Abstract
Background
The effects of subcutaneous closed-suction Blake drain for preventing incisional surgical site infections (SSIs) after colorectal surgery have never been evaluated in a randomized controlled trial (RCT). Thus, we performed a RCT to evaluate the clinical benefits of using a subcutaneous closed-suction Blake drain in patients undergoing colorectal surgery.
Method
Consecutive patients who underwent colorectal surgery were enrolled in this study. Patients were randomly assigned to the subcutaneous closed-suction drainage arm or the control (no subcutaneous drainage) arm. The primary endpoint was incidence rate of incisional SSIs. And, we performed logistic regression analysis to detect predictive factors for incisional SSIs after colorectal surgery.
Results
From November 2012 to September 2014, a total of 240 patients were enrolled in this study. One-hundred-seventeen patients who were treated by the control arm and 112 patients by the subcutaneous drainage arm were judged to be eligible for analysis. The incidence of incisional SSIs rate was 8.7 % in the overall patients. The incidence of incisional SSIs rate was 12.8 % in the control arm and 4.5 % in the subcutaneous drainage arm. There was significantly reduction of the incidence in the subcutaneous drainage arm than in the control arm (p = 0.025). Logistic regression analysis demonstrated that thickness of subcutaneous fat >3.0 cm, forced expiratory volume in 1 s as percent of forced vital capacity (FEV1.0 %) >70 %, and subcutaneous drain were independent predictors of postoperative incisional SSIs (p = 0.008, p = 0.004, and p = 0.017, respectively).
Conclusion
The results of our RCT suggest that a subcutaneous Blake drain is beneficial for preventing incisional SSIs in patients undergoing colorectal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical site infections (SSIs), including wound infection (incisional SSI), are still a major problem in general surgery. The morbidity, increased length of stay, delay in further treatment (adjuvant chemotherapy or radiation), and the significant psychological effects on patients have been well demonstrated in the literature [1]. Despite the execution of such preventive strategies as preoperative antibiotic prophylaxis [1] and antiseptic skin cleansing [1], SSI rate remains above 15 % after general abdominal surgery in prospective trials [2, 3]. Moreover, the incidence of SSIs after colorectal surgery has been reported to be as high as 20 % and up to 32 % from randomized controlled trials (RCTs) [2, 3].
In colorectal surgery, endogenous pathogens from the patient’s gastrointestinal tract cause incisional SSI [1] and the existence of fluid collection in subcutaneous layer is thought to encourage bacterial growth, resulting in increased SSIs [4]. It was proposed that clearing contaminated secretions from the wound might reduce the incidence of surgical site infection [5]. Insertion of subcutaneous closed-suction Blake drain removes fluids from the subcutaneous layer in the early postoperative phase before they become infected, resulting in a reduction of incisional SSIs.
Recently, two RCTs have examined subcutaneous closed-suction drainage systems as a means to prevent incisional SSIs in digestive surgery, but these studies were not found effective in preventing SSIs [6, 7]. In a subgroup analysis of one RCT, in patients with colorectal malignancies and lower abdominal incisions, this drainage system was found effective [7].
The effects of subcutaneous closed-suction Blake drain for preventing incisional SSIs after colorectal surgery have never been evaluated in a RCT. Thus, we performed a RCT to evaluate the clinical benefits of using a subcutaneous closed-suction Blake drain in patients undergoing colorectal surgery.
Materials and methods
Patients
This study was approved by the Ethics Advisory Committee of Yokosuka Kyosai Hospital before initiation and was registered in the UMIN Clinical Trials Registry as UMIN000010281 (http://www.umin.ac.jp/ctr/index.htm). Consecutive patients who underwent colorectal surgery in the Department of Surgery, Yokosuka Kyosai Hospital, were enrolled in this study. The inclusion criteria were ages over 20 years. The exclusion criteria were appendectomy, hernia repair surgery, laparoscopic operation without additional skin incision, re-do operations before the end of wound healing of the primary operation, history of radiotherapy in lower abdomen, and emergency operation. The procedure used during this study was explained, and written informed consent was obtained from all of the patients.
Randomization and masking
Patients were randomly assigned to the subcutaneous closed-suction drainage group or the control group using minimization method according to location (colon/rectum). The allocated procedure was not masked from investigator or patients.
Procedure
Three consultant surgeons who specialized in colorectal open surgery and one consultant surgeon who were specialized in colorectal laparoscopic surgery involved in this study. Each consultant surgeons had performed 200 or more open colorectal surgery, respectively. Moreover, one consultant laparoscopic surgeon was qualified by an Endoscopic Surgical Skill Qualification System of the Japan Society for Endoscopic Surgery [8].
Skin incision was performed with a scalpel; subcutaneous fat and linea alba were dissected by electrical cautery. During the operation, wound protection was achieved by a dual-ring drape device. Wound closure was done using 1-Vicryl® for the fascia layer and 4–0 PDS® subcuticular sutures for the skin. Prophylactic intra-operative wound irrigation with 1000 ml saline was routinely performed before skin closure. Subcutaneous closed-suction drains were inserted if the patient was randomized in the subcutaneous drainage group. In these cases, 15-Fr silicon flexible drains (Blake drains, Ethicon, Somerville, NJ, USA) were used for the drainage tube. The exit of the drains was separate from the incisions. The device was connected to a low pressure (40–80 mmHg), continuous aspiration reservoir to allow the full length of the wound to be drained. Drains were removed on the fifth day after operation.
The prophylactic antibiotic regimens were performed as follows: flomoxef sodium was injected intravenously within 30 min before skin incision. In patients who underwent operations lasting longer than 3 h, flomoxef sodium was injected intravenously every 3 h, as recommended by the CDC guidelines [1].
Diagnosis of incisional SSIs
All patients were monitored for postoperative incisional SSIs, which were included superficial and deep SSIs. The surgeons performed a physical examination every day from the operating day until discharge. After hospital discharge, all patients were followed at the hospital as an outpatient on day 14 and day 30.
The diagnosis of SSI was based on the definition of the CDC guideline: (1) purulent discharge with or without laboratory confirmation from the superficial incision; (2) organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision; (3) at least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, or feat and superficial incision are deliberately opened by surgeon, unless incision is culture-negative; and (4) diagnosis of superficial SSI by the surgeon or attending physician. According to these definition, incisional SSIs were defined as such findings occurring within 30 day after surgery. The severity of SSIs was assessed by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Each SSI was diagnosed and confirmed by a surgeon other than patient’s primary surgeon.
Statistical analysis
The primary endpoint for this study was incidence rate of incisional SSIs. It was calculated that 95 patients would be required in this study to demonstrate a reduction in incidence rate of SSI from 15 to 5 % at the 5 % significance level with power of 80 %. The data were presented as a mean and standard deviation or as a median and variance. Statistical analysis was performed using the SPSS statistical software (SPSS Inc., Chicago, USA). Differences between categorical variables were tested with Pearson’s chi-squared test. Differences between continuous variables were tested with the Mann-Whitney U test. Probability (p) values were considered to be statistically significant at a level of p < 0.05. And, we performed logistic regression analysis to detect predictive factors for incisional SSIs after colorectal surgery.
Results
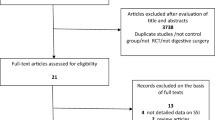
From November 2012 to September 2014, a total of 240 patients were enrolled in this study. Patients were randomized to the control arm (120 patients) and the subcutaneous drainage arm (120 patients). After randomization, three patients marked a deviation from study protocol which was treated by the control arm. These three patients could not be followed at the hospital as an outpatient on day 30 because of changing hospital. Six patients marked a deviation from study protocol, and two patients delayed surgery, which was treated by the subcutaneous drainage arm. Of these six patients, three patients could not be followed at the hospital as an outpatient on day 30 because of changing hospital. One patient did not undergo colorectal surgery because tumor location was ileum. One patient underwent laparoscopic operation without additional skin incision. In one patient, subcutaneous drain was not inserted. Therefore, 117 patients who were treated by the control arm and 112 patients who were treated by the subcutaneous drainage arm were judged to be eligible for analysis (Fig. 1). The clinicopathological characteristics of 229 patients are presented in Table 1. Baseline factors were well balanced between the arms. Surgical procedure and outcomes are summarized in Table 2. Operation time, blood loss, wound length, thickness of subcutaneous fat, and laparotomy rate were similar between the control arm and subcutaneous drainage arm.
The incidence of incisional SSIs
The incidence of incisional SSIs rate was 8.7 % (20/229) in the overall patients. The incidence of incisional SSIs rate was 12.8 % (15/117) in the control arm and 4.5 % (5/112) in the subcutaneous drainage arm. There was significantly reduction of the incidence in the subcutaneous drainage arm than in the control arm (p = 0.025). The incidence of superficial SSIs in the subcutaneous drainage arm was significantly smaller than in the control arm (control arm 10.3 % (12/117) and subcutaneous drainage arm 3.6 % (4/112); p = 0.047). The incidence of incisional SSIs over grade 3 rate was 1.7 % (2/117) in the control arm and 0 % (0/112) in the subcutaneous arm (Table 3).
Predictive factors for incisional SSIs
Based on univariate analysis, thickness of subcutaneous fat >3.0 cm, forced expiratory volume in 1 s as percent of forced vital capacity (FEV1.0 %) <70 %, and subcutaneous drain significantly predicted postoperative incisional SSIs (Table 4). Moreover, logistic regression analysis demonstrated that thickness of subcutaneous fat >3.0 cm, FEV1.0 % >70 %, and subcutaneous drain were independent predictors of postoperative incisional SSIs (p = 0.008, p = 0.004, and p = 0.017, respectively).
Discussion
Usefulness of subcutaneous drain for wound infection has been reported mainly in the obstetrics and gynecological region. Among prospective RCTs of obstetrics and gynecological region [9, 10], cholecystectomy [11], and gastrointestinal region [6, 7] ever reported, it has been reported that subcutaneous drain has no effect for reduction of wound infection in the obstetrics and gynecological region as a result of meta-analysis including RCT [12]. In consideration of a low occurrence rate of wound infection during surgery of obstetrics and gynecological region ranging from 3.8 to 10.0 % even in high-risk group [12], effect of intervention by subcutaneous drain is believed to be poor.
It has been reported on effects of subcutaneous drain on wound infection also in gastrointestinal region. Some retrospective studies have reported that subcutaneous drain reduces wound infection in case of colorectal surgery [13], hepatectomy [14], and closure of ileostomy [15], which have high risk of wound infection. With two reports of prospective RCTs ever made, on the other hand, Baier PK et al. have reported that no usefulness of subcutaneous drain on wound infection was not recognized in 200 cases of gastrointestinal surgeries [6]. In addition, Kaya E. et al. have reported that usefulness of subcutaneous drain was not recognized in 402 cases of gastrointestinal surgeries but that it significantly reduced wound infection in colorectal malignancies and lower abdominal incisions as a result of subgroup analysis [7]. It is controversial in gastrointestinal region whether subcutaneous drain is useful for wound infection prevention. SSI occurrence rate is still at high level as a report has indicated that incisional SSI occurrence rate in colorectal surgery exceeds 20 % [2, 3]. From those described above, it is believed that subcutaneous drain is likely to be useful as long as it is indicated limitedly to cases with high risk of wound infection occurrence such as colorectal surgeries. Without any report ever made on prospective RCT performed only for colorectal surgery using a closed-suction Blake drain for subcutaneous area, this report is the first one in such setting with a result that subcutaneous drain placement significantly reduces wound infection.
Problem in postoperative subcutaneous tissue is that suture of subcutaneous fat is easy to cause tissue necrosis, but dead space is formed subcutaneously if it is not sutured [16]. With a formation of seroma by effusion and blood retained in the dead space, such environment is believed to be formed that bacteria contaminated during surgery is easy to proliferate [12, 13]. It is believed that incisional SSI may be reduced by removing effusion and blood that would induce proliferation of bacteria earlier after surgery before infection by placing a subcutaneous drain. Moreover, it is believed that wound healing is enhanced advantageously by promoting division and proliferative capacity of fibroblast cells as well as enhancing blood flow and granulation assisted by suction effect [17]. However, it is required to pay attention to the period of placement because placement of drain as a foreign matter on the subcutaneous tissue may cause retrograde infection with a risk that the drain itself could work as a culture if the placement continued for a long period of time [18]. Wound infection is often developed from day 5 on after surgery [3]. In the present study, median value of wound infection development timing was day 8 after surgery. In postoperative negative-pressure incision therapy, period of placement execution after surgery is for 5 days or longer [19–21]. Therefore, as incisional SSI may not be prevented if subcutaneous drain was removed within 72 h since its placement [6, 7], the day of subcutaneous drain removal was set at day 5 after surgery in this study. As the result, incisional SSI occurrence rate became significantly smaller to 4.5 % in subcutaneous drainage arm compared with control arm. Further, any case was not recognized that developed incisional SSI based on retrograde infection caused by 5 days of placement.
In reports ever made, drains with multiple side holes have been used such as Redon drain [6], Redi-vac [9], and Jackson-Pratt [10, 22]. Such drains may damage adipose tissues by a strong negative pressure from small suction holes [14]. Since this small hole is easily clogged if adipose tissue got stuck in the hole, it is possible that subcutaneous area is not drained evenly. While there is a report using a Penrose drain [23], open drain is not recommended from a perspective of retrograde infection. So, in this study, we used silastic closed-suction Blake drain with four channels along the sides with a solid core center. This drain prevents effusion from the full length of the wound through a slit, without causing tissue damage [18].
In this study, in the multivariate analysis, thickness of subcutaneous fat >3.0 cm, FEV1.0 % <70 %, and subcutaneous drain correlated with incisional SSIs. A lot of reports have been made up to now on obesity as the risk factors of wound infection [24, 25]. According to Lee et al., abdominal subcutaneous fat thickness measured by CT scanning was significantly thicker in SSI onset group in a study for 655 cases of patients who experienced median abdominal laparotomy; they reported that abdominal subcutaneous fat thickness is an independent risk factor of superficial SSI [26]. Fujii et al. have also reported on 152 cases of patients who received elective colorectal surgery that abdominal subcutaneous fat thickness was an independent risk factor of SSI occurrence as a result of multivariate analysis [25]. In addition, a volume less than 70 % of FEV 1.0 % was proved to be an independent risk factor in this study, whereas Segal CG et al. [27]. and Moghadamyeghaneh Z et al. [28] have reported COPD as a risk factor of superficial SSI and wound dehiscence, respectively. With a lot of reports that smoking is an independent risk factor for surgical site infection occurrence [29, 30], reduction in FEV 1.0 % may have been affected by smoking in the past. From the results of this study, it is suggested that thickness of subcutaneous fat and reduction in FEV 1.0 % work as useful indicators of incisional SSI occurrence.
One limitation of this study is that it was performed within a single specialized institution. Thus, further studies of multiinstitutions and a larger population are needed to confirm our findings and to determine recommendations for the routine use of a subcutaneous drain in colorectal surgery.
In conclusion, the results of our randomized controlled trial suggest that a subcutaneous Blake drain is beneficial for preventing incisional SSIs in patients undergoing colorectal surgery, and logistic regression analysis demonstrates that thickness of subcutaneous fat >3.0 cm, FEV% >70 %, and subcutaneous drain were independent predictors of postoperative incisional SSIs.
References
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control 27(2):97–132
Pinkney TD, Calvert M, Bartlett DC, Gheorghe A, Redman V, Dowswell G, Hawkins W, Mak T, Youssef H, Richardson C, Hornby S, Magill L, Haslop R, Wilson S, Morton D (2013) Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI trial). BMJ 347:f4305. doi:10.1136/bmj.f4305
Mihaljevic AL, Schirren R, Ozer M, Ottl S, Grun S, Michalski CW, Erkan M, Jager C, Reiser-Erkan C, Kehl V, Schuster T, Roder J, Clauer U, Orlitsch C, Hoffmann TF, Lange R, Harzenetter T, Steiner P, Michalski M, Henkel K, Stadler J, Pistorius GA, Jahn A, Obermaier R, Unger R, Strunk R, Willeke F, Vogelsang H, Halve B, Dietl KH, Hilgenstock H, Meyer A, Kramling HJ, Wagner M, Schoenberg MH, Zeller F, Schmidt J, Friess H, Kleeff J (2014) Multicenter double-blinded randomized controlled trial of standard abdominal wound edge protection with surgical dressings versus coverage with a sterile circular polyethylene drape for prevention of surgical site infections: a CHIR-net trial (BaFO; NCT01181206). Ann Surg 260(5):730–737 . doi:10.1097/SLA.0000000000000954discussion 737-739
Panici PB, Zullo MA, Casalino B, Angioli R, Muzii L (2003) Subcutaneous drainage versus no drainage after minilaparotomy in gynecologic benign conditions: a randomized study. Am J Obstet Gynecol 188(1):71–75
Colli A, Camara ML (2011) First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg 6:160. doi:10.1186/1749-8090-6-160
Baier PK, Gluck NC, Baumgartner U, Adam U, Fischer A, Hopt UT (2010) Subcutaneous Redon drains do not reduce the incidence of surgical site infections after laparotomy. A randomized controlled trial on 200 patients. Int J Color Dis 25(5):639–643. doi:10.1007/s00384-010-0884-y
Kaya E, Paksoy E, Ozturk E, Sigirli D, Bilgel H (2010) Subcutaneous closed-suction drainage does not affect surgical site infection rate following elective abdominal operations: a prospective randomized clinical trial. Acta Chir Belg 110(4):457–462
Mori T, Kimura T, Kitajima M (2010) Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minimally invasive therapy & allied technologies : MITAT : official journal of the Society for Minimally Invasive Therapy 19(1):18–23. doi:10.3109/13645700903492969
Al-Inany H, Youssef G, Abd ElMaguid A, Abdel Hamid M, Naguib A (2002) Value of subcutaneous drainage system in obese females undergoing cesarean section using Pfannenstiel incision. Gynecol Obstet Investig 53(2):75–78
Magann EF, Chauhan SP, Rodts-Palenik S, Bufkin L, Martin JN Jr, Morrison JC (2002) Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol 186(6):1119–1123
Chowdri NA, Qadri SA, Parray FQ, Gagloo MA (2007) Role of subcutaneous drains in obese patients undergoing elective cholecystectomy: a cohort study. Int J Surg 5(6):404–407. doi:10.1016/j.ijsu.2007.05.011
Hellums EK, Lin MG, Ramsey PS (2007) Prophylactic subcutaneous drainage for prevention of wound complications after cesarean delivery—a metaanalysis. Am J Obstet Gynecol 197(3):229–235. doi:10.1016/j.ajog.2007.05.023
Fujii T, Tabe Y, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H (2011) Effects of subcutaneous drain for the prevention of incisional SSI in high-risk patients undergoing colorectal surgery. Int J Color Dis 26(9):1151–1155. doi:10.1007/s00384-011-1228-2
Tsujita E, Yamashita Y, Takeishi K, Matsuyama A, Tsutsui S, Matsuda H, Taketomi A, Shirabe K, Ishida T, Maehara Y (2012) Subcuticular absorbable suture with subcutaneous drainage system prevents incisional SSI after hepatectomy for hepatocellular carcinoma. World J Surg 36(7):1651–1656. doi:10.1007/s00268-012-1524-1
Pan HD, Wang L, Peng YF, Li M, Yao YF, Zhao J, Zhan TC, Du CZ, Gu J (2015) Subcutaneous vacuum drains reduce surgical site infection after primary closure of defunctioning ileostomy. Int J Color Dis 30(7):977–982. doi:10.1007/s00384-015-2168-z
Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH (2006) The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 118(2):390–397 . doi:10.1097/01.prs.0000227675.63744.afDiscussion 398-400
Borgquist O, Ingemansson R, Malmsjo M (2010) Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg 125(2):502–509. doi:10.1097/PRS.0b013e3181c82e1f
Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S (2014) Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepato-biliary-pancreat Sci 21(7):509–517. doi:10.1002/jhbp.93
Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG (2009) Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 16(2):140–146. doi:10.1177/1553350609334821
Stannard JP, Atkins BZ, O’Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE (2009) Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy/Wound Manag 55(8):58–66
Chadi SA, Kidane B, Britto K, Brackstone M, Ott MC (2014) Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon rectum 57(8):999–1006. doi:10.1097/DCR.0000000000000161
Ramsey PS, White AM, Guinn DA, Lu GC, Ramin SM, Davies JK, Neely CL, Newby C, Fonseca L, Case AS, Kaslow RA, Kirby RS, Rouse DJ, Hauth JC (2005) Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol 105(5 Pt 1):967–973. doi:10.1097/01.AOG.0000158866.68311.d1
Imada S, Noura S, Ohue M, Shingai T, Sueda T, Kishi K, Yamada T, Ohigashi H, Yano M, Ishikawa O (2013) Efficacy of subcutaneous penrose drains for surgical site infections in colorectal surgery. World J Gastrointest Surg 5(4):110–114. doi:10.4240/wjgs.v5.i4.110
Bamgbade OA, Rutter TW, Nafiu OO, Dorje P (2007) Postoperative complications in obese and nonobese patients. World J Surg 31(3):556–560 . doi:10.1007/s00268-006-0305-0discussion 561
Fujii T, Tsutsumi S, Matsumoto A, Fukasawa T, Tabe Y, Yajima R, Asao T, Kuwano H (2010) Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig Surg 27(4):331–335. doi:10.1159/000297521
Lee JS, Terjimanian MN, Tishberg LM, Alawieh AZ, Harbaugh CM, Sheetz KH, Holcombe SA, Wang SC, Sonnenday CJ, Englesbe MJ (2011) Surgical site infection and analytic morphometric assessment of body composition in patients undergoing midline laparotomy. J Am Coll Surg 213(2):236–244. doi:10.1016/j.jamcollsurg.2011.04.008
Segal CG, Waller DK, Tilley B, Piller L, Bilimoria K (2014) An evaluation of differences in risk factors for individual types of surgical site infections after colon surgery. Surgery 156(5):1253–1260. doi:10.1016/j.surg.2014.05.010
Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Mills S, Pigazzi A, Nguyen NT, Stamos MJ (2015) Wound disruption following colorectal operations. World J Surg 39(12):2999–3007. doi:10.1007/s00268-015-3208-0
Thomsen T, Tonnesen H, Moller AM (2009) Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. The British journal of surgery 96(5):451–461. doi:10.1002/bjs.6591
Hawn MT, Houston TK, Campagna EJ, Graham LA, Singh J, Bishop M, Henderson WG (2011) The attributable risk of smoking on surgical complications. Ann Surg 254(6):914–920. doi:10.1097/SLA.0b013e31822d7f81
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jun Watanabe, Mitsuyoshi Ota, Yusuke Suwa, Atsushi Ishibe, Kazuteru Watanabe, Hidenobu Masui, Kaoru Nagahori, and Itaru Endo have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Watanabe, J., Ota, M., Kawamoto, M. et al. A randomized controlled trial of subcutaneous closed-suction Blake drains for the prevention of incisional surgical site infection after colorectal surgery. Int J Colorectal Dis 32, 391–398 (2017). https://doi.org/10.1007/s00384-016-2687-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2687-2