (SD) and median (range). Since some patients had multiple surgical procedures, preoperative and outcome variables were compared between ERAS group and non-ERAS group through generalized linear models with generalized estimating equations to account for within-patient correlation.
In addition, ERAS patients were matched with non-ERAS patients using propensity score matching. The calipers used were ± 0.15 (0.25*SD of logit propensity scores) for the probability of ERAS procedure. Control patients were selected randomly from the control group, defined with the calipers. The propensity of an ERAS protocol surgery was estimated through a logistic regression model. The response variable was ERAS procedure (yes or no). Independent variables were age at surgery, sex, body mass index (BMI), Charlson Comorbidity Index, ASA class, wound class, surgeon, extensiveness of the procedure, and surgery type. Standardized differences were calculated when the matched baseline variables were compared between the ERAS group and the non-ERAS group. Generalized linear models with the generalized estimating equations approach were used to assess differences in outcome variables between the two matched groups with the following distribution and link function: LOS (γ distribution and log-link function), pain scores (normal distribution and identity link function), and complication and readmission (binary distribution and logit link function).
All statistical tests were two-sided with the α level set at 0.05 for statistical significance. The analysis was performed with statistical software (SAS version 9.4; SAS Institute Inc). The Mayo Clinic Institutional Review Board approved this retrospective study. This study followed the reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology.
Results
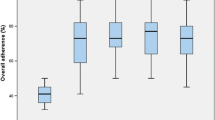
The population was 1556 patients undergoing 1915 operations (861 ERAS vs 1054 non-ERAS) during the study period. Baseline demographic characteristics were compared between the groups (Table 2). In the unmatched analysis, patients in the ERAS group were younger, had lower Charlson Comorbidity Index and ASA scores, and were more likely to undergo minimally invasive surgery. However, after propensity score matching, the resulting groups showed no significant differences in regard to patient characteristics. Among operations, 698 pairs of procedures were propensity matched on the basis of baseline variables. Standardized differences before and after matching were plotted (Fig. 1). Median LOS was significantly shorter in the ERAS group than the non-ERAS group (3 vs 5 days, P < .001) (Table 3). During the study period, LOS showed a decreasing trend overall; however, a significant decrease was observed with subsequent stabilization after protocol implementation in 2013 (Fig. 2). Postoperative bleeding was less in the ERAS group than the non-ERAS group (3.9 vs 6.4%, P = .03). Postoperative pain scores in the ERAS group were significantly lower than in the non-ERAS group up to postoperative day 2 but were not statistically different at discharge (Fig. 3). Finally, 30-day readmission, reoperation, anastomotic leakage, and SSIs were statistically similar between the two groups. During 2013, compliance with the ERAS pathway was 80%; it subsequently increased to 92% in 2016.
Discussion
While ERAS has evolved since its early description, its adoption has increased largely because of improved patient outcomes compared with traditional care. Our study of the implementation of an ERAS pathway demonstrated three observations. First, the ERAS pathway implemented at another institution (MCR) was successfully validated at our institution (MCJ) with nearly identical results. Second, ERAS implementation was associated with shorter LOS and better pain control. Third, the implementation was not associated with higher rates of re-admissions or postoperative complications.
The findings of this ERAS study are largely congruent with other reported studies [8, 9, 15, 17, 19,20,21,22]. However, in a recent Cochrane review, Bond-Smith et al. [23] highlighted elevated risk of bias and unstandardized pathways across different sites in the studies included in the meta-analysis; thus, given the uncertainty of the validity of the results, a routine implementation of the ERAS protocol might be challenging. Other meta-analyses of ERAS protocol report favorable outcomes but are unable to show that these outcomes are generalizable because of the heterogeneity of the included studies [19, 20].
The present study is distinctive in that the ERAS protocol was first implemented at a different hospital (MCR) and was applied with a similar patient population, measuring the same primary outcome of LOS. In addition, the propensity score matching ensured that patients in each group shared preoperative characteristics and had similar lengths of surgery (a surrogate for procedural complexity) and modes of surgery (minimally invasive or open). Any unmeasurable differences between surgeons were minimized. This study found the same difference in LOS as seen at MCR. Despite the difficulty in comparing these outcomes to previous studies, our results not only replicate but also further demonstrate the potential benefits of ERAS when previously defined protocol standards are implemented [4, 9, 15, 24].
As in the MCR study, the MCJ ERAS patients reported lower postoperative pain scores than those receiving usual care. In addition, they were discharged with the same pain score; they arrived at the “ideal” pain score faster and were not discharged with higher pain scores than the control group. Complications in the ERAS group were less overall, and the 30-day readmission was the same as in the control group. This outcome is especially important because a historical concern in the literature about the ERAS pathway is that although patients enrolled in ERAS return home sooner, they might be at risk for a higher readmission rate (i.e., they may be discharged prematurely) [25, 26]. In this MCJ study, we found that this was not the case, a finding similar to the MCR study.
We wanted to assess the true incidence of SSIs. As previously mentioned, NSQIP and NHSN infection rates are discordant with each other and with the individual hospital’s internal data [27, 28]. When initially assessing the SSI rate for the present study, we found that the NSQIP and NHSN rates were indeed discordant to each other and were lower than the rates found with the criterion described in the Box. It is impossible to know how many infections the algorithm missed, but SSIs were not the main focus of this work. Nevertheless, all efforts were made to ensure completeness of the analyzed data. The SSI numbers between the two groups were similar and within the acceptable range of today’s standards [27, 28].
Box surgical site infection criteria with presence of any 1 category, code, or treatment occurring within 30 days of discharge
CCS diagnosis category | |
2, septicemia (except in labor) | |
3, bacterial infection, unspecified site | |
135, intestinal infection | |
148, peritonitis and intestinal abscess | |
197, skin and subcutaneous tissue infection | |
238, complication of surgical procedure or medical care | |
CCS procedure category | |
168, incision and drainage; skin subcutaneous tissue and fascia | |
ICD-9/ICD-10 specific codes | |
998.5 | |
998.59 | |
T81.4XXA | |
K68.11 | |
CPT-4 code | |
49,021 | |
49,061 | |
Antibiotics administered in absence of UTI or pneumonia | |
Cefazolin | |
Cephalexin | |
Amoxicillin | |
Ciprofloxacin | |
Metronidazole |
Initial compliance with the ERAS protocol was 80%; a peak compliance of 92% was reached within 2 months. On the basis of this model, achievement of a LOS decrease may be possible within 4 months of complete ERAS implementation. This is an important consideration for any financial modeling, although the financial effect and effort necessary for implementation vary by institution. Most institutions have realized a financial advantage with ERAS implementation [9, 14, 29, 30].
Limitations of this study include its retrospective design, with the biases therein. The study period was 8 years long; therefore, there is risk for lead time bias; however, median LOS before ERAS implementation in non-ERAS group was 5.0 days each year from 2009 through 2012. A stable trend can also be seen in the ERAS group after the ERAS implementation, with a median LOS of 3.0 days each year from 2014 to 2016. Therefore, it is unlikely that time significantly influenced our results. Propensity matching was used, thereby balancing the treatment and control groups across the covariates identified as significant. A risk exists that other unidentified variables could also influence the outcomes. The use of propensity score matching led to an exclusion of 30 and 18% of the study population from the first and the second time periods respectively. While the exclusion of patients is often a limitation of this type of analysis, there is no difference between matched and unmatched records among the surgical procedures (p = 0.204). Furthermore, in the multivariable analysis adjusting for the same covariates in the original population, results are similar compared to the matched analysis. In addition, this was a single-institution experience, which may be not representative sample. Further validation from other institutions will be beneficial.
In conclusion, this study supports that ERAS is associated with decreased LOS, improved pain control, and no additional complications than a traditional pathway. It validates the specific ERAS protocol implemented at a different site and shows that it is reproducible in hospitals with similar population of patients. This protocol would likely benefit all patients undergoing elective colorectal surgery. Future direction for this group is the description of the financial effect of ERAS implementation at MCJ and comparison with MCR.
References
Anderson AD, McNaught CE, MacFie J et al (2003) Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg 90(12):1497–1504
Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbolle P, Hendel HW, Rosenberg J, Kehlet H (2002) Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg 89(4):446–453
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78(5):606–617
Moiniche S, Bulow S, Hesselfeldt P et al (1995) Convalescence and hospital stay after colonic surgery with balanced analgesia, early oral feeding, and enforced mobilisation. The Eur J Surg 161(4):283–288
Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, Boutros M, McClane J, Feldman LS, Steele SR (2017) Clinical practice guidelines for enhanced recovery after Colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 60(8):761–784
Delaney CP, Brady K, Woconish D, Parmar SP, Champagne BJ (2012) Towards optimizing perioperative colorectal care: outcomes for 1,000 consecutive laparoscopic colon procedures using enhanced recovery pathways. Am J Surg 203(3):353–355 discussion 5-6
Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J, Enhanced Recovery After Surgery Study Group (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146(5):571–577
Larson DW, Lovely JK, Cima RR, Dozois EJ, Chua H, Wolff BG, Pemberton JH, Devine RR, Huebner M (2014) Outcomes after implementation of a multimodal standard care pathway for laparoscopic colorectal surgery. Br J Surg 101(8):1023–1030
Stephen AE, Berger DL (2003) Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery 133(3):277–282
Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt M, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH, Enhanced Recovery After Surgery (ERAS) Group (2009) Consensus review of optimal perioperative care in colorectal surgery: enhanced recovery after surgery (ERAS) group recommendations. Arch Surg 144(10):961–969
Kahokehr A, Sammour T, Zargar-Shoshtari K et al (2009) Implementation of ERAS and how to overcome the barriers. Int J Surg (London, England) 7(1):16–19
Ljungqvist O (2015) Sustainability after structured implementation of ERAS protocols. World J Surg 39(2):534–535
Song W, Wang K, Zhang RJ, Dai QX, Zou SB (2016) The enhanced recovery after surgery (ERAS) program in liver surgery: a meta-analysis of randomized controlled trials. Springerplus 5:207
Spanjersberg WR, Reurings J, Keus F, et al. 2011 Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. (2):Cd007635
Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38(6):1531–1541
Rawlinson A, Kang P, Evans J, Khanna A (2011) A systematic review of enhanced recovery protocols in colorectal surgery. Ann R Coll Surg Engl 93(8):583–588
Lovely JK, Maxson PM, Jacob AK, Cima RR, Horlocker TT, Hebl JR, Harmsen WS, Huebner M, Larson DW (2012) Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg 99(1):120–126
Moriarty JP, Daniels PR, Manning DM, O’Meara JG, Ou NN, Berg TM, Haag JD, Roellinger DL, Naessens JM (2017) Going beyond administrative data: retrospective evaluation of an algorithm using the electronic health record to help identify bleeding events among hospitalized medical patients on warfarin. Am J Med Qual 32(4):391–396
Varadhan KK, Neal KR, Dejong CH et al (2010) The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr (Edinburgh, Scotland) 29(4):434–440
Lv L, Shao YF, Zhou YB (2012) The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Color Dis 27(12):1549–1554
Wind J, Hofland J, Preckel B, Hollmann MW, Bossuyt PMM, Gouma DJ, van Berge Henegouwen MI, Fuhring JW, Dejong CHC, van Dam RM, Cuesta MA, Noordhuis A, de Jong D, van Zalingen E, Engel AF, Goei TH, de Stoppelaar IE, van Tets WF, van Wagensveld BA, Swart A, van den Elsen MJLJ, Gerhards MF, de Wit LT, Siepel MAM, van Geloven AAW, Juttmann JW, Clevers W, Bemelman WA (2006) Perioperative strategy in colonic surgery; laparoscopy and/or fast track multimodal management versus standard care (LAFA trial). BMC Surg 6:16
Wind J, Polle SW, Fung Kon Jin PH et al (2006) Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 93(7):800–809
Bond-Smith G, Belgaumkar AP, Davidson BR et al (2016) Enhanced recovery protocols for major upper gastrointestinal, liver and pancreatic surgery. Cochrane Database Syst Rev 2:Cd011382
Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF (2014) Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 101(3):172–188
Hendren S, Morris AM, Zhang W, Dimick J (2011) Early discharge and hospital readmission after colectomy for cancer. Dis Colon Rectum 54(11):1362–1367
Andersen J, Hjort-Jakobsen D, Christiansen PS, Kehlet H (2007) Readmission rates after a planned hospital stay of 2 versus 3 days in fast-track colonic surgery. Br J Surg 94(7):890–893
Bergquist JR, Thiels CA, Etzioni DA, Habermann EB, Cima RR (2016) Failure of colorectal surgical site infection predictive models applied to an independent dataset: do they add value or just confusion? J Am Coll Surg 222(4):431–438
Etzioni DA, Lessow CL, Lucas HD, Merchea A, Madura JA, Mahabir R, Mishra N, Wasif N, Mathur AK, Chang YHH, Cima RR, Habermann EB (2018) Infectious surgical complications are not dichotomous: characterizing discordance between administrative data and registry data. Ann Surg 267(1):81–87
Stone AB, Grant MC, Pio Roda C, Hobson D, Pawlik T, Wu CL, Wick EC (2016) Implementation costs of an enhanced recovery after surgery program in the United States: a financial model and sensitivity analysis based on experiences at a quaternary academic medical center. J Am Coll Surg 222(3):219–225
Nelson G, Kiyang LN, Crumley ET, Chuck A, Nguyen T, Faris P, Wasylak T, Basualdo-Hammond C, McKay S, Ljungqvist O, Gramlich LM (2016) Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: the ERAS Alberta colorectal surgery experience. World J Surg 40(5):1092–1103
Funding
Funding provided by Robert D. and Patricia E. Kern Center for Science of Health Care Delivery. The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
All authors made substantial contribution to the conception and design of the work, as well as the acquisition, analysis, and interpretation of data. All authors were critically involved in drafting and revising this project for important intellectual content. Final approval of the version to be published was given by all authors, and they agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
The Mayo Clinic Institutional Review Board approved this retrospective study. This study followed the reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This manuscript was presented as a “quick shot presentation” at the 13th annual Academic Surgical Congress, January 30-February 1, 2018, Jacksonville, Florida.
Rights and permissions
About this article
Cite this article
Lemini, R., Spaulding, A.C., Naessens, J.M. et al. ERAS protocol validation in a propensity-matched cohort of patients undergoing colorectal surgery. Int J Colorectal Dis 33, 1543–1550 (2018). https://doi.org/10.1007/s00384-018-3133-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-3133-4