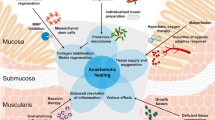
Abstract
Purpose
Current surgical dogma dictates that tissue ischemia and hypoxia are major contributing factors in anastomotic leak despite scant evidence. The aim of this study was to determine if tissue hypoxia is a feature of anastomotic leakage in rats following colon resection and segmental devascularization.
Methods
Rats were randomly assigned to undergo sham operation, segmental colon devascularization alone, colectomy alone, or segmental devascularization plus colectomy. Tissue hypoxia present at the colon anastomosis site across the various treatment groups was determined at sacrifice on postoperative day 6. Pimonidazole HCl was injected 30 min prior to sacrifice. Anastomotic tissues were examined and scored for healing versus leakage using an anastomotic healing score (AHS). Collagen content, hypoxia, enteric smooth muscle and periendothelial stromal patterning, and apoptosis were evaluated histologically.
Results
No differences in tissue hypoxia were noted in the 16% of anastomotic tissues with poor healing compared to the remaining 84% of rats whose anastomoses healed well. No significant changes were found in cell death in the submucosa of any group. Consistent with previous findings, poor healing was associated with lower collagen content. Submucosal thickness correlated with increased arteriole diameter (R 2 = 0.25, p < 0.005).
Conclusions
These results demonstrate that tissue hypoxia is not a distinctive feature of anastomotic tissues that fail to heal and leak, even when their blood supply is interrupted. These findings suggest that compensatory factors may mitigate the effects of ischemia and hypoxia during healing of anastomotic tissues and that the process of leakage involves factors beyond their acute effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When a well-constructed anastomosis performed by an expert surgeon leaks, it has become axiomatic to attribute the cause to ischemia or patient-related factors. Although there is little evidence to support such attributions, the logic seems to be based on the assertion that “what else can it be?” [1, 2]. The two anatomic structures at greatest risk for anastomotic leak rate are the esophagus and the rectum. Operating within these sites poses significant challenges, as they often require division of the feeding blood vessels in order to allow the divided segments to reach one another to form a tension-free anastomosis.
Yet, the effort to document ischemia and/or hypoxia following anastomotic surgery has been challenging. Boyle and colleagues used laser Doppler to determine colonic blood flow in ten patients following colon resection and anastomosis [3]. Despite demonstrating a 57% decrease in blood flow at the time of surgery, no leaks developed in their cohort. Vignali and colleagues prospectively studied 55 patients undergoing colon resection for cancer and investigated blood flow in the proximal and distal segments constituting the anastomosis before and after vascular ligation, and again, decreased blood flow was not predictive of anastomotic leak [4]. Finally, Shouten examined the histology of anastomotic tissues excised during emergency surgery for leakage in 14 patients and found no evidence for inadequate blood flow, based on microvessel density quantification using CD31 staining [5]. Furthermore, not only the lumen of the intestine but the mucosa and serosa normally function in low or very low oxygen concentrations (52 to <1 mmHg pO2) [6]. Therefore, the assertion that hypoxia plays a major role in the pathogenesis of anastomotic leak remains unconfirmed.
Our group has previously described a model of anastomotic leakage in rats in which a low colon segment is excised and a colorectal anastomosis is performed with segmental devascularization by dividing the feeding blood supply 1 cm above and 1 cm below the anastomosis. We demonstrated a key role for the commensal pathogen Enterococcus faecalis in the pathogenesis of leakage in this model independent of hypoxia [7]. However, the potential role for tissue hypoxia in the pathogenesis of leak in this model was not formally addressed. Therefore, the aims of the present study were to determine the effect of segmental devascularization of a colon segment following anastomotic surgery on tissue hypoxia in rats.
Methods
Animals
All experiments were approved by the University of Chicago Institutional Animal Care and Use Committee (IACUC). Two hundred fifty to three hundred grams male Wistar rats (Charles River Laboratory, Chicago, IL) were used for all experiments and were allowed unrestricted access to rat chow and tap water. Seventy-two animals were randomized to four groups, consisting of (a) sham operation (laparotomy without colon manipulation), (b) segmental devascularization of a distal colon segment alone, with division of 2 cm of mesenteric blood supply, (c) distal colectomy only, and (d) devascularization plus colectomy. Prior to surgery, the rats were anesthetized with 40–80 mg/kg ketamine and 5–10 mg/kg xylazine via intraperitoneal injection (IP). All rats were subjected to laparotomy. Animals randomized to colectomy underwent 1.0 cm distal colon resection with end-to-end recto-sigmoid anastomosis using 12 interrupted 6–0 non-absorbable monofilament sutures, with the distal resection margin 1 cm above the peritoneal reflection. Immediately following completion of the anastomosis, a “leak test” for anastomotic integrity was performed by infusing 5 ml of sterile 0.9% saline into the rectum, occluding the bowel proximally, and observing for extravasation. Rats in the devascularization group underwent division of 2 cm of the colonic mesentery, beginning at the peritoneal reflection and moving proximally. On postoperative day 6 (POD6), all rats were injected with pimonidazole HCl (Hypoxyprobe kit, Hypoxyprobe Inc., Burlington MA) and sacrificed 30 min later. A previously developed anastomotic healing score (AHS) was used to evaluate healing at the time of sacrifice [8]. The anastomotic site was inked, excised, and placed in formaldehyde for histologic analysis. One rat in the devascularization plus colectomy group died of complications from anesthesia and was therefore excluded from the analysis.
Anastomotic healing score
The AHS was assigned by two blinded observers at the time of sacrifice, prior to histological analysis, as previously described [8]. Briefly, AHS is graded from 0 to 4. AHS0 corresponds to normal healing, with no adhesion or inflammation and complete apposition of tissues. AHS1 indicates flimsy adhesions to the adjacent tissues, without other abnormalities. AHS2 denotes dense adhesions involving the omentum and small bowel. AHS3 specifies gross phlegmonous change (hard and inflamed anastomotic tissue). AHS4 indicates macroscopic intestinal tissue separation and abscess formation.
Hypoxia evaluation
Hypoxia in tissues was quantified using a commercially available pimonidazole HCl kit (Hypoxyprobe®, as above). The rats received 15 mg pimonidazole HCl IP and sacrificed 30 min later. The anastomotic site was excised, placed in formaldehyde, and embedded in paraffin. Five-micrometer sections were then immunostained, using a biotinylated goat anti-mouse secondary antibody (Vector Labs, Burlingame, CA) as previously described [9]. Two negative controls were used: a tissue incubated without primary antibody and a distal colon tissue from a rat which did not undergo pimonidazole HCl injection.
Intestinal collagen content
Masson’s trichrome staining was to evaluate collagen content in formaldehyde-fixed, paraffin-embedded 5-μM sections, as previously described [7] (Human Tissue Resource Center at The University of Chicago).
Hypoxia and collagen quantification
Slides were scanned at ×20 magnification on an Aperio ScanScope XT whole slide scanner and then annotated using an Aperio ImageScope software, with annotation layers generated for the submucosal layer of each tissue sample. An ImageScope deconvolution algorithm was first applied to identify the red, green, and blue values of each color channel for both immunostains. The values were processed using an ImageScope colocalization algorithm for the trichrome stain and dual colocalization and cytoplasmic algorithms for the pimonidazole immunostain.
Quantification of apoptosis in submucosa
Apoptosis was visualized using fluorescent terminal dUTP nick-end labeling (TUNEL kit, EMD Millipore, Billerica, MA) according to the manufacturer’s instructions. In order to visualize apoptotic signal in the submucosal enteric smooth muscle and periendothelial vascular stromal cells, tissues were double-stained for both TUNEL and αSMA, using Alexafluor 488 goat anti-rabbit (ThermoFisher Scientific, Waltham, MA), as a secondary antibody, as previously described [10]. Pictures were taken with Axioscop2 plus, using an Axiocam 503 mono camera, and Zen 2.0 software (Zeiss, Oberkochen, Germany) was used to process and quantify fluorescent images. Apoptotic nuclei (identified by TUNEL and 4′,6-diamidino-2-phenylindole (DAPI) dual staining) were then manually counted in order to ensure accuracy.
Intestinal layers and arteriole visualization
The muscularis mucosa and inner muscularis were visualized by immunohistochemistry for anti-α-smooth muscle actin (αSMA). Similarly, the arterioles were identified by αSMA-positive periendothelial vascular stroma, as previously described [10], using a rabbit anti-αSMA primary antibody (ThermoFisher Scientific, Waltham, MA) and biotinylated goat anti-rabbit secondary antibody (Vector Labs, Burlingame, CA).
Necrosis
Tissue necrosis was analyzed by hematoxylin and eosin staining (H&E). Stained slides were scanned at ×20 resolution in a PannoramicScanner and Pannoramic Viewer software (3D Histech, Budapest, Hungary; Integrated Light microscopy Core Facility at The University of Chicago).
Mucosal and submucosal thickness measurements and arteriolar quantification
The mucosal layer was identified in the villi luminal to the αSMA-positive enteric smooth muscle. The submucosal layer was localized by identifying tissue between the two αSMA-positive enteric smooth muscle layers (examples, Figs. 2–5). Submucosal layer thickness was quantified using the Pannoramic Viewer by measuring the distance between the mucosa and inner muscularis every 400 μM and plotting the average of these distances for each tissue. Arteriole quantification was performed on αSMA-stained sections and confirmed on trichrome-stained sections using ImageScope (Leica Biosystems, Nussloch, Germany; Human Tissue Resource Center at The University of Chicago).
Statistical analyses
Descriptive statistics were examined to determine distribution. Mann-Whitney U test was used to interrogate between-group differences of percentage and intensity of hypoxia stain, TUNEL+DAPI+ stain, and layer thicknesses, while Kruskal-Wallis was used to test differences between mean AHS grading, both using the Prism software (GraphPad). Regression and significance of the ratio of arteriole diameter to thickness within the submucosal layer was determined using STATA SE version 14.0. A level of 0.05 was deemed significant.
Results
Devascularization of a colon segment is associated with poor anastomotic healing
The mean and standard deviation of AHS by group is shown in Fig. 1a. Sham (0 ± 0), devascularization only (Devasc) (0 ± 0) and colectomy only (Colec) (0.22 ± 0.44) control groups did not differ from each other, while Devasc + Colec (1.31 ± 1.15) was statistically different from controls (p < 0.001). Frequency and percentage of AHS is shown in Fig. 1b. Rats undergoing colectomy only (n = 9) healed normally with AHS of 0–1. Eighty-two percent (37/44) of rats subjected to colectomy plus segmental devascularization healed well with an AHS of 0–2. However, the remaining 16% (7/44) displayed poor healing, with AHS scores of 3–4 (Fig. 1b), characterized by dense adhesions with gross abscess at the anastomotic site (AHS3) and gross leak with visible anastomotic dehiscence (AHS4) (Shakhsheer et al., Journal of Gastrointestinal Surgery, accepted).
Devascularization plus colectomy induces colon leak on postoperative day 6. a Sham, devascularization (Devasc), and colectomy (Colec) control groups have a significantly lower mean anastomotic healing score (AHS) than devasculirization + colectomy combined. AHS scores are as follows: AHS0 = complete healing (no adhesion or inflammation and complete apposition of tissues), AHS1 = flimsy adhesions to adjacent tissues only, AHS2 = dense adhesions involving the omentum and small bowel, AHS3 = gross phlegmon, AHS4 = gross intestinal tissue separation and abcess. b A significantly higher percentage of rats in devascularization + colectomy are in AHS groups 1–4 than sham, devascularized, or colectomy alone controls. Percentage of rats, number of rats in group/total number of animals, rows add to 100%. Font size represents percentage of animals per AHS group. One way Anovas were run in GraphPad software. **p<0.0001
Hypoxia does not correlate with anastomotic healing grade
Pimonidazole HCl forms adducts that bind to proteins only in hypoxic tissues (pO2 < 10 mmHg) [11] and can be readily detected by immunohistochemistry. Pimonidazole immunohistochemistry has been shown to predict pO2 electrode measurements of tissue oxygenation [12]. Pimonidazole staining was quantified in the submucosal layer adjacent to the anastomotic site (tissue orientation is shown in Fig. 2a) for two reasons: intestinal mucosal pO2 under normal conditions is less than 10 mmHg (the threshold of detection for pimonidazole immunostaining) [6]. Therefore, as predicted, the mucosa in all groups including that in the sham-operated controls demonstrated pimonidazole binding. Second, the submucosal layer demonstrated significant changes in other important markers as described in Figs. 3, 4, and 5. The entire submucosal layer in each section was manually selected on the software, and the stain was normalized to the area of each tissue. Pimonidazole staining was observed in the submucosal layer of all groups, including sham-operated controls (Fig. 2b, top row). Quantification demonstrated no statistical difference in the percentage of pimonidazole-positive area between controls (sham-operated, devascularized, or colectomy only) and any AHS group (Fig. 2b, c). Finally, we quantified the intensity of pimonidazole stain and found no difference between any of the groups according to AHS (Fig. 2d). No staining was detected in tissues from rats that did not receive pimonidazole or tissues that were not incubated with primary antibody (Fig. 2a, right panel, and data not shown). Pimonidazole staining in controls was consistent with prior reports demonstrating that the intestine is normally moderately hypoxic [13]. Our assessment confirms that tissue oxygen levels were not altered 6 days after colectomy and/or devascularization regardless of AHS grade, either by extent or overall percentage of area. Pimonidazole staining and quantification were performed twice with similar results; one representative experiment is shown (Fig. 2c).
Hypoxia does not differentiate healing versus leaking anastomotic tissues. a–d Evaluation of hypoxia with pimonidazole HCl (Hypoxyprobe). Tissue orientation (a, left panel) highlights the area of submucosa (between dotted lines) except AHS4 where loss of morphology was apparent. Pimonidazole immunostaining in non-treated rats (b, right panel). d Rat colon sections were immunostained for azole adducts (red stain, black arrowheads), and counterstained with hematoxylin (blue). Representative images of controls or Devasc+Colec. No statistical difference in b percentage of immunopositive area or c immunostaining intensity was found between groups. The entire mucosal or submucosal layer from the tissues of 3–7 rats per group were manually selected at ×10 and quantified using the Aperio software. The results were plotted and the statistics ran on GraphPad software
No evidence of differences in apoptosis in submucosa or in arterioles. a ×2 H&E analysis shows no cell death in any control or AHS group. Top panel indicates tissue orientation with regard to the intestinal lumen; asterisk denotes the anastomotic site. b Orientation in top panel: submucosa is between the white dotted lines. Representative images of TUNEL (red) and DAPI (blue) in the submucosa of Controls or Devasc plus Colec groups. The white asterisk indicates TUNEL+DAPI overlap, and the white arrowheads highlight aSMA-positive arterioles (green): no apoptotic blood vessels were observed in any group. c Quantification of TUNEL+ (red) and DAPI+ (blue) cells relative to the area in submucosa. ×40 images of 3–4 tissues from each group were photographed, and only overlapping staining was manually counted and normalized to the area of submucosa. Measured on the Zeiss Zen software and the data was analyzed on Prism (GraphPad). *p<0.05
Histologic analysis fails to demonstrate evidence of tissue hypoxia following colon resection and devascularization
We sought to test the hypothesis that devascularization in this model causes poor anastomotic healing as a result of tissue ischemia by examining apoptosis and necrosis in the intestinal tissues harvested on postoperative day 6. This approach was based on colonic and intestinal ischemia resulting in the subsequent atrophy and fibrosis of surface epithelium and apoptotic regions evident on H&E [14]. We did not identify any areas of necrosis or fibrosis in the tissues of any group by H&E (Fig. 3a). Even in anastomotic tissues from the rats with poor healing (i.e., AHS4), where loss of morphology was observed, there was no apparent mucosal, submucosal, or serosal necrosis evident (Fig. 3a, bottom panel). Using TUNEL staining to detect apoptosis, αSMA immunostaining as a marker for arteriolar smooth muscle, and DAPI to distinguish nuclei, we did not observe any overlap in the submucosa of any of the tissues (n = 28) or groups examined (Fig. 3b) that would indicate necrosis/apoptosis. We also examined vascular regression, a potential indicator of ischemia, which can be detected as “ghost vessels”, consisting of an empty collagen sleeve devoid of endothelial and vascular mural cells [15]. We found no histological evidence of such vascular regression (Fig. 3b, data not shown). Having observed no apoptotic cells in αSMA-positive vascular mural cells, we quantified non-vascular cell death in the submucosa by double immunostaining for TUNEL and DAPI (Fig. 3c). Except for intestinal tissues displaying gross leaks (i.e., AHS4), TUNEL staining was barely detectable; we therefore highlighted these events with a white asterisk symbol in Fig. 3b. We found a significantly higher number of apoptotic cells in AHS3 specimens as compared to controls (Fig. 3c, p = 0.04). These apoptotic events, however, were not clustered; they were evenly distributed throughout the tissue, and TUNEL intensity was low compared to AHS4 (Fig. 3b, white asterisk), suggesting that these events were not related to insufficient oxygenation. AHS4 tissues did present multiple double-positive TUNEL + DAPI nuclei, also distributed throughout the tissue, in no particular pattern in reference to the anastomotic site or existing blood vessels (Fig. 3b, lower panel). Taken together, these data provide no evidence of hypoxic contribution to cell death or anastomotic disruption at POD6 based on these findings.
Leaking anastomoses display decreased and discontinuous collagen fibers
We have previously reported that collagenase-producing bacteria can cause anastomotic leak in association with depletion of intestinal collagen at anastomotic tissues [7, 16]. In the present study, shorter collagen fibers were found in the submucosa of AHS grades 1–4 than in controls (Fig. 4, blue stain, arrows). These findings are consistent with our previous reports.
Intestinal leak is correlated with loss of collagen in submucosa. a Tissue orientation indicating the mucosa (top), submucosa (between dotted lines), and inner muscularis (bottom). b Progressive loss of continuous collagen fibers, blue stain (black arrowheads) in the submucosal layer in healing scores 1 through 4 compared to controls (black arrows). ×5 images
Submucosal layer thickness and vasculature rearrangements are increased in correlation with the AHS
The muscularis mucosa and inner muscularis express αSMA. We quantified the thickness of the submucosal and mucosal layers in αSMA-stained sections by measuring the distance between the muscularis mucosa and inner muscularis (Fig. 5a). The submucosal layer was significantly thicker in AHS 1–3 as compared to sham controls (Fig. 5b, c). On the other hand, we found no difference in the thickness of mucosal layers of any AHS compared to sham-operated, devascularized, or colectomy only controls (Fig. 5d).
Increased AHS correlates with increased submucosal, but not mucosal thickness, and increased arteriolar diameter. (a–b) aSMA immunostaining denotes muscularis mucosa and inner muscularis, allowing for measurement of submucosal thickness (area between dotted lines). Also immunostained are the arterioles in this layer (black arrowheads). a Orientation of tissue and b representative images of controls and Devasc+Colec groups showing increased submucosal layer thickness in AHS1–3 as well as increased arteriole diameter. c Devasc+Colec AHS groups 1–3 demonstrate increased submucosal thickness compared to the other groups. d The mucosal layer thickness was not different in any group. e Positive correlation between arteriole diameter and submucosal thickness, with AHS1–3 tissues (blue dots) cluster on the upper right side of the line. Five to seven tissues per group were measured using Pannoramic viewer and the data analyzed on GraphPad. *p<0.05 Mann-Whitney test
Alterations in extracellular matrix, including collagen degradation, can promote dynamic shifts in vascular architecture [17]. Periendothelial vascular mural cells, found in arterioles, express αSMA, allowing measurement of the diameter of vessels in the submucosal layer. We have previously shown that changes in arteriolar diameter can provide quantitative information about vascular remodeling in response to microenvironmental changes (e.g., adaptation to specific molecular cues) [18]. Here, we found that mean arteriole diameter significantly correlated with submucosal thickness (Fig. 5e, R 2 = 0.25, p < 0.005), indicating that as this layer increased in thickness, arterioles correspondingly increased in diameter. Furthermore, the samples displaying AHS1–3 clustered in the upper right end of the distribution (Fig. 5e, blue dots), suggests an association between thicker submucosa and greater arteriolar diameter in the setting of anastomotic leak. However, we did not detect differences between the number of arterioles in any group (data not shown), suggesting that the proliferative signals typically elicited by sustained hypoxia may not have been present. These data suggest that the increase in arteriolar diameter may reflect a distinct and efficient response repertoire in anastomotic tissues, maintaining oxygenation via compensatory mechanisms which do not involve vessel proliferation.
Discussion
The mechanism of anastomotic leak is invariably attributed to some process of ischemia, despite the weak to non-existent evidence for this claim. Work from our laboratory has generated two novel models of anastomotic leak, involving either radiation exposure or segmental devascularization of the anastomotic area, each of which model aspects of ischemia. In our models, however, intestinal bacteria and not ischemia appeared to be the key contributors to leak. Yet there is little doubt that the process of intestinal surgery imposes decreases in blood flow which necessarily limits oxygen delivery, with tissue hypoxia resulting from the combined effects of pneumoperitoneum, manipulation of tissues, clamping, dividing of blood vessels, and/or stretching segments of intestine together to achieve apposition. We posit that the effect of this initial stress is likely to be compensated for by the ability of tissues to autoregulate their regional blood supply, yet the extent to which this compensatory response might occur following surgery has never been studied. As such, the claim that anastomotic leak is caused by ischemia or tissue hypoxia remains unconfirmed.
Our studies and the work of others clearly demonstrate that even in the face of grossly ischemic tissues, without the presence of intestinal bacteria, leaks do not occur [7, 19]. Therefore, in the present study, we sought to decouple hypoxia from anastomotic leak using a model of segmental devascularization of the anastomotic area. Our findings provide compelling evidence for the lack of change in hypoxia in leaking tissues as compared to all other groups (including non-treated controls), both prior to and at postoperative day 6. In fact, all tissues examined had detectable and comparable hypoxia levels at postoperative day 6. An increase in hypoxia would be predicted to induce VEGF-A expression, followed by an increase in blood vessel numbers (proliferation). Although we detected vascular remodeling in poorly healed anastomotic tissues, we found no changes in blood vessel density in the submucosal layer of the intestinal tissues following anastomotic surgery and devascularization or devascularization alone, suggesting there are no changes in proliferative signals to blood vessels. It is possible that vessel remodeling compensates for changes in blood supply after segmental devascularization only. However, since these changes were not observed in this group, we hypothesize that the loss of collagen may represent a unique environmental cue contributing to shifts in vascular architecture.
In the present study, we were able to decouple sustained hypoxia as a causative factor of anastomotic leak. However, while devascularization was required to produce anastomotic leak in our model and that this is caused by tissue hypoxia, it was not supported by our findings raising the question of a yet-to-be discovered mechanism. Our current and previous findings suggest that devascularized conditions may support a suitable environment for bacteria to express phenotypes that promote anastomotic leaks, independent of tissue hypoxia [20]. Additional factors may include recruitment of immune cells and other environmental cues reflecting the combined effects of surgery and adaptation to a disrupted blood supply.
References
Goligher JC, Graham NG, De Dombal FT (1970) Anastomotic dehiscence after anterior resection of rectum and sigmoid. Br J Surg 57:109–118
Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K (2013) Do we really know why colorectal anastomoses leak? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 17:1698–1707
Boyle NH, Manifold D, Jordan MH, Mason RC (2000) Intraoperative assessment of colonic perfusion using scanning laser Doppler flowmetry during colonic resection. J Am Coll Surg 191:504–510
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon rectum 43:76–82
Schouten SB, De Bruin AF, Gosselink MP et al (2014) Is microvessel density correlated with anastomotic leakage after low anterior resection? Hepato-Gastroenterology 61:90–93
Zheng L, Kelly CJ, Colgan SP (2015) Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 309:C350–C360
Shogan BD, Belogortseva N, Luong PM et al (2015) Collagen degradation and MMP9 activation by enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 7:286ra68
Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley S, Zaborin A, Guyton KL, Krezalek M, Smith DP, Ajami NJ, Petrosino JF, Fleming ID, Belogortseva N, Zaborina O, Alverdy JC (2016) Morphine promotes colonization of anastomotic tissues with collagenase - Producing enterococcus faecalis and causes leak. J Gastrointest Surg 20(10):1744–1751
Hernandez SL, Banerjee D, Garcia A et al (2013) Notch and VEGF pathways play distinct but complementary roles in tumor angiogenesis. Vascular cell 5:17
Funahashi Y, Hernandez SL, Das I et al (2008) A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res 68:4727–4735
Chapman JD, Franko AJ, Sharplin JA (1981) Marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer 43:546–550
Pogue BW, Paulsen KD, O’Hara JA, Wilmot CM, Swartz HM (2001) Estimation of oxygen distribution in RIF-1 tumors by diffusion model-based interpretation of pimonidazole hypoxia and eppendorf measurements. Radiat Res 155:15–25
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114:1098–1106
Kumar V, Abbas AK, Aster JC, Robbins SL (2013) Robbins basic pathology, 9th edn. Elsevier/Saunders, Philadelphia, PA
Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM (2003) Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 163:1801–1815
Olivas AD, Shogan BD, Valuckaite V et al (2012) Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One 7:e44326
Edgar LT, Underwood CJ, Guilkey JE, Hoying JB, Weiss JA (2014) Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS One 9:e85178
Huang J, Bae JO, Tsai JP et al (2009) Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol 34:79–87
Cohn I Jr, Rives JD (1955) Antibiotic protection of colon anastomoses. Ann Surg 141:707–717
Patel NJ, Zaborina O, Wu L et al (2007) Recognition of intestinal epithelial HIF-1alpha activation by Pseudomonas aeruginosa. American journal of physiology Gastrointestinal and liver physiology 292:G134–G142
Acknowledgments
We thank the Human Tissue Resource Center at The University of Chicago and the Integrated Light microscopy Core Facility at The University of Chicago for their technical assistance whose work was compensated according to The University of Chicago Core Facilities Fees. This work was in part funded by NIH grant 2R01GM062344-13A1 and NIH NIDDK grant #2T35DK062719-27.
Authors’ contributions
Conception and design: HSL, SBA, ZO, AJ, KJK
Development of methodology: HSL, SBA, GK, LB
Acquisition of data: HSL, SBA, GK, LB, DM, BN, ZA
Analysis and interpretation of the data: HSL, SBA, LB, ZO, AJ, KJK
Writing, review, and/or revision of the manuscript: HSL, SBA, ZO, AJ, KJK
Administrative, technical, or material support: HSL, SBA, DAM, BN, LB
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Hernandez SL and Alverdy J contributed equally.
Rights and permissions
About this article
Cite this article
Shakhsheer, B.A., Lec, B., Zaborin, A. et al. Lack of evidence for tissue hypoxia as a contributing factor in anastomotic leak following colon anastomosis and segmental devascularization in rats. Int J Colorectal Dis 32, 539–547 (2017). https://doi.org/10.1007/s00384-016-2737-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2737-9