Abstract
Purpose
Short bowel syndrome (SBS) patients require long-term parenteral nutrition following massive bowel resection, which causes intestinal failure-associated liver disease (IFALD). Previous reports have shown that glucagon-like peptide-2 (GLP-2) resulted in the bowel adaptation for SBS. The aim of this study was to evaluate the effect of GLP-2 for IFALD in a parenterally fed rat model.
Methods
Using rat, a catheter was placed in the jugular vein, and 90% small bowel resection (SBR) was performed. Animals were divided into three groups: SBR and total parenteral nutrition (TPN) (SBS/TPN group), SBR and TPN plus GLP-2 at 1 µg/kg/h [SBS/TPN/GLP-2 (low) group], and SBR and TPN plus GLP-2 at 10 µg/kg/h [SBS/TPN/GLP-2 (high) group]. On day 13, the liver was harvested and analyzed by using nonalcoholic fatty liver disease (NAFLD) score.
Results
Histologically, hepatic steatosis in the SBS/TPN group and SBS/TPN/GLP-2 (high) group was observed. Both steatosis and lobular inflammation score in the SBS/TPN/GLP-2 (low) group were significantly lower compared with those in the other two groups (p < 0.05). Active NAFLD score in the SBS/TPN/GLP-2 (low) group was significantly lower compared with that in the SBS/TPN/GLP-2 (high) group (p < 0.01).
Conclusion
Low-dose GLP-2 intravenous administration improves hepatic steatosis of IFALD following in an SBS parenterally fed rat model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short bowel syndrome (SBS) is defined as the spectrum caused by the insufficient absorption of nutrients due to inadequate small intestinal length after massive bowel resection of the small intestine [1]. In children, the main causes of SBS are midgut volvulus with malrotation, necrotizing enterocolitis, and extensive aganglionosis in Hirschsprung’s disease. These patients require long-term parenteral nutrition (PN), which causes intestinal failure-associated liver disease (IFALD). IFALD is a life-threatening complication in children with intestinal failure. The clinical spectrum of IFALD includes hepatic steatosis, cholestasis, cholelithiasis, and hepatic fibrosis [2]. The clinical findings of IFALD tend to differ between adults and neonates and infants. Cholestasis, which is also known as parenteral nutrition-associated cholestasis, occurs in 40–60% of neonates and infants; hepatic steatosis, which is also known as non-alcoholic fatty liver disease (NAFLD) occurs in 40–55% of adults [2].
In our previous studies on IFALD using a parenterally fed rat model of SBS, hepatic steatosis was observed histologically. And Kakino et al. presented that tumor necrosis factor-α (TNF-α) was the pivotal role to develop and progress hepatic steatosis of NAFLD [3]. Our colleagues showed that the exogenous administration of ghrelin and fish oil (omega-3), which have the anti-inflammatory effect, was effective for treating the hepatic steatosis of IFALD [4, 5].
Glucagon-like peptide-2 (GLP-2) is a 33-amino acid peptide of the pituitary adenylate cyclase-activating glucagon superfamily, which is secreted from ileal and colonic enteroendocrine L cells [6]. Previous studies have demonstrated that the administration of GLP-2 results in intestinal hypertrophy by increasing the crypt cell proliferation rate, which then results in an increased villus height, crypt depth, bowel length and an overall mucosal surface area through the GLP-2 receptor. The GLP-2 receptor is mainly expressed by the enteroendocrine cells of the gastrointestinal tract [7]. Hence this agent mimics the features of spontaneous bowel adaptation following massive bowel resection. Additionally, Lim et al. showed that the exogenous administration of GLP-2 attenuated cholestasis using a parenterally fed neonatal piglet model without bowel resection [8].
However, no studies have described the effect of GLP-2 on hepatic steatosis in IFALD in a parenterally fed rat model of SBS. The aim of this study was to evaluate the effect of GLP-2 on IFALD in a parenterally fed rat model of SBS.
Methods
Animal preparation
Seven-week-old male Sprague–Dawley (SD) rats (body weight, 200–240 g; purchased from Kyudo Co., Ltd., Saga, Japan) were maintained and used in this study. The animals were individually housed in metabolic cages with ad libitum access to the standard rat chow and water and were acclimatized to their environment for 6 days before the experiments. The animals were maintained under standardized temperature (23 ± 1 °C) and humidity (50% ± 10%) and a 12-h light–dark cycle (lights on at 7:00 a.m.). All the experimental procedures were approved by the Laboratory Animal Committees of Kagoshima University Graduate School and were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals (approval number: MD18032).
Study design
The animals were fasted overnight, and after jugular vein catheterization and 90% small bowel reduction (SBR), were randomly divided into following three treatment groups (n = 9 per group): (1) the 90% SBR and total parenteral nutrition (TPN) alone group (SBS/TPN group) (2) the 90% SBR and TPN plus low-dose GLP-2 (1 µg/kg/h) group [SBS/TPN/GLP-2 (low) group], and (3) the 90% SBR and TPN plus high-dose GLP-2 (10 µg/kg/h) group [SBS/TPN/GLP-2 (high) group]. The previous study reported that GLP-2 at 10 µg/kg/h induced intestinal adaptation and that GLP-2 at 1 µg/kg/h did not, following massive bowel resection [7], so we determined that the administration of 1 µg/kg/h is “low dose” and 10 µg/kg/h is “high dose”. GLP-2 (1–33, Bachem Ag, Bubendorf, Switzerland) was dissolved in distilled water and administered intravenously. On Day 13, the animals were anesthetized, weighed, and blood was obtained from the heart, then sacrificed and harvesting the liver tissue for the analysis for the assessment of hepatocellular injury. In our previous study, histological findings of liver disease were shown in an SBS rat model infused for 13 days, so we determined that the experimental period for this study should be 13 days after surgery.
The surgical procedure and maintenance methods
For surgery, the animals were anesthetized with isoflurane (1.5% inhalation by mask), and an intravenous catheter was inserted into the right jugular vein. A silastic catheter with an outside diameter of 1.2 mm (NIPRO Co., Ltd., Osaka, Japan) was used, tunneled out of the back, and attached to a standard swivel device (LOMIR BIOMEDICAL INC., Quebec, Canada). For the SBR-operated rats, the intestinal length was measured in the standard fashion, and 90% SBR operation was performed, leaving 5 cm of the ileum above the ileocecal valve anastomosed to the jejunum, 5 cm below the ligament of Treitz. Bowel anastomosis was completed with the aid of an operating microscope, using interrupted 6-0 silk sutures (Alfresa Pharma Corporation, Osaka, Japan); the abdominal incision was closed with 3-0 polyglycolic sutures (Ethicon Inc., Cincinnati, OH, USA). All animals received cefazolin (50 mg/kg, subcutaneously; Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to prevent postoperative infection and buprenorphine (0.01 mg/kg, subcutaneously; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) for analgesia and were allowed free access to water immediately after surgery.
TPN was delivered via a multichannel syringe pump (KDS Legato 200 Series Syringe Pump Series; KD Scientific, Inc., Holliston, MA, USA). After catheterization, the SBR animals were maintained with low-concentration TPN solution at 60 mL/day; NEOPAREN® No. 2 (Otsuka Pharmaceutical Co., Ltd.) to which 20% Intralipos® (Otsuka Pharmaceutical Co., Ltd.) had been added. The composition of the TPN solution was as follows (in g/L): amino acids 25, glucose 145, and soy bean oil 33.3. The solution also contained the following electrolytes (final mM/L): 41.6 Na+, 22.5 K+, 41.6 Cl−, 4.1 Ca2+, and 4.1 Mg2+. After 24 h, the composition of the TPN solution was switched to (in g/L) amino acids 31.6, glucose 203, and soy bean oil 33.3, with similar electrolyte additives. The TPN solution was delivered at a rate of 60 mL/day, providing equivalent isocaloric/isonitrogenous nutritional support to all TPN-fed animals at 76.4 kcal/rat/day (1.9 g protein, 2.0 g fat, and 12.2 g carbohydrates).
On day 13, after an overnight fast, all the rats were anesthetized by isoflurane inhalation. Blood was obtained from the heart and immediately centrifuged at 1500×g for 15 min at 4 °C. All the serum samples were stored until use at − 80 °C. After the blood collection, the animals were euthanized by exsanguination. Liver samples were fixed with 10% formaldehyde for a histological analysis. Paraffin sections of formalin-fixed tissue were cut at a thickness of 3 µm for staining with hematoxylin and eosin (H&E).
The biochemical test of the liver function
To evaluate the extent of liver injury, the levels of serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), lactase dehydrogenase (LDH), total bilirubin (T-Bil), triglyceride (TG), and total cholesterol (T-CHO) were measured using the Japan Society of Clinical Chemistry standardized matching method. All measurements were performed by Clinical Pathology Laboratory, Inc., Kagoshima, Japan.
Histological analysis
For the histological analysis of the liver specimens, we evaluated the degree of lipid accumulation (steatosis score), the number of positive macrophages or T lymphocytes in ten randomly selected fields (inflammation score), and the degree of liver cell ballooning injury (ballooning score). Finally, we evaluated the extent of liver dysfunction using the NAFLD score, which was calculated from the steatosis score, the inflammation score, and the ballooning score. The three scores were determined based on the histological findings [9], steatosis score [no lipid droplets (score = 0); lipid droplets in < 33% of the hepatocytes (score = 1); lipid droplets in 33–66% of the hepatocytes (score = 2); and lipid droplets in > 66% of the hepatocytes (score = 3)], inflammation score [no inflammation (score = 0); < 10 inflammatory foci, each consisting of > 5 inflammatory cells (score = 1); 10 inflammatory foci (score = 2); or uncountable diffuse or fused inflammatory foci (score = 3)]; and ballooning score [none (score = 0); few balloon cells (score = 1) or many balloon cells/prominent ballooning (score = 2)]. Two independent observers who were blinded to the physical outcome and other biological and pathological data of each sample evaluated all of the histological slides. In the case of disagreement, a third board-certified pathologist determined a consensus score.
Real-time quantitative polymerase chain reaction (qPCR) of TNF-α in the liver tissue
We evaluated the TNF-α in the liver tissue using real-time qPCR. The first-standard cDNA was synthesized using SuperScript IV® Reverse Transcriptase (Thermo Fisher Scientifics, Waltham, MA, USA) with oligo (dT) primer. Each cDNA sample was then diluted with RNase/DNase-free water to 1.25 ng template RNA/μL. The expression of each gene was analyzed by qPCR using the Bio-Rad CFX96 system (BioRad Laboratories, Inc., Hercules, CA, USA). Standard DNA was generated by blocks double stranded DNA fragments synthesis (Integrated DNA Technologies, Inc., Skokie, IL, USA). These measurements were performed by Repertoire Genesis Inc., Osaka, Japan.
Statistical analysis
The data are presented as mean ± standard deviation (SD). The statistical analyses of the data were performed using a two-factor factorial analysis of variance (ANOVA) followed by Tukey–Kramer’s multiple-comparison post hoc test. p values of < 0.05 were considered to indicate statistical significance.
Results
Serum tests of the liver function
There were no significant differences in the serum levels of AST, LDH, ALT, LDH, T-Bil, TG and T-CHO (Table 1).
The representative histological findings of liver specimens
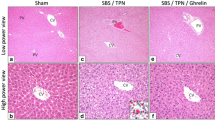
Representative histological findings of the liver tissue specimens (H&E staining) are shown in Fig. 1. The upper and lower panels show a low-power view (100×) and high-power view (200×), respectively. The examination of H&E-stained sections revealed that the liver specimens of the rats in the SBS/TPN group (Fig. 1a, b) and the SBS/TPN/GLP-2 (high) group (Fig. 1e, f) had an increased number of lipid droplets in comparison to the SBS/TPN/GLP-2 (low) group (Fig. 1c, d).
Representative histological findings of the liver tissue specimens (H&E staining). The upper panels show a low-power view (×100). The lower panels show a high-power view (×200). The examination of the H&E stained sections revealed that the liver specimens of the rats in the SBS/TPN (a, b) and SBS/TPN/GLP-2 (high) (e, f) groups had an increased number of lipid droplets in comparison to the SBS/TPN/GLP-2 (low) group (c, d)
The SBS/TPN/GLP-2 (low) group showed mild steatosis (Fig. 1d), while the SBS/TPN and SBS/TPN/GLP-2 (high) groups showed moderate-to-severe steatosis (Fig. 1b, f).
The histological analysis using the NAFLD score
Based on the histological findings, NAFLD score was calculated; the results are shown in Fig. 2. The steatosis score in the SBS/TPN/GLP-2 (low) group was lower than that in the other two groups [steatosis score: SBS/TPN/GLP-2 (low) group 0.22 ± 0.44, vs. SBS/TPN group 1.44 ± 0.88 (p < 0.05), vs. SBS/TPN/GLP-2 (high) group 1.56 ± 1.01 (p < 0.01)] (Fig. 2a). The lobular inflammation score in the SBS/TPN/GLP-2 (low) group was also lower in comparison to the other groups [lobular inflammation score: SBS/TPN/GLP-2 (low) group 0.56 ± 0.53, vs. SBS/TPN group 1.22 ± 0.44 (p < 0.05), vs. SBS/TPN/GLP-2 (high) 1.22 ± 0.67 (p < 0.01)] (Fig. 2b). The SBS/TPN/GLP-2 (high) group showed a higher hepatocyte ballooning score in comparison to the other two groups [hepatocyte ballooning score; SBS/TPN/GLP-2 (high) 1.44 ± 0.73, vs. SBS/TPN 0.33 ± 0.50 (p < 0.01), vs. SBS/TPN/GLP-2 (low) 0.67 ± 0.71 (p < 0.05)] (Fig. 2c). Finally, the NAFLD score of the SBS/TPN/GLP-2 (high) group was significantly higher in comparison to the SBS/TPN/GLP-2 (low) group [NAFLD activity score; SBS/TPN/GLP-2 (low) 1.50 ± 1.41 vs. SBS/TPN/GLP-2 (high) 4.22 ± 1.79 (p < 0.01)] (Fig. 2d).
The expression of TNF-α in the liver tissue
No significant differences were observed in TNF-α expression in the liver tissue among three groups (Fig. 3).
Discussion
Short bowel syndrome patients who require long-term PN for the provision of fluids and nutrients may be at a high risk of developing IFALD, a life-threatening complication [1]. The clinical spectrum of IFALD includes cholestasis and steatosis. GLP-2 is a therapeutic agent that has an intestinotrophic effect on the bowel adaptation in SBS patients. Lim et al. reported that the exogeneous administration of GLP-2 improved cholestasis using parenterally fed neonatal piglet model [8]. However, in our previous study using a parenterally fed SBS rat model, hepatic steatosis of IFALD which was known as NAFLD, was observed [4, 5]. The present study was conducted to evaluate the effect on GLP-2 in the hepatic steatosis of IFALD induced by long-term TPN following massive bowel resection.
The major findings of the present study were as follows; (1) The intravenous administration of low-dose GLP-2 (1 µg/kg/h) suppressed hepatic steatosis. On the other hand (2) the administration of high-dose GLP-2 (10 µg/kg/h) aggravated hepatic steatosis. (3) There were no significant differences in the expression of the TNF-α in the liver among three groups.
In present study, in terms of histological findings and NAFLD score, low-dose GLP-2 administration at 1 μg/kg/h suppressed hepatic steatosis, and high-dose GLP-2 administration at 10 μg/kg/h aggravated hepatic steatosis. And according to our previous study, in which the mucosal atrophy was induced by parenterally fed SBS rat model, high-dose GLP-2 administration at 10 μg/kg/h improved the mucosal atrophy, but low-dose GLP-2 administration at 1 μg/kg/h did not improve the mucosal atrophy [7]. Lim et al. showed that the administration of GLP-2 attenuated the hepatic cholestasis and also improved the mucosal atrophy observed in a parenterally fed neonatal piglet model without bowel resection [8]. Their dose of GLP-2 was effective for treating both cholestasis in IFALD and intestinal mucosal atrophy. This discrepancy may be associated with difference between animals (e.g., pigs or rats), the experimental model (e.g., with or without massive bowel resection), and animal age (e.g., neonates or adult). The dosage of GLP-2 in the high-dose group (10 μg/kg/h) might have been too much for the liver of our SBS rat model. Further studies are needed to clarify the appropriate GLP-2 dose for intestinal adaptation and the attenuation of the hepatic steatosis in IFALD following massive bowel resection using our rat model.
In this study, the only low-dose administration of GLP-2 attenuated hepatic steatosis, which was the novel effect of GLP-2. Previous study presented that TNF-α was the pivotal role to develop and progress hepatic steatosis of NAFLD [3]. Since several studies which showed that GLP-2 induced the suppression of TNF-α production in the intestinal tissue such as an anti-inflammatory effect through enteric neuron where GLP-2 receptors existed [10, 11], we expected that GLP-2 administration would attenuate the hepatic steatosis with suppression of TNF-α. However, there were no significances in the expression of TNF-α in the liver among three groups. Generally, GLP-2 receptors are expressed in the enteroendocrine cells of the stomach, small intestine and colon, and are slightly expressed on the hepatocytes or nonparenchymal cells in the liver [12]. Hence, the attenuation of hepatic steatosis induced by PN following massive bowel resection with administration of low-dose GLP-2, may be associated with the indirect effect of GLP-2 receptors. Shi et al. showed that GLP-2 had glucoregulatory effect without through GLP-2 receptor [13]. Further studies are required to clarify the mechanisms underlying the attenuation of steatosis observed by the only low-dose GLP-2 in our parenterally fed rat model of SBS.
Surprisingly, rats treated with high-dose GLP-2 [the SBS/TPN/GLP-2 (high) group] showed a deterioration of hepatic steatosis in comparison to those that did not receive GLP-2 therapy (the SBS/TPN group). Taher et al. reported that the administration of GLP-2 at a dose of 250 μg/kg/day, which was almost the same as the dose administered to the high-dose group, in present study, induced hepatic steatosis in a mouse model that received a high-fat diet, and that the mechanism was accompanied by de novo lipogenesis in the liver [14]. They demonstrated that GLP-2 played a lipogenic role in the liver via the increase in the lipogenic gene expression and the induction of hepatic steatosis.
A GLP-2 analogue, Teduglutide was used in clinical state [15]. But to our knowledge, hepatic steatosis induced by high-dose GLP-2 was not reported clinically. However, according to our results, the administration of GLP-2 would cause hepatic steatosis. Furthermore, and most important points, no significant differences were observed in the serum transaminase and bilirubin levels or lipid profiles of the three groups, even though the high-dose GLP-2 group developed severe hepatic steatosis histologically. Attention should be paid to the potential development of hepatic steatosis by the high-dose administration of GLP-2.
Conclusion
Our study revealed that the administration of low-dose GLP-2 attenuated hepatic steatosis, which was the novel effect, in a parenterally fed rat model of SBS and that high-dose GLP-2 induced severe steatosis. Further studies are needed to clarify the appropriate dose of GLP-2 and the mechanism underlying the suppression of hepatic steatosis in IFALD.
References
Coletta R, Khalil BA, Morabito A (2014) Short bowel syndrome in children: surgical and medical perspectives. Semin Pediatr Surg 23:291–297. https://doi.org/10.1053/j.sempedsurg.2014.09.010
Kelly DA (2006) Intestinal failure-associated liver disease: what do we know today? Gastroenterology 130(2 Suppl 1):S70–S77. https://doi.org/10.1053/j.gastro.2005.10.066
Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, Soejima E, Tajiri Y, Yamada K (2018) Pivotal role of TNF-alpha in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res 50:80–87. https://doi.org/10.1055/s-0043-118666
Onishi S, Kaji T, Yamada W, Nakame K, Moriguchi T, Sugita K, Yamada K, Kawano T, Mukai M, Souda M, Yamada S, Yoshioka T, Tanimoto A, Ieiri S (2016) The administration of ghrelin improved hepatocellular injury following parenteral feeding in a rat model of short bowel syndrome. Pediatr Surg Int 32(12):1165–1171. https://doi.org/10.1007/s00383-016-3975-1
Machigashira S, Kaji T, Onishi S, Yamada W, Yano K, Yamada K, Masuya R, Kawano T, Nakame K, Mukai M, Ieiri S (2018) The protective effect of fish oil lipid emulsions on intestinal failure-associated liver disease in a rat model of short-bowel syndrome. Pediatr Surg Int 34(2):203–209. https://doi.org/10.1007/s00383-017-4190-4
Sigalet DL (2001) ALX-0600 (NPS Allelix Corp). Curr Opin Investig Drugs 2(4):505–509
Kaji T, Tanaka H, Holst J, Redstone H, Wallace L, de Heuval E, Sigalet DL (2008) The effects of variations in dose and method of administration on glucagon like peptide-2 activity in the rat. Eur J Pharmacol 596(1–3):138–145. https://doi.org/10.1016/j.ejphar.2008.07.070
Lim DW, Wales PW, Josephson JK, Nation PN, Wizzard PR, Sergi CM, Field CJ, Sigalet DL, Turner JM (2016) Glucagon-like peptide 2 improves cholestasis in parenteral nutrition-associated liver disease. J Parenter Enteral Nutr 40(1):14–21. https://doi.org/10.1177/0148607114551968
Nabeshima A, Yamada S, Guo X, Tanimoto A, Wang KY, Shimajiri S, Kimura S, Tasaki T, Noguchi H, Kitada S, Watanabe T, Fujii J, Kohno K, Sasaguri Y (2013) Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid Redox Signal 19(17):1983–1998. https://doi.org/10.1089/ars.2012.4946
Nakame K, Kaji T, Mukai M, Shinyama S, Matsufuji H (2016) The protective and anti-inflammatory effects of glucagon-like peptide-2 in an experimental rat model of necrotizing enterocolitis. Peptides 75:1–7. https://doi.org/10.1016/j.peptides.2015.07.025
Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA (2007) Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293(1):G211–G221. https://doi.org/10.1152/ajpgi.00530.2006
El-Jamal N, Erdual E, Neunlist M, Koriche D, Dubuquoy C, Maggiotto F, Chevalier J, Berrebi D, Dubuquoy L, Boulanger E, Cortot A, Desreumaux P (2014) Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol 307(3):G274–G285. https://doi.org/10.1152/ajpgi.00389.2012
Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Li D, Burrin DG, Chan L, Guan X (2013) Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab 18(1):86–98. https://doi.org/10.1016/j.cmet.2013.06.014
Taher J, Baker C, Alvares D, Ijaz L, Hussain M, Adeli K (2018) GLP-2 dysregulates hepatic lipoprotein metabolism, inducing fatty liver and VLDL overproduction in male hamsters and mice. Endocrinology 159(9):3340–3350. https://doi.org/10.1210/en.2018-00416
Schoeler M, Klag T, Wendler J, Bernhard S, Adolph M, Kirschniak A, Goetz M, Malek N, Wehkamp J (2018) GLP-2 analog teduglutide significantly reduces need for parenteral nutrition and stool frequency in a real-life setting. Therap Adv Gastroenterol 11:1–11. https://doi.org/10.1177/1756284818793343
Acknowledgements
We thank Mr. Brain Quinn for his comments and help with the manuscript. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS: 19K10485, 19K09150, 19K09078, 19K03084, 19K18061, 19K17304, 19K18032, 18K08578, 18K16262 17K10555, 17K11514, 17K10183, 17K11515, 16K10466, 16K10094, 16K10095, 16K10434, 16H07090) and Grant for the experimental research in the Japanese Society for Parenteral and Enteral Nutrition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yano, K., Kaji, T., Onishi, S. et al. Novel effect of glucagon-like peptide-2 for hepatocellular injury in a parenterally fed rat model of short bowel syndrome. Pediatr Surg Int 35, 1345–1351 (2019). https://doi.org/10.1007/s00383-019-04560-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-019-04560-8