Abstract
Purpose
To investigate the feasibility of the re-patent EHPVO (r-EHPVO) as an animal model of Rex shunt and the effectiveness of Rex shunt in improving abnormal portal hemodynamics and portal venous pathology of EHPVO.
Methods
A total of 18 New Zealand white rabbits were randomly divided into three groups: normal control (NC) group, extrahepatic portal venous obstruction (EHPVO) group, and r-EHPVO group. The main portal vein was dissected only in the NC group. The main portal vein was narrowed by a cannula in the EHPVO group. The cannula narrowing the main portal vein was removed to restore the portal blood flow into the liver on day 14 in the r-EHPVO group. The portal pressure, splenic size, blood flow velocity, and diameter of the portal vein were measured on days 14 and 28. The shear stress (SS) and circumferential stress (CS) of the portal vein were calculated. The proximal end of the main portal vein was collected on day 28 for further pathological analysis, and the thickness and area of the intima and media were measured by Image J software. The portal pressure, splenic size, SS, CS, intima and media thickness, the ratio of intimal to medial area (I/M), and the ratio of intimal area to the sum of intimal and medial area (I/I + M) were compared among the three groups. The correlation between SS and intimal thickness and between CS and medial thickness were analyzed.
Results
On day 28, the portal pressure of the EHPVO group was significantly higher than that of the NC and r-EHPVO groups, but no significant difference was detected in the portal pressure between r-EHPVO and NC groups. The length and thickness of the spleen in the EHPVO and r-EHPVO groups were significantly higher than those in the NC group (P < 0.01) but were significantly lower in the r-EHPVO group than those in the EHPVO group (P < 0.05). The SS was significantly lower in the EHPVO group than in NC and r-EHPVO groups (P < 0.05) but was significantly higher in the NC group than in the r-EHPVO group (P = 0.003). The CS was significantly higher in the EHPVO and r-EHPVO groups than that in the NC group (P < 0.05) but was significantly lower in the r-EHPVO group than that in the EHPVO group (P < 0.001). The intimal thickness, I/M, and I/I + M of the EHPVO group were significantly higher than those of the NC and r-EHPVO groups (P < 0.05), but no significant difference was observed between the NC and r-EHPVO groups (P > 0.05). The SS is negatively related to intimal thickness (r = – 0.799, P < 0.001).
Conclusion
The r-EHPVO model is feasible as an animal model of the Rex shunt. The Rex shunt could be beneficial to improving the abnormal portal hemodynamic and portal venous intimal hyperplasia by restoring the portal blood flow into the liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extrahepatic portal venous obstruction (EHPVO) is a major cause leading to portal hypertension in children, which results in upper gastrointestinal bleeding due to the obstruction of portal blood flow into the liver. The abnormal portal hemodynamic changes in decreased shear stress (SS) and increased circumferential stress (CS) were reported in the EHPVO animal, which were related to the esophageal varices [1]. Rex shunt was considered an ideal surgical method for treating EHPVO due to its reconstruction of the portal blood flow into the liver [2,3,4,5]. Rex shunt refers to a surgery wherein the extrahepatic portal blood is drained into the left branch of the intrahepatic portal vein in Rex recesses by bypassing or transposing a grafted vein, through which a hepatopetal portal blood is reconstructed. No study has yet reported whether the Rex shunt could change this hemodynamic situation of portal hypertension. In the present study, a re-patent EHPVO (r-EHPVO) model was established based on the feature of Rex shunt restoring portal blood flow into the liver and used as the animal model of Rex shunt, in which the portal blood flow was restored by removing a cannula narrowing the main portal vein in the EHPVO animal model. Furthermore, the changes in portal hemodynamics and portal venous pathology among EHPVO, normal portal vein, and r-EHPVO models were compared to evaluate the efficiency of Rex shunt in improving abnormal portal hemodynamics and portal venous pathology of EHPVO.
Materials and methods
A total of 18 New Zealand white rabbits (body weight: 4–5 kg; gender: male; age: adult) were randomly divided into three groups (6 cases in each group): normal control (NC), extrahepatic portal venous obstruction (EHPVO), and r-EHPVO. This study was approved by the ethics committee of the Capital Institute of Pediatrics, Beijing, China.
Establishment of animal model
NC group: at porta hepatis, the main portal vein was dissected, and the portal pressure and the splenic length and thickness were measured.
After 14 days, a laparotomy was performed again. The portal pressure, blood flow velocity (FV), and diameter of the main portal vein were measured. Blood sample was collected for a routine blood test.
After 28 days of the first operation, the portal pressure, splenic length and thickness, and blood FV and diameter of the main portal vein were measured again. Blood sample was collected for routine blood tests. The proximal end of the main portal vein was collected for pathological analysis.
EHPVO group: first, the main portal vein was dissected at porta hepatis. A cannula was placed outside the main portal vein to narrow the main portal vein by tightening the suture around the cannula, through which the lumen of the main portal vein was narrowed to 20%. This cannula was made using an infusion tube, the length of the cannula was about 0.5 cm, and its diameter was about 20% of the main portal vein (Fig. 1). Then, the color of the small intestine was observed for 10 min. This operation was completed where there was no obvious congestion. Before and after narrowing the main portal vein, the portal pressure and splenic length and thickness were measured.
After 14 days, a laparotomy was performed again. During the operation, the portal pressure and blood FV, and diameter of the main portal vein were measured again. The blood sample was collected for the routine blood test.
After 28 days of the first operation, the portal pressure, splenic length and thickness, and blood FV and diameter of the main portal vein were measured again. The blood sample was collected for routine blood test. The proximal end of the main portal vein was collected for further pathological analysis.
r-EHPVO group: firstly, the main portal vein was narrowed with a cannula to establish the EHPVO model, and the portal pressure and splenic length and thickness were measured. After 14 days, the cannula narrowing the main portal vein was removed (Fig. 2), and the portal pressure, blood FV, and diameter of the main portal vein were measured. The blood sample was collected for routine blood test.
After 28 days of the first operation (after 14 days of removing the cannula), the portal pressure, splenic length and thickness, and blood FV and diameter of the main portal vein were measured again. The blood sample was collected for routine blood test. The proximal end of the main portal vein was collected for further pathological analysis.
Measurement of portal pressure
A 1F tube filled with heparin saline and connected with a pressure transducer was inserted into a small intestinal vein, and the portal pressure was measured by the Mikro Tip blood pressure basic system (ADInstruments, Australia).
Measurement of splenic size and diameter of the main portal vein
The splenic length and thickness and diameter of the proximal end of the main portal vein were measured using Vernier calipers.
Measurement of blood FV in the portal vein
The FV of the proximal end of the main portal vein was measured using an ALC-BFS Doppler blood flow measurement system (ALCBIO, ALC-BFS_TY7203). The exact portion of the portal vein measuring the FV was the lower portion of the main portal vein between the mesenteric vein and the cannula narrowing the portal vein.
Routine blood test
The levels of red blood cell (RBC), white blood cell (WBC), hemoglobin (Hb), and platelets (PLT) were detected on a hematology analyzer (Mindray, BC2800Vet).
Calculation of SS and CS in portal vein
The SS of the main portal vein was calculated using the following formula [1, 6]: SS = 4FV × η/π × r3 (η is the blood viscosity; FV is the flow velocity of the portal vein; r is the radius of the main portal vein).
The CS of the main portal vein was calculated by the formula [1, 6], CS = Δ P × r / h (r is the radius of main portal vein; Δ P is the transmural pressure gradient, which is the portal pressure in this study; h is the wall thickness, which is about 0.1 mm in this study). The portal pressure of the lower portion between the mesenteric vein and the cannula is increased due to the obstruction of portal blood flow into the liver after narrowing the portal vein using the cannula. Therefore, to measure the SS and CS of portal hypertension, the lower portion of the portal vein was the exact portion of the portal vein measuring the SS and CS.
Pathology of the portal vein
The pathological sections of the portal vein tissue were subjected to hematoxylin–eosin (HE) staining, and the images were collected under an electronic microscope. The intimal and medial thickness of the vascular wall was measured by image analysis software Image J 1.53 as follows (Fig. 3a): The thickness of the intima and media were measured at zero, three, six, and nine o’clock of the vascular section image, and the thickness of the intima and media was obtained as an average of the four values.
The areas of the intima and media of the vascular wall were measured using Image J 1.53 as follows: The measuring area was selected with the free selection tool (Fig. 3b), and then the selected area was measured. The ratio of the intimal to medial area (I/M) and the ratio of the intimal area to the sum of the intimal and medial area (I/I + M) were calculated.
Statistical analysis
SPSS13.0 was used for statistical analysis. One-way analysis of variance (ANOVA) was used to compare the portal pressure, splenic size, the levels of RBC, WBC, Hb, and PLT, SS, CS, the intimal and medial thickness, I/M, and I/I + M among NC, EHPVO, and r-EHPVO groups. Paired sample t-test was used for the comparison of portal pressure, splenic size, the levels of RBC, WBC, Hb, and PLT, SS, and CS among different time points in the same group. Pearson’s correlation analysis was used to analyze the correlation between SS and intimal thickness and between CS and medial thickness. P < 0.05 indicated a statistically significant difference.
Results
The time point that a rabbit underwent the first, second, and third operation was days 0, 14, and 28, respectively.
Portal pressure
On day 0, the portal pressure was increased significantly after narrowing the main portal vein than that before narrowing the main portal vein in the EHPVO and r-EHPVO groups (P < 0.001) but was significantly higher than that in the NC group (P < 0.001).
On day 14, the portal pressure of the EHPVO and r-EHPVO groups was significantly higher than that of the NC group (P < 0.001). No significant difference was observed between the EHPVO and r-EHPVO groups before the removal of the cannula (P = 0.677). The portal pressure was significantly decreased after the removal of the cannula than that before the removal of the cannula in the r-EHPVO group (P < 0.001).
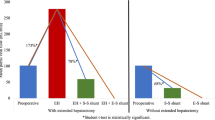
On day 28, the portal pressure of the EHPVO group was significantly higher than that of the NC and r-EHPVO groups (P < 0.05), while no significant difference was detected between the r-EHPVO and NC groups (P = 0.079).
The portal pressure on day 28 was significantly lower than on day 14 in the r-EHPVO group (P < 0.05). The detailed results are shown in Table 1.
Splenic size
On day 28, the length and thickness of the spleen in the EHPVO and r-EHPVO groups were significantly higher than those in the NC group (P < 0.001), while they were significantly lower in the r-EHPVO group compared to the EHPVO group (P < 0.05).
The length and thickness of the spleen were significantly increased on day 28 than on day 0 in the EHPVO and r-EHPVO groups (P < 0.01). The detailed results are shown in Table 2.
Routine blood test
On day 0, no significant difference was observed in the WBC, RBC, Hb, and PLT levels among three groups.
On day 14, the levels of RBC, Hb, and PLT in the EHPVO and r-EHPVO groups were significantly decreased than those in the NC group (P < 0.05), while no significant difference was detected between the EHPVO and r-EHPVO groups.
On day 28, the RBC, Hb, and PLT levels in the EHPVO and r-EHPVO groups were significantly decreased than in the NC group (P < 0.05). The RBC, Hb, and PLT levels were significantly increased in the r-EHPVO group compared to the EHPVO group (P < 0.05).
In the r-EHPVO group, the PLT level significantly increased on day 28 than on day 14 (P < 0.05). The detailed results were shown in Table 3.
SS of the portal vein
On day 14, the SS in the EHPVO and r-EHPVO groups was significantly lower than that in the NC group (P < 0.05). The SS of the EHPVO group did not differ significantly from that of the r-EHPVO group before the removal of the cannula (P = 0.656) but was significantly lower than that of the r-EHPVO group after the removal of the cannula (P = 0.003).
On day 28, the SS in the EHPVO group was significantly lower than in the NC and r-EHPVO groups (P < 0.001). The SS in the NC group was significantly higher than that in the r-EHPVO group (P = 0.003).
The SS after the removal of the cannula was significantly higher than that before the removal of the cannula in the r-EHPVO group on day 14 (P = 0.001). The SS in the r-EHPVO group was significantly higher on day 28 than that before the removal of the cannula on day 14 (P < 0.001) but was not significantly different from that after the removal of the cannula on day 14 in the r-EHPVO group (P = 0.317). The detailed results were shown in Table 4.
CS of the portal vein
On day 14, the CS in the EHPVO and r-EHPVO groups was significantly higher than that in the NC group (P < 0.001). On the other hand, the CS of the EHPVO group did not differ significantly from that of the r-EHPVO group before the removal of the cannula (P = 0.521) but was significantly higher than that of the r-EHPVO group after the removal of the cannula (P < 0.001).
On day 28, the CS in the EHPVO and r-EHPVO groups was significantly higher than that in the NC group (P < 0.05), while the CS in the r-EHPVO group was significantly lower than that in the EHPVO group (P < 0.001).
The CS in the r-EHPVO group after the removal of the cannula was significantly lower than that before the removal of the cannula in the r-EHPVO group on day 14 (P = 0.001). The CS of the r-EHPVO group on day 28 was significantly lower than that before the removal of the cannula on day 14 (P = 0.001) but was not significantly different from that after the removal of the cannula in the r-EHPVO group on day 14 (P = 0.052). The detailed results are shown in Table 4.
Changes in intima and media in the portal vein
The intimal thickness of the EHPVO group was significantly higher than that of the NC and r-EHPVO groups (P < 0.05), but no significant difference was observed between the NC and r-EHPVO groups (P = 0.071). Also, no significant difference was detected in the medial thickness among the three groups (P > 0.05).
The I/M of the EHPVO group was significantly higher than that in the NC and r-EHPVO groups (P < 0.05), but no significant difference was observed between the NC and r-EHPVO groups (P = 0.104).
The I/I + M of the EHPVO group was significantly higher than that of the NC and r-EHPVO groups (P < 0.05), but no significant difference was observed between the NC and r-EHPVO groups (P = 0.089). The detailed results are shown in Table 5.
Correlation between hemodynamic and pathological changes in the portal vein
The SS was negatively related to intimal thickness (r = – 0.799, P < 0.001) (Fig. 4a), and the CS was positively related to the medial thickness (r = 0.792, P < 0.001) (Fig. 4b).
Discussion
Feasibility of the r-EHPVO model as the animal model of Rex shunt
In this study, the animal model of EHPVO was established by narrowing the trunk of the portal vein with a cannula. The results showed that the portal pressure and spleen size on days 14 and 28 in the EHPVO group were significantly higher than those in the NC group, indicating that narrowing the main portal vein with a cannula increased the portal pressure and splenic size, which were the typical manifestations of portal hypertension. The routine blood test showed that RBC, Hb, and PLT in the EHPVO group were significantly lower than in the NC group on days 14 and 28; these results could be ascribed to hypersplenism. Thus, it can be inferred that the EHPVO model was established successfully by narrowing the main portal vein with a cannula in this study.
Although the main portal vein was narrowed using a cannula in the EHPVO model, the main portal vein was not obstructed completely without portal venous thrombosis. Therefore, the portal blood flow into the liver could be restored after the removal of the cannula in the r-EHPVO model. In the present study, the portal pressure of the r-EHPVO group was significantly decreased after the removal of the cannula. In addition, the hypersplenism and splenic size were significantly relieved after the removal of the cannula in the r-EHPVO group. These outcomes suggested that the portal blood flow was restored after cannula removal. The basic function of the Rex shunt in the treatment of EHPVO is the restoration of portal blood flow into the liver through the bypass vein [2,3,4,5], which is similar to the reconstruction of portal blood flow by removal of the narrowed cannula in the r-EHPVO model. Therefore, the r-EHPVO could be used as an animal model of Rex shunt to assess the function of restoring portal blood flow and the effectiveness of relieving portal hypertension.
However, EHPVO in children is mainly caused by the thrombosis of the portal vein, which is different from the EHPVO model based on the partial narrowing of the portal vein in this study. Although the different etiologies of EHPVO between human and animal models might affect the efficacy evaluation after Rex shunt, both EHPVO in the animal model and humans have extrahepatic portal vein obstruction, reduced portal blood flow into the liver, portal hypertension, splenomegaly, and hypersplenism. Nonetheless, the modified hemodynamics in the portal venous system caused by EHPVO may be similar between the animal model and humans.
Abnormal portal hemodynamics and portal venous pathology in EHPVO
Portal pressure is affected by the resistance of portal inflow and outflow. When the portal blood flow into the liver is obstructed, the portal pressure is increased, which alters the portal venous hemodynamics [1]. SS and CS are two major indexes in the evaluation of hemodynamic changes. SS is a tangential force acting on the endothelia of blood vessels along the blood flow, determined by the blood FV and viscosity and the morphology of blood vessels. CS is a tangential force acting on the vascular wall in the vertical direction of blood flow, affected by the pressure gradient between inside and outside the vascular wall. The hemodynamics are stable in a normal situation wherein there are no collateral branches, enlarged vascular diameter, and other morphological changes. In this study, the SS was significantly decreased, and the CS was significantly increased in the EHPVO group, which confirmed the abnormal hemodynamic changes due to decreased SS and increased CS in portal hypertension, as described previously [1].
Hemodynamic changes play a critical role in intimal hyperplasia of the grafted vein. Intimal hyperplasia and thickening of the vascular wall were the results of arterialized adaptation of grafted veins after hemodynamic changes [7, 8]. The theory of decreased SS leading to atherosclerosis revealed that atherosclerotic injury usually occurs on the inner lining of the vascular wall [9]. Therefore, the decreased SS may lead to intimal hyperplasia [10, 11]. In this study, the intimal thickness of the EHPVO group was significantly higher than that of the NC and r-EHPVO groups and was negatively correlated with the level of SS. These findings suggested that decreased SS might be a cause of intimal hyperplasia. The mechanisms may be as follows: 1. Altered SS can induce endothelial cells to express a variety of cytokines, including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and nitric oxide synthase (NOS). The decreased SS upregulates PDGF mRNA and protein expression. PDGF and FGF induce intimal hyperplasia [12,13,14]. 2. When SS is decreased, the platelets and leukocytes accumulate and adhere to the vascular wall [15], resulting in the local release of cytokines at a high concentration, which further stimulates the proliferation of smooth muscle cells in media and their migration into the intima.
In addition, the I/M and I/I + M in the EHPVO group were significantly higher than that of the NC and r-EHPVO groups, suggesting that intimal hyperplasia is a major of the thickening of the vascular wall in EHPVO.
Effectiveness of improving the abnormal portal hemodynamics and portal venous pathology by Rex shunt
In this study, the SS was significantly increased, and the CS was significantly decreased in the r-EHPVO group after restoring the portal blood flow into the liver, suggesting that the abnormal hemodynamic changes of decreased SS and increased CS of portal hypertension were reversed through the restoration of portal blood flow into the liver. Owing to this correlation between the decreased SS and intimal hyperplasia, the intima thickness, I/M, and I/I + M were also significantly decreased in the r-EHPVO group after removing the cannula narrowing the portal vein, suggesting that the intimal hyperplasia could be improved through reconstruction of the hepatopetal blood flow. These findings indicated that the Rex shunt restores the portal blood flow into the liver and improves the abnormal portal hemodynamics and intimal hyperplasia.
However, on day 28, the SS in the r-EHPVO group was lower than that in the NC group, and the CS in the r-EHPVO group was higher than that in the NC group, suggesting that the hepatopetal portal blood flow was not completely restored. Some EHPVO patients still had esophageal varices and splenomegaly after the Rex shunt [16], which might be related to the incomplete relief of the abnormal portal hemodynamics. Moreover, we did not analyze the structure of the liver and the intrahepatic portal vein due to our focus on the correlation between EHPVO and portal hemodynamics. Inevitably, the volume of the liver and caliber of the intrahepatic portal vein decline in EHPVO, which might be improved after the Rex shunt. Therefore, we speculated that exploring the structure of the liver and the intrahepatic portal vein in EHPVO and Rex shunt using our animal model would be beneficial in future studies.
Conclusion
The r-EHPVO model is feasible to be used as an animal model of the Rex shunt. The Rex shunt could be beneficial in improving the abnormal portal hemodynamic and portal intimal hyperplasia by restoring the portal blood flow into the liver.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhang Z, Tang S, Zhu L, Wu G, Jiang Z, Shi B (2008) Stresses in portal venous system of pre-hepatic portal hypertension (PHT) rabbits. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 25(6):1322–1326
Superina R, Shneider B, Emre S, Sarin S, de Ville de Goyet J (2006) Surgical guidelines for the management of extrahepatic portal vein obstruction. Pediatr Transplant 10(8):908–913
de Ville de Goyet J, Alberti D, Falchetti D, Rigamonti W, Matricardi L, Clapuyt P, et al. (1999) Treatment of extrahepatic portal hypertension in children by mesenteric-to-left portal vein bypass: a new physiological procedure. Eur J Surg 165(8):777–781
Bambini DA, Superina R, Almond PS, Whitington PF, Alonso E (2000) Experience with the Rex shunt (mesenterico-left portal bypass) in children with extrahepatic portal hypertension. J Pediatr Surg 35(1):13–18; discussion 18–19
Lautz TB, Keys LA, Melvin JC, Ito J, Superina RA (2013) Advantages of the meso-Rex bypass compared with portosystemic shunts in the management of extrahepatic portal vein obstruction in children. J Am Coll Surg 216(1):83–89
Ballermann BJ, Dardik A, Eng E, Liu A (1998) Shear stress and the endothelium. Kidney Int Suppl 67:S100–S108
Wu J, Zhang C (2009) Neointimal hyperplasia, vein graft remodeling, and long-term patency. Am J Physiol Heart Circ Physiol 297(4):H1194–H1195
Berceli SA, Tran-Son-Tay R, Garbey M, Jiang Z (2009) Hemodynamically driven vein graft remodeling: a systems biology approach. Vascular 17 Suppl 1(Suppl 1):S2–S9
Caro CG, Fitz-Gerald JM, Schroter RC (1971) Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci 177(1046):109–159
Ojha M (1994) Wall shear stress temporal gradient and anastomotic intimal hyperplasia. Circ Res 74(6):1227–1231
Giddens DP, Zarins CK, Glagov S (1993) The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng 115(4B):588–594
Randone B, Sterpetti AV, Stipa F, Proietti P, Aromatario C, Guglielmi MB et al (1997) Growth factors and myointimal hyperplasia in experimental aortic allografts. Eur J Vasc Endovasc Surg 13(1):66–71
Sterpetti AV, Cucina A, Randone B, Graziano P, Stipa F, Corvino V et al (1999) Basic fibroblast growth factor and myointimal hyperplasia after experimental polytetrafluoroethylene arterial grafting. Eur J Surg 165(8):772–776
Sterpetti AV, Cucina A, Randone B, Guglielmi MB, Fragale A, Cavallaro A (1999) Increased production of cytokines and growth factors by aortic allografts: a possible explanation for myointimal hyperplasia formation. Eur Surg Res 31(4):297–304
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282(21):2035–2042
Zhang JS, Li L, Cheng W (2017) The optimal procedure of modified Rex shunt for the treatment of extrahepatic portal hypertension in children. J Vasc Surg Venous Lymphat Disord 5(6):805–809
Funding
This study has received funding from National Nature Science Foundation of China (Code: 81770595 and 82170679).
Author information
Authors and Affiliations
Contributions
JS was a major contributor in performing the study and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the ethics committee of Capital Institute of Pediatrics.
Ethical statement
All methods used in this study were carried out in accordance with relevant guidelines and regulations. The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, JS., Li, L. Effectiveness of Rex shunt for improving the abnormal portal hemodynamics and portal venous pathology in EHPVO animal model. Pediatr Surg Int 39, 192 (2023). https://doi.org/10.1007/s00383-023-05480-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05480-4