Abstract
Undescended testis (UDT) is defined as failure of a testis to descend into the scrotum. It is one of the most common reasons for consultation in pediatric surgery and urology with an incidence of 3% in live-born male infants. Decades ago, classical studies established that the failure of a testis to descend alters the development of its germ cells increasing the risk of infertility and testicular cancer in adulthood. More recent publications have rebutted some of the myths and raised controversies regarding the management of these patients, which, far from being limited to surgical treatment, should include pathophysiological and prognostic aspects for a comprehensive approach to the condition. Therefore, here we present an updated review divided into two parts: the first assessing the pathophysiological aspects and risks of these patients focused on fertility and cancer, and the second addressing the different treatment options for UDT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Failure of a testis to descend into the scrotal sac is one of the main reasons for consultation in pediatric urology. The prevalence of undescended testis (UDT) is between 1 and 8% in full-term born male infants, increasing to up to 30% in premature male neonates [1,2,3]. In around 50% of these infants, descent of the testis occurs spontaneously within the first months of life, and subsequent incidence is 3% at 3 months of life and 1% at 1 year [2,3,4]. Postnatal descent is related to the temporary activation of the hypothalamus–pituitary–gonadal axis, a phenomenon known as the “minipuberty”, leading to an increase in reproductive hormone levels between 2 and 4 months of life. After this age, the probability of spontaneous descent is low [5].
UDT is a congenital condition in the majority of cases; however, in a group of patients, UDT is acquired late in childhood with an incidence of 1–2% [6]. In these patients, testicular descent into the scrotum is well documented, but over time, the testicle does not remain in this position. This may be the course of a retractile testicle that over time becomes stuck in the inguinal canal. Some authors have hypothesized that this may be due to failure of elongation of the spermatic cord during growth, as the distance between the inguinal canal and the scrotum duplicates during childhood when the pelvis becomes larger [7]. Finally, UDT may be secondary to a scarring process after inguinal hernioplasty or hydrocelectomy.
Risk factors associated with UDT are maternal hypertension (especially in cases of severe and early-onset preeclampsia), maternal smoking during pregnancy, and the use of analgesics (mainly paracetamol) during the first and second trimesters of pregnancy [8, 41, 42].
Maternal obesity, alcohol consumption during pregnancy and maternal exposure to estrogens have been associated with UDT, but in has not been possible to prove that they are statistically significant [41, 42].
Pathophysiology
Normal testicular descent
Although a continuous phenomenon, normal testicular descent in the fetus has been described to occur in two main phases: the intra-abdominal and the inguinoscrotal phase. The large majority of UDT is the result of an alteration in the second phase and are anchored between the internal inguinal ring and the root of the scrotum [3, 5]. Failures in the intra-abdominal phase are the cause of around 5% of UDT and clinically lead an intra-abdominal testis [2, 3].
The intra-abdominal phase occurs between weeks 8 and 15 of gestation and results in a testicle that reaches the internal inguinal ring, which is the entrance to the future inguinal canal. The inguinoscrotal phase takes place between weeks 23 and 35 of gestation and during this time, the testicle passes the inguinal canal to settle in the scrotum [3].
Fetal testicular hormones are vital for adequate descent and are the main cause of failure. It is thought that an insufficient placental or pituitary stimulus on the developing testis leads to inadequate production of androgens and insulin-like factor 3 (INSL3) by Leydig cells and of anti-Müllerian hormone (AMH) by Sertoli cells [1, 2, 4].
Intra-abdominal phase
In the intra-abdominal phase, there is increasing relevance of the action of INSL3 and AMH on the gubernaculum, a mesenchymal structure initially located between the lower pole of the testis and the inguinal region. INSL3 and AMH action leads to shortening of the gubernaculum resulting in descent of the testis to the vicinity of the internal inguinal ring, where it is anchored to avoid ascend during growth of the abdominal cavity of the fetus [5]. Patients with persistent Müllerian ducts and gene mutations determinant for AMH and AMH receptor production were observed to have an abnormally long gubernaculum. On the other hand, although mutations in the genes regulating production or expression of INSL3 receptors are rare in individuals with congenital UDT, low levels of this hormone were found in cord blood of these patients, as shown by a prospective case–control study by Fénichel et al. [17]. Indeed, if the development of the gubernaculum is interrupted, the testicles move freely and remain located in the abdomen.
In a recent review, Sarila and Hutson identifies other factors involved in the failure of the intra-abdominal phase of testicular descent, highlighting defects in the synthesis of AMH or INSL3, defects in their respective cell receptors, mutations of the Hoxo-10 gene, anatomical anomalies in the anchorage of the gubernaculum in the abdominal wall and agenesis of the gubernaculum (which is a theoretical cause rather than something clinically proven) [10].
Inguinoscrotal phase
In the inguinal phase of the descent, the testicle, epididymis and gubernaculum migrate “in block” following the genital branch of the genitofemoral nerve under the influence of androgenic action and intra-abdominal pressure. Androgen action indirectly controls migration of the gubernaculum by its effects on the inguinoscrotal fat pad, which releases neurotrophins that act on the sensory branches of the genitofemoral nerve, which in turn, release the neurotransmitter calcitonin gene-related peptide (CGRP), producing a chemotactic gradient that guides the gubernaculum on its passage [3, 5]. In case of minor androgen deficiencies, one side may be more affected than the other, leading to unilateral UDT [5]. The androgen action on the gubernaculum is also direct, activating androgen receptors which allows its proper development [10].
A fetal peritoneal evagination, known as processus vaginalis, accompanies these structures in their descent. At the end of the inguinal phase of testis descent, the proximal processus vaginalis is obliterated and the gubernaculum regresses and becomes a fibrous structure, called scrotal ligament, adhering to the scrotum. This final phase in testicular descent is complex and is also mediated by CGRP released by genitofemoral nerve [10].
Consequences of failure of testicular descent
The scrotal temperature is 4 °C less than that of body and the human testicle adapts its biochemical and physiologic processes to that temperature [16].
Pluripotential human germ cell (gonocytes) undergo important changes during the first year of life, differentiating into unipotential type A spermatogonia from 3 months of life, a transformation that is critical for spermatogenesis. This differentiation takes place concomitantly to the aforementioned minipuberty, which drives the sudden increase in gonadotrophin and testosterone production. Gonocytes that do not differentiate undergo apoptosis.
Type A spermatogonia slowly mature into type B spermatogonia and around 4 years of age, become primary spermatocytes, the form in which they will remain in the seminiferous tubules until puberty.
Both apoptosis and the differentiation from gonocytes to A spermatogonia occur at 33 ºC [16]. If the testicle has not descended, cell transformation and apoptosis take place under thermal stress, resulting in a lower number of A spermatogonia available for spermatogenesis and persistence of pluripotenial cells that may eventually become malignant. This explains why patients with UDT are at increased risk of infertility and testicular tumor development.
In patient with acquired UDT, these processes have taken place physiologically; however, there is also evidence of germ-cell alteration in these cases, although the severity is less than congenital UDTs [13].
Classification
Multiple classifications have been developed for UDT. Some consider the location of the testis in its lowest, tension-free position classifying them into high scrotal, supra-scrotal and nonpalpable testis.
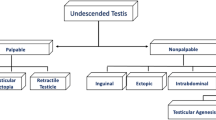
We believe that, from a practical point of view, it is convenient to use those that classify UDT into palpable and nonpalpable testis, since it is more helpful to guide treatment (Fig. 1). In some patients, the UDT is located intra-abdominally but immediately adjacent to the internal inguinal ring and with a Valsalva maneuver it may slide into the inguinal canal. These cases are called “peeping testis” and may be palpable on some occasions and nonpalpable on others.
Nonpalpable UDT
Between 10 and 20% of the UDT are nonpalpable [2, 4]. Most of them are due to intra-abdominal location, including peeping testicles or vanishing testis secondary to in utero torsion. Others causes include: an inguinal location with varying degrees of dysplasia or atrophy and testicular regression syndrome (TRS).
Anorchidism or TRS is a rare condition that account for 0.5–4% of UDT. It is characterized by 46, XY presenting with male phenotypic genitalia, bilateral absence of the testicles and elevated gonadotropins. It occurs in about 1:20.000 males and the cause is unclear. It has been proposed to be caused by an intrauterine vascular accident; if occurs during the second half of gestation, causing testosterone deficiency and impaired penile growth, is associated with micropenis (50% of cases). No genetic factors have been definitively linked to anorchidism, although connections to the steroidogenic factor 1 (SF1, NR5A1) gene have been explored [11, 12].
Palpable UDT
Around 80% of UDT are palpable and they are generally located in the inguinal canal.
Ectopic testis, observed outside the normal path of descent, is less common finding and can be found as a supra-aponeurotic inguinal pouch (most frequently) or with a location in the suprapubic, perineal, femoral region or even in the contralateral scrotum.
The so-called retractile testicle is a scrotal testis that retracts to an undescended inguinal position, but descends or is easily manipulated into the scrotum without tension, and remains there until the cremasteric reflex is activated. This condition is not considered to be pathological, but needs to be closely monitored by the specialist as over time around 30% may evolve into acquired UDT [14, 15] or they may progressively decrease the volume of the affected testicle [14], requiring orchiopexy.
UDT and infertility
The association of UDT with decreased fertility has been broadly debated in the literature. Currently, early treatment is strongly recommended to preserve fertility potential in these patients.
Fertility rate versus paternity rate
It is well known that unilateral UDT is associated with a decreased fertility rate, defined as the number of descendants born per sexual relationship for a couple, individual or population, compared to the general population; whereas paternity rate, the actual potential to become a father, does not differ significantly. Conversely, patients with bilateral UDT have lower rates of both parameters [15]. In 2004, Peter Lee analyzed almost 700 patients treated for UDT and compared them to a similar number of controls. He observed that de paternity rate was 65% in patients with bilateral UDT, 89.7% in patients with unilateral UDT and 93.2% in the control group. Time to conception did not differ between the three groups [36].
When evaluating the sperm of these patients, Lee found that 54% of those who had a history of bilateral UDT had a sperm density of less than 5 million/mL compared to 9% of those who had unilateral UDT. This finding confirms the increased risk of patients with bilateral UDT, but also suggest that other factors determining paternity rate should be considered, especially when counseling the parents [36].
In a recent systematic review [40], the fertility and hormonal function in adults with bilateral UDT presenting with infertility or testicular cancer, who have not undergone orchidopexy was evaluated. The results of 157 spermiograms of the patients before undergoing unilateral or bilateral orchidopexy, found azoospermia in 100% of them. In 51 patients, the results of a spermiogram performed on average 1 year after orchidopexy were reported, finding sperm in 11 cases. Testicular sperm extraction (TESE) after orchidopexy was reported in 22 patients who persisted with azoospermia in the ejaculate, obtaining sperm in 10 patients. These findings confirm that the probability of regaining fertility in untreated adults with bilateral UDT is very low, although not impossible [40].
Relationship between initial location UDT and fertility
Tasian et al. evaluated the risk of germ-cell loss in patients with palpable and nonpalpable UDT and concluded that the latter had a 50% increased risk of germ-cell depletion [37]. Muncey et al. found that both, the concentration of sperm in the ejaculate and the amount of germ cells post-orchidopexy, were higher in patients with inguinal UDT than in the abdominal ones [40].
Remarkably, when analyzing the group of patients with unilateral UDT, Lee did not find a significant difference in paternity rate according to initial location [36], confirming that histological alterations of a UDT by themselves are not sufficient to alter paternity potential of patients with one healthy testicle and that only bilaterality significantly compromises fertility.
Relationship between age at orchidopexy and fertility
Based on hormone as well as histology studies, a better fertility prognosis was observed in patients undergoing orchidopexy before 2 years [36, 37, 39]. Coughlin et al. showed that inhibin B levels are higher and follicle-stimulating hormone (FSH) levels lower in patients who underwent orchidopexy before 2 years of life [39]. In a testicles histological study of 274 patients with UDT undergoing orchidopexy, Tasian et al. found that age at surgery is a clinical predictor of future histological findings with significantly higher germ cells count in patients who underwent surgery before 18 months [37].
In previously mentioned systematic review of adult patients with untreated bilateral UDT, Muncey found that testosterone plasmatic levels were < 300 ng/dL in 9 patients (11%). In the univariate analysis, neither the location nor the age of the patient was statistically significant variables in plasma testosterone levels [40].
In summary, it seems to be clear that the age of orchidopexy is a determining factor in the prognosis of the histology of the affected testicle with less impact on that of hormonal function. This explains the lower paternity rate in patients with untreated or late-treated bilateral UDT.
UDT and testicular cancer
Testicular cancer is the most frequent solid malignancy in men between 14 and 44 years in western countries. Its incidence varies between 1 and 9 per 100,000 men, depending on the geographic region studied and it is estimated that it has been increasing in the last 2 decades. In Chile, a gross rate of 8 per 100,000 inhabitants was recorded between 2000 and 2014, reaching 12 per 100,000 men in “Los Ríos” region [19, 20].
It is well known that UDT patients are at significantly higher risk of developing cancer in the affected testis. Therefore, they should be taught how to self-examine the testicles. Studies that were published 40 or 50 years ago, when patients were rarely treated before puberty, described an eight- to tenfold higher risk of cancer then in the general population [21,22,23]. More recent studies have reported a relatively lower risk, as UDT are treated earlier. Dieckmann et al. estimated an overall relative risk of 4.8 in a 2004 meta-analysis including 21 studies [24], while Pettersson et al. published a relative risk of testicular cancer in men who underwent orchidopexy before puberty of 2.23 in a cohort of more than 16,000 patients treated for UDT [25].
On the other hand, in adult patients with testicular cancer, 5% were found to have a history of UDT [26].
UDT localization and risk of testicular cancer
Patients with intra-abdominal UDT were described to have a higher risk of developing cancer than those with inguinal UDT [27]. Noteworthy, in different studied conducted in patients with testicular cancer, UDT were considered to have been intra-abdominal, but the exact pre-operative location was not documented. Nevertheless, in other reports, UDT location was adequately reported. Ford et al. performed biopsies of UDT in adult patients and found a 25% rate of carcinoma in situ (CIS) in intra-abdominal versus 7% in inguinal UDT [28]. This finding in relevant considering that the risk of malignant transformation of CIS is as high as 50% [29].
Impact of pre- and post-pubertal orchiopexy on the risk of cancer
In 2007, two studies provided evidence that the risk of developing UDT-related cancer diminishes after orchidopexy. In a meta-analysis, Walsh et al. [31] showed that testicular cancer was six times more common in untreated patients or in those treated after puberty than those who underwent earlier surgery. In above-mentioned study evaluating more than 16,000 patients, Pettersson et al. found that the relative risk to develop cancer ranged between 2.02 and 2.35 in boys who underwent orchidopexy prior to puberty, while the relative risk was between 5.06 and 6.24 in those who were treated after puberty [25]. Therefore, we may say that orchidopexy before puberty significantly reduces the risk of cancer in UDT, although it remains higher than that in the general population.
Histology
Classical studies mainly including patients that were not treated before puberty, agreed that the most common histological type of UDT-related cancer is seminoma (70–80% of the cases) [24], while non-seminomatous types, including embryonal tumors and teratocarcinoma, account for 30% [21,22,23, 28]. Nevertheless, more recent studies evaluating patients that were treated earlier in life have minimized this difference, and in some, an inversed ratio was found [27], which suggest that testicular descent before puberty decreases the risk of cancer; however, it makes it more likely to find non-seminomatous histology.
In summary, the predominant histological type of testicular tumors in UDT patients is seminoma. Although the ratio of seminoma to non-seminoma is lower in patients who underwent pre-pubertal treatment than in those who did not, it is still doubtful if orchidopexy changes tumor histology or if the overall reduction in cancer risk also leads to a reduction of this ratio.
Histological type of the tumor has also been reported to vary according to the initial location of the UDT, with a higher rate of seminoma in intra-abdominal compared to inguinal testis [22]. This hypothesis, however, may not be correct as most studies reporting UDT-related cancer do not explicitly document the initial location of the testis and assume that testicles that underwent orchidopexy were initially located intra-abdominally.
Risk of cancer in the contralateral testicle
Although multiple publications state that patients with unilateral UDT have an elevated risk of developing tumor in the normally descended contralateral testicle, there is evidence that this is not true. Large series published as early as 1980s, such as the studies by Fonger et al. [21] in more than 600 patients and by Batata et al. [22], showed that less than 1% of tumors occur in the contralateral testis, which is a risk that is similar to that in the general population.
Cancer risk in patients with UDT associated with differences of sex development (DSD)
Children with UDT associated with DSD and presence of Y chromosome constitutes a different group of patients. Depending on the specific condition, they might be at risk of developing gonadoblastoma (noninvasive neoplasm precursor of malignant germ-cell tumors). For example, patients with DSD due to partial androgen insensitivity syndrome (PAÍS) or testis determination (e.g., Denys–Drash or Frasier syndrome) have a 30% and 60% increased risk of developing tumors, respectively. Therefore, the need for prophylactic gonadectomy has been considered in some of these cases [32].
Risk of cancer in testicular remnants
The finding of a nonpalpable testis may point to an intra-abdominal testis, but other diagnoses should be considered. Vanishing testes or nubbins, consisting of the spermatic cord with a small nodule of fibrous tissue at the end, are the cause of nonpalpable testis in around 50% of the cases.
The etiology of vanishing testes is thought to be related to a vascular accident early in fetal development affecting a normally developing testicle. Around 90% of these remnants are located in the inguinal canal o scrotum [11, 33] and the typical laparoscopic finding is a closed internal inguinal ring with atrophied spermatic vessels entering the canal. The probabilities of finding germ cells in these remnants is around 5% and this has been shown to occur only in case of the rare laparoscopic finding an open inguinal ring with normal spermatic vessels [33]. Given de low probability of the presence of germ cells, there is practically no risk of malignant transformation. In the literature, only one report describes the finding of intratubular germ neoplasia in a 9-year-old patient [34]. Nevertheless, remnant resection remains controversial as shown by a survey administered to pediatric urologists in 2013; in a patient with a clinical, ultrasonography or laparoscopic diagnosis of a inguinal vanishing testis, 56% would not perform any inguinal intervention and would only respect the remnant during inguinal exploration [35].
Other risks associated with UDT
Other risk that stem from the failure of fixation and location of the UDT should be taken into account and must be discussed with the parents of these patients.
Testicular torsion
In the absence of complete testicular descent, the previously described mechanisms of fixation to the scrotum do no occur, which is associated with an increased risk of testicular torsion [2].
When this occurs, the patient generally presents to the emergency department with a history of inguinal or inguinoscrotal pain associated with nausea and/or vomiting. On physical examination, the most common finding is swelling in the groin with inflammatory signs of variable intensity depending on the duration of the torsion. In this setting, the main differential diagnosis is a complicated inguinal hernia, while absence of the ipsilateral testis in its scrotal sack is the finding that points to testicular torsion.
Severe testicular trauma
As mentioned above, the inguinal location in the most common in UDT. In this position close to the pubic bone, the testicle is more prone to attrition in case of trauma to that area and may be more severely injuries than when it is located in the scrotum.
Discussion
UDT is a common reason for consultation with the pediatric urologist or surgeon. Given the risks related to this condition, we consider the review of some of concepts surrounding UDT that remain controversial of utmost importance of our daily clinical practice.
First, it seems relevant to emphasize the importance of the clinical examination in the patient evaluation, as it is fundamental for their classification into palpable or nonpalpable UDT. Based on this classification, the most appropriate approach will be decided on for each.
The main risks for these patients are long term consisting of the possibility of decreased fertility and the development of cancer. Nevertheless, other risks presenting in childhood, such as testicular torsion and severe testicular trauma, should also be kept in mind.
It should be emphasized that early orchidopexy significantly diminishes the likelihood of UDT-associated cancer. Fortunately, patients with UDT who reach adolescence without being treated are less than 1%; however, special care should be taken in this age group, as the high risk of tumor development in the future, justifies orchidectomy as the best therapeutic option. This issue will be more thoroughly discussed in the article “The undescended testis in children and adolescents part 2”.
Finally, regarding fertility, histological alterations should be carefully distinguished from the compromise of paternity rate these patients may have, which may truly worry the parents. Currently, evidence has shown that only in patients with bilateral UDT, the paternity rate is affected and that early orchidopexy significantly decreases this risk.
Conclusions
In the daily practice of the pediatric surgeon, UDT is a highly prevalent condition around which controversies have arisen with the advancement of knowledge. In this review, we analyze the pathophysiological aspects especially focusing on the risk of infertility and cancer in these patients with the aim to offer them the most up-to-date comprehensive treatment. In “part 2” of this article, treatment updates will be reviewed.
References
Elder JS (2016) Surgical management of the undescended testis: recent advances and controversies. Eur J Pediatr Surg 26(5):418–426
Sepúlveda X, Egaña PL (2016) Current management of non-palpable testes: a literature review and clinical results. Transl Pediatr 5(4):233–239
Mäkelä JA, Koskenniemi JJ, Virtanen HE et al (2019) Testis development. Endocr Rev 40(4):857–905
Berkowitz GS, Kapiński RH, Dolin SE et al (1993) Prevalence and natural history of cryptorchidism. Pediatrics 92:44–49
Vikraman J, Hutson JM, Li R, Thorup J (2016) The undescended testis: clinical management and scientific advances. Semin Pediatr Surg 25(4):241–248
Van der Plas EM, Zijip GW, Frieling FM et al (2013) Long-term testicular volume after orchidopexy at diagnosis of acquired undescended testis. J Urol 190(1):257–262
Clarnette TD, Rowe D, Hasthorpe S, Hutson JM (1997) Incomplete disappearance of the processes vaginalis as a cause of ascending testis. J Urol 157(5):1889–1891
Hakonsen LB, Ernst A, Ramlau-Hansen CH (2014) Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl 16(1):39–49
Brauner R, Neve M, Allali S, Trivin C et al (2011) Clinical, biological and genetic analysis of anorchia in 26 boys. PLoS ONE 6(8):e23292
Sarila G, Hutson J, Vikraman J (2021) Testicular descent: a review of a complex, multistaged process to identify potential hidden causes od UTD. J Ped Surg 8:30
Belman AB, Rushton HG (2001) Is the vanished testis always a scrotal event? BJU Int 87:480–483
Heksch R, Matheson M, Tishelman A, Swatz J et al (2019) Testicular regression syndrome: practice variation in diagnosis and management. Endocr Pract 25(8):779–786
Cortes D, Thorup J, Visfeldt J (2000) Hormonal treatment may harm the germ cells in 1 to 3-year-old boys with cryptorchidism. J Urol 163:1290–1292
López PJ, Angel L, Rodríguez J (2009) Abordaje Laparoscópico en el testículo no palpable. Rev Chil Pediatr 80(3):225–230
Barthold JS, Gonzalez R (2003) The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. J Urol 170:2396–2401
Radmayr C, Bogaert G, Dogan HS, Nijman JM, Silay MS, Stein R, Tekgül S. EAU guidelines on pediatric urology. European Association of Urology 2021
Fénichel P, Lahlou N, Coquillard P et al (2015) Cord blood insulin-like peptide 3 (INSL3) but not testosterone is reduced in idiopathic cryptorchidism. Clin Endocrinol (Oxf) 82(2):242–247
Hutson JM, Li R, Southwell BR, Petersen BL et al (2012) Germ cell development in the postnatal testis. The key to prevent malignancy in cryptorchidism? Font Endocrinol (Lausanne) 3:176
Cheng L, Albers P, Berney D et al (2018) Testicular cancer. Nat Rev Dis Primers 4(1):29
- Ebel L, Fonerón A, Troncoso L, Fonerón A. Sociedad Chilena de Urologia. Manual de Urologia. Segunda Edición. Capitulo 29.
Fonger JD, Filler RM, Rider WD, Thomas GM (1981) Testicular tumours in maldescended testes. Can J Surg 24(4):353–355
Batata MA, Whitmore WF Jr, Chu FC, Hilaris BS, Loh J et al (1980) Cryptorchidism and testicular cancer. J Urol 124:382
Gehring GG, Rodriguez FR, Woodhead DM (1974) Malignant degeneration of cryptorchid testes following orchiopexy. J Urol 112:354
Dieckman KP, Pichlmeier U (2004) Clinical epidemiology of testicular germ cell tumors. World J Urol 22:2
Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O (2007) Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med 356(18):1835–1841
Ryang SH, Jung JH, Eom M, Song JM, Chung HC, Chae Y, Lee CM, Kim KJ (2015) The incidence and histological characteristics of intratubular germ cell neoplasia in postpubertal cryptorchid testis. Korean J Urol 56(7):515–518. https://doi.org/10.4111/kju.2015.56.7.515
Wood HM, Elder JS (2009) Cryptorchidism and testicular cancer: separating fact from fiction. J Urol 181(2):452–461
Ford TF, Parkinson MC, Pryor J (1985) The undescended testis in adult life. B Urol 57:181
Dieckmann KP, Skakkebaek NE (1999) Carcinoma in situ of the testis: review of biological and clinical features. Int J Cancer 83:815–822
Rogers E, Tehan S, Gallagher H et al (1998) The role of orchiectomy in the management of postpubertal cryptorchidism. J Urol 159(3):851–854
Walsh TJ, Dall’Era MA, Croughan MS, Carroll PR, Turek PJ (2007) Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol 178(4 Pt 1):1440–1446
Pyle LC, Nathanson KL (2017) A practical guide for evaluating gonadal germ cell tumor predisposition in differences of sex development. Am Jour Med Genet 175C:304–314
Woodford E, Eliezer D, Deshpande A, Kumar R (2018) Is excision of testicular nubbin necessary in vanishing testis syndrome? J Pediatr Surg 53(12):2495–2497
Rozanski T, Wojno K, Bloom D (1996) The remnant orchiectomy. J Urol 155:712–714
Broderick KM, Martin BG, Herndon CD, Joseph DB, Kitchens DM (2013) The current state of surgical practice for neonatal torsion: a survey of pediatric urologists. J Pediatr Urol 9(5):542–545
Lee PA (2005) Fertility after cryptorchidism: epidemiology and other outcome studies. Urology 66(2):427–431
Tasian GE, Hittelman AB, Kim GE, DiSandro MJ, Baskin LS (2009) Age at orchiopexy and testis palpability predict germ and Leydig cell loss: clinical predictors of adverse histological features of cryptorchidism. J Urol 182(2):704–709
Hadziselimovic F, Hoecht B (2008) Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin Padiatr 220(5):302–307
Coughlin MT, Bellinger MF, Lee PA (1999) Age at unilateral orchiopexy: effect on hormone levels and sperm count in adulthood. J Urol 162(3 Pt 2):986–988
Muncey W, Dutta R, Terlecki R, Lynn W, Scaberry K (2021) Fertility potential in adult men treated for uncorrected bilateral cryptorchidism: a systematic literature review and analysis of case reports. Andrology 9(3):781–791
- Kolon T., Herndon A., Baker L., et al. AUA guidelines “evaluation and tratment of cryptorchidism”. 2018.
Zhang L, Wang X, Zheng X (2015) Research article: maternal gestational smoking, diabetes, alcohol drinking, pre-pregnancy obesity and the risk of cryptorchidism: a systematic review and meta-analysis of observational studies. PLoS ONE 10:e0119006
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Echeverría Sepúlveda, M.P., Yankovic Barceló, F. & Lopez Egaña, PJ. The undescended testis in children and adolescents. Part 1: pathophysiology, classification, and fertility- and cancer-related controversies. Pediatr Surg Int 38, 781–787 (2022). https://doi.org/10.1007/s00383-022-05110-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05110-5