Abstract
Purpose
Long-term parenteral nutrition following massive bowel resection causes liver dysfunction, such as intestinal failure-associated liver disease (IFALD). IFALD includes two different states, cholestasis and steatosis, which represents a life-threatening complication. The previous reports have shown the protective role of ghrelin in the liver. The aim of this study was to evaluate the effects of the administration of ghrelin in the liver in a parenterally fed rat model of short bowel syndrome (SBS).
Methods
Rats underwent jugular vein catheterization, and were divided into three groups: 90 % small bowel resection (90 % SBR) and TPN (SBS/TPN group), 90 % SBR and TPN plus ghrelin (SBS/TPN/ghrelin group), and sham operation with normal chow (sham group). Ghrelin was administered continuously at a dose of 10 μg/kg/day. On day 13, all rats were euthanized. The serum chemistry was analyzed, the lipid content of the liver was measured, and the liver tissue was histologically analyzed.
Result
The AST and LDH levels significantly increased, and the accumulation of lipids in the liver was observed in the TPN/SBS group. The accumulation of lipids in the liver of the rats in the SBS/TPN group was attenuated by the administration of ghrelin.
Conclusion
The administration of ghrelin has a therapeutic potential for IFALD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short bowel syndrome (SBS) is defined as the spectrum of malabsorption that occurs after massive bowel resection of the small intestine for congenital or acquired lesions [1]. The three most common causes of SBS in children are necrotizing enterocolitis, intestinal atresia, and midgut volvulus [2]. In this particularly challenging group of patients, the long-term parenteral nutrition (PN) causes significant problems, including liver dysfunction, such as intestinal failure-associated liver disease (IFALD). IFALD is a life-threatening complication in children with intestinal failure. The clinical spectrum of IFALD includes hepatic steatosis, cholestasis, cholelithiasis, and hepatic fibrosis [3]. IFALD differs in adults and children. Cholestasis, which is also known as parenteral nutrition associated cholestasis (PNAC), occurs in 40–60 % of neonates and infants; hepatic steatosis, which is also known as non-alcoholic fatty liver dysfunction (NAFLD) occurs in 40–55 % of adults; biliary sludge and cholelithiasis occur in both adults and children [4]. Omega three lipid emulsions are thought to improve the cholestasis associated with IFALD [5], but treatments for IFALD are often based on repeated trial-and-error.
Ghrelin is a 28-amino-acid peptide with an n-octanoylation modification on serine 3 that is mainly produced by the X/A-like endocrine cells of the gastrointestinal tract [6]. Ghrelin has a vast range of physiological functions, including orexigenic, metabolic, and hormonal functions [7]. Ghrelin is presumed to be a ligand of the growth hormone (GH) secretagogue receptors and is reported to stimulate the release of GH and insulin-like growth factor 1 (IGF-1) [8]. Recent studies have found that exogenous ghrelin has a range of effects, including a trophic effect on the intestinal mucosa [9], the modulation of the release of anti-inflammatory cytokines [10], the suppression of insulin secretion in normal individuals [11], and the attenuation of hepatocellular injury and liver fibrosis [12].
We hypothesized that ghrelin might have therapeutic potential in liver injury, such as IFALD, which is caused in SBS patients by parenteral feeding. The aim of the present study was to investigate the effects of the administration of ghrelin on a parenterally fed rat model of SBS.
Methods
Animal preparation
Seven-week-old male Sprague–Dawley (SD) rats (body weight, 200–240 g; purchased from Kyudo Co., Ltd., Saga, Japan) were used in this experiment. The animals were individually housed in metabolic cages with ad libitum access to the standard rat chow and water and were acclimatized to their environment for 6 days before the experiments. The animals were maintained under standardized temperature (23 ± 1 °C) and humidity (50 % ± 10 %) and a 12-h light–dark cycle (lights on at 7:00 a.m.). All the experimental procedures were approved by the Laboratory Animal Committees of Kagoshima University Graduate School and were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals (Approval number: MD15083).
Study design
The animals were fasted overnight. After the placement of a central venous catheter, they were randomly assigned to one of the following three treatment groups (n = 9 per group): (1) the sham operation and oral feeding with normal chow plus vehicle group (sham group); (2) the 90 % small bowel resection (SBR) and total parenteral nutrition (TPN) alone group (SBS/TPN group); and (3) the 90 % SBR and TPN plus ghrelin group (SBS/TPN/Ghrelin group). Ghrelin (Peptide Institute Inc., Osaka, Japan) was dissolved in distilled water and administered intravenously. On day 13, the animals were anesthetized, weighed, and then sacrificed for the assessment of the liver morphology and tissue harvesting for the histological analysis. The morphological changes in the liver were shown to appear within 6 or 7 days in a rat model of total parenteral nutrition [13, 14]. Given that a considerable number of SBS patients require PN for approximately 1 year, we determined that the experimental period for this study should be 13 days after surgery.
The surgical procedure and maintenance methods
For surgery, the animals were anesthetized with isoflurane (1.5 % inhalation by mask), and an intravenous catheter was inserted into the right jugular vein. A silastic catheter with an outside diameter of 1.2 mm (NIPRO Co., Ltd., Osaka, Japan) was used, tunneled out of the back, and attached to a standard swivel device (LOMIR BIOMEDICAL INC., Quebec, Canada). For the SBR-operated rats, the intestinal length was measured in the standard fashion, and a 90 % SBR operation was performed, leaving 5 cm of the ileum above the ileocecal valve anastomosed to the jejunum, 5 cm below the ligament of Treitz. Bowel anastomosis was completed with the aid of an operating microscope, using interrupted 6–0 silk sutures (Alfresa Pharma Corporation, Osaka, Japan); the abdominal incision was closed with 3–0 polyglycolic sutures (Ethicon Inc., Cincinnati, OH, USA.). In the sham group, the ileum was transected at 5 cm above the ileocecal valve and re-anastomosed. All animals received cefazolin (50 mg/kg, subcutaneously; Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to prevent postoperative infection and buprenorphine (0.01 mg/kg, subcutaneously; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) for analgesia and were allowed free access to water immediately after surgery.
TPN was delivered via a multichannel syringe pump (KDS Legato 200 Series Syringe Pump Series; KD Scientific, Inc., Holliston, MA, USA). After catheterization, the animals in the sham group were maintained with saline (Otsuka Normal Saline; Otsuka Pharmaceutical Co., Ltd.) at 60 ml/day, while the SBR animals were maintained with low-concentration TPN solution at 60 ml/day; NEOPAREN® No. 2 (Otsuka Pharmaceutical Co., Ltd.) to which 20 % Intralipos® (Otsuka Pharmaceutical Co., Ltd.) had been added. The composition of the TPN solution was as follows (in g/L): amino acids 25, dextrose 145, and soy bean oil 33.3. The solution also contained the following electrolytes (final mM/L): 41.6 Na+, 22.5 K+, 41.6 Cl−, 4.1 Ca2+, and 4.1 Mg2+. After 24 h, the composition of the TPN solution was switched to (in g/L) amino acids 31.6, glucose 203, and soy bean oil 33.3, with similar electrolyte additives. The TPN solution was delivered at a rate of 60 ml/day, providing equivalent isocaloric/isonitrogenous nutritional support to all TPN-fed animals at 76.4 kcal/rat/day (1.9 g protein, 2.0 g fat, and 12.2 g carbohydrates). The animals in the sham group were fed 23 g of normal rat chow per day (79 kcal/rat/day).
On day 13, after an overnight fast, all the rats were anesthetized by isoflurane inhalation. Blood was obtained from the heart and immediately centrifuged at 1500×g for 15 min at 4 °C. All the serum samples were stored until use at −80 °C. After the blood collection, the animals were euthanized by exsanguination. Liver samples were taken and stored at −80 °C for the measurement of the triglyceride (TG), free fatty acid (FFA), and cholesteryl ester (CE) content. Additional liver samples were fixed with 10 % formaldehyde for a histological analysis. Paraffin sections of formalin-fixed tissue were cut at a thickness of 3 μm for staining with hematoxylin and eosin (H&E), oil red O, and Masson’s trichrome.
The biochemical test of the liver function
To evaluate the extent of liver injury, the levels of serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), lactase dehydrogenase (LDH), total bilirubin (T-Bil), total cholesterol (T-CHO), and TG were measured using the Japan Society of Clinical Chemistry standardized matching method. All the measurements were performed by Clinical Pathology Laboratory, Inc., Kagoshima, Japan.
Histological analysis
For the histological analysis of the liver specimens, we evaluated the degree of lipid accumulation (steatosis score), the number of positive macrophages or T lymphocytes in ten randomly selected fields (inflammation score), and the degree of liver cell ballooning injury (ballooning score). Finally, we evaluated the extent of liver dysfunction using the NAFLD score, which was calculated from the steatosis score, the inflammation score, and the ballooning score. The three scores were determined based on the histological findings [15]: steatosis score [no lipid droplets (score = 0); lipid droplets in <33 % of the hepatocytes (score = 1); lipid droplets in 33–66 % of the hepatocytes (score = 2); and lipid droplets in >66 % of the hepatocytes (score = 3)], inflammation score [no inflammation (score = 0); <10 inflammatory foci, each consisting of >5 inflammatory cells (score = 1); ≥10 inflammatory foci (score = 2); or uncountable diffuse or fused inflammatory foci (score = 3)]; and ballooning score [none (score = 0); few balloon cells (score = 1) or many balloon cells/prominent ballooning (score = 2)]. Liver fibrosis was quantified by Masson’s trichrome staining.
Two independent observers who were blinded to the physical outcome and other biological and pathological data for each sample evaluated all the histological slides. In the case of disagreement, a third board-certified pathologist determined a consensus score.
The analysis of the lipid content of the liver specimens
To examine the hepatic lipid profiles, each snap frozen tissue specimen (30 mg) was homogenized and extracted with chloroform–methanol (2/1 v/v), as described previously [15]. The organic phase was dried and resolubilized in 2-propanol. Then, the TG, FFA, and CE contents were determined using the commercial assay kits (Wako Pure Chemical Co., Osaka, Japan).
Statistical analysis
The data are presented as the mean values ± standard deviation (SD). The statistical analyses of the data were performed using a two-factor factorial analysis of variance (ANOVA) followed by Tukey’s multiple-comparison post-hoc test. p values of <0.05 were considered to indicate statistical significance.
Results
The serum tests of the liver function
The serum levels of AST and LDH were significantly increased in the SBS/TPN group in comparison with the sham group, which means that hepatocyte injury was caused in the TPN-fed SBS rat model (Sham vs. SBS/TPN, AST: p < 0.05, LDH: p < 0.05, Table 1). The administration of ghrelin maintained the AST and LDH values to levels that were similar to the sham group (Sham vs. SBS/TPN/Ghrelin, AST: p = 0.98, LDH: p = 0.91, Table 1). The serum TG and T-CHO levels in the SBS/TPN group were significantly increased in comparison with the sham group. The administration of ghrelin also increased the serum T-CHO and TG levels in comparison with the sham group. The T-Bil levels in the three groups did not differ to a statistically significant extent. Hepatic cholestasis was not observed in the SBS/TPN group.
The histological analysis of the liver
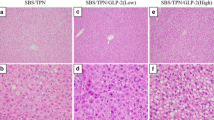
H&E staining of the rat liver sections revealed lipid accumulation and inflammatory cells in the liver of the rats in the SBS/TPN group (Fig. 1c, d). In addition, oil-red-O staining of the frozen sections confirmed the accumulation of hepatic lipids in the SBS/TPN group (Fig. 1d). With regard to the NAFLD score, the steatosis and inflammatory scores but not the ballooning score in the SBS/TPN group were significantly increased in comparison with the sham group [Sham vs. SBS/TPN: steatosis score, 0.00 ± 0.00 vs. 1.44 ± 0.88 (p < 0.01); inflammation score: 0.56 ± 0.5 vs. 1.22 ± 0.44 (p < 0.05), Table 2]. Consequently, the NAFLD score in the SBS/TPN group was significantly increased in comparison with the sham group [Sham vs. SBS/TPN: 0.56 ± 0.53 vs. 3.00 ± 1.12, (p < 0.01), Table 2]. Histologically, the administration of ghrelin significantly attenuated liver injury (Fig. 1e, f). The fat droplets (steatosis score) and inflammatory cells (inflammation score), which are both the components of the NAFLD score, in the SBS/TPN/Ghrelin group were significantly decreased in comparison with the SBS/TPN group [SBS/TPN vs. SBS/TPN/Ghrelin: steatosis score, 1.44 ± 0.88 vs. 0.56 ± 0.53 (p < 0.05); inflammation score, 1.22 ± 0.44 vs. 0.44 ± 0.53 (p < 0.01), NAFLD score: 3.00 ± 1.12 vs. 1.00 ± 0.87 (p < 0.01), Table 2]. Perivenular and pericellular fibrosis, as determined by Masson’s trichrome staining, were rarely seen in any of the liver specimens from the three groups.
Histological analysis of the liver tissue specimens. The H&E-stained sections revealed that the liver specimens of the rats in the SBS/TPN group (c, d) had an increased number of lipid droplets in comparison with the two other groups [the sham group (a, b) and the SBS/TPN/Ghrelin group (e, f)]. Oil-red-O staining confirmed the accumulation lipids in the liver specimens from the SBS/TPN group (d: inset). CV central vein, PV portal vein. Original magnification: ×100 (low) and ×400 (high)
The lipid content of the liver
The hepatic TG, FFA, and CE levels in the SBS/TPN group were significantly increased in comparison with the sham group (Sham vs. SBS/TPN, TG: p < 0.01, FFA: p < 0.01, CE: p < 0.01, Table 3). Hepatic TG, FFA, and CE in the SBS/TPN/Ghrelin group were significantly decreased in comparison with the SBS/TPN group [SBS/TPN vs. SBS/TPN/Ghrelin, TG (p < 0.05), FFA (p < 0.05), CE (p < 0.05); Table 3]. Although the administration of ghrelin significantly prevented the accumulation of lipids in the liver, the TG level in the SBS/TPN/Ghrelin group was significantly increased in comparison with the sham group (Table 3).
Discussion
IFALD is associated with two types of liver dysfunction: cholestasis and steatosis [4]. IFALD has the potential progress to biliary cirrhosis and non-alchoholic steatohepatitis (NASH) and eventually to decompensated liver failure; it, therefore, represents a significant life-threatening complication. It would be very valuable to discover an optimal therapy for the treatment of IFALD.
In the current study, we reveal for the first time that the exogenous administration of ghrelin attenuated hepatocellular injury in a parenterally fed rat model of SBS. The major new findings of this study are as follows: (1) TPN for 13 days following 90 % SBR induced hepatic steatosis without histological cholestasis. (2) The administration of ghrelin attenuated the histological changes and decreased the levels of TG, FFA, and CE in the liver. (3) The administration of ghrelin improved the serum AST and LDH levels and increased the serum T-CHO level.
In the present study, the parenteral feeding of rats after 90 % SBR caused an increase in the serum AST and LDH levels, whereas the T-Bil level did not increase. The histological examinations revealed the accumulation of lipids but not cholestasis or fibrosis. The steatosis and inflammation scores, which are both components of the NAFLD score, were significantly increased. These findings suggest that only steatosis had occurred and that cholestasis and fibrosis of the liver had not developed. The previous studies have reported that cholestasis is commonly observed in neonates and infants [3]. This may be related to the immaturity of the neonatal liver, the increased frequency of SBS in this age group, or bacterial translocation leading to sepsis and hepatic damage [3]. The use of 8-weeks-adult rats in the present study might be the reason why we observed steatosis but not cholestasis.
A previous study reported that the excessive infusion of carbohydrates created hepatic steatosis in a rat model after 6 days of TPN [13]. We previously reported that 6 days of TPN with appropriate nutrients did not induce hepatic steatosis in the rat model without bowel resection (the data were not shown) [9]. This suggests that an appropriate nutrient composition and adequate catheter handling in TPN are necessary for avoiding liver injury. In fact, massive bowel resection is strongly associated with the initiation of IFALD. Piglet [16] and mouse [17] models of IFALD have also been reported; however, there have been no studies of hepatocellular injury in rats after massive bowel resection. Thus, the present parenterally fed rat model of massive bowel resection was useful for investigating IFALD.
The serum levels of AST and LDH in the SBS/TPN/Ghrelin group were similar to those in the sham group. As the serum AST and LDH levels had deviated from the normal liver enzyme levels, ghrelin would be expected to attenuate the hepatocyte injury. Li et al. reported that exogenous ghrelin attenuated the apoptosis of hepatocytes [12], which would be associated with the improvement of histological hepatocellular injury and the unchanged AST and LDH levels that were observed in the SBS/TPN/Ghrelin group. The serum level of T-CHO was increased in the SBS/TPN/Ghrelin group in comparison with the sham and SBS/TPN group. This may be another effect of ghrelin. Asakawa et al. reported that the repeated administration of ghrelin significantly increased adiposity with a concomitant increase in cholesterol under a high-fat diet [18]. As the mechanism underlying this effect has not been clarified, further studies are needed.
Omega-3 lipid emulsion is one of the most interesting treatments for IFALD to have emerged in the recent years. Gura et al. first reported the reversal of cholestasis in two patients [19]. Since jaundice and histological cholestasis in the liver were not observed in this study, we did not evaluate the efficacy of ghrelin against cholestasis. However, as a recent study reported that ω-3 polyunsaturated fatty acid (EPA and DHA) supplementation was effective in treating the hepatic steatosis induced by a fat diet [20], ω-3 lipid emulsion might be an effective treatment for the condition that was observed in the present study.
According to the “two-hit” theory of NAFLD initiation and progression, hepatic steatosis is the first hit, while further second hits, such as oxidative stress, inflammation, and apoptosis, can cause NASH. In this study, the first hit was 90 % SBR and TPN because of the histological presence of steatosis and inflammation and the subsequent occurrence of further events, such as bacterial translocation, catheter-related blood stream infection, and further surgery as second hits could be expected to cause NASH and life-threatening conditions. Histological hepatic steatosis was not observed in the SBS/TPN/Ghrelin group. Li et al. [12] reported that the administration of ghrelin improved histological hepatic steatosis in a high-fat diet-fed rat model of NAFLD. They clarified that the administration of ghrelin attenuated inflammation and oxidative stress in the liver. Since inflammation and oxidative stress are key events in the progression from simple steatosis to NASH [21, 22], the retardation of these processes by the administration of ghrelin may reverse the development of hepatic steatosis. Further studies will be needed to clarify the mechanism underlying the improved histology and fat composition of the liver, which were achieved by the administration of ghrelin.
In conclusion, this is the first experimental study to investigate the efficacy of the exogenous administration of ghrelin in treating the hepatic damage caused by TPN following 90 % SBR based on the results of histological analyses and serum examinations. Ghrelin has a potential application in the treatment of hepatic steatosis associated with IFALD. Further studies will be necessary to elucidate the mechanism underlying the ghrelin-induced recovery from hepatocellular injury for a clinical trial.
References
Wales PW, de Silva N, Kim JH, Lecce L, Sandhu A, Moore AM (2005) Neonatal short bowel syndrome: a cohort study. J Pediatr Surg 40(5):755–762. doi:10.1016/j.jpedsurg.2005.01.037
McMellen ME, Wakeman D, Longshore SW, McDuffie LA, Warner BW (2010) Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg 19(1):35–43. doi:10.1053/j.sempedsurg.2009.11.010
Kelly DA (2006) Intestinal failure-associated liver disease: what do we know today? Gastroenterology 130(2 Suppl 1):S70–S77. doi:10.1053/j.gastro.2005.10.066
Kelly DA (1998) Liver complications of pediatric parenteral nutrition–epidemiology. Nutrition 14(1):153–157
Diamond IR, Grant RC, Pencharz PB, de Silva N, Feldman BM, Fitzgerald P, Sigalet D, Dicken B, Turner J, Marchand V, Ling SC, Moore AM, Avitzur Y, Wales PW (2016) Preventing the progression of intestinal failure-associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. JPEN J Parenter Enteral Nutr. doi:10.1177/0148607115626921
Stengel A, Tache Y (2012) Ghrelin—a pleiotropic hormone secreted from endocrine x/a-like cells of the stomach. Front Neurosci 6:24. doi:10.3389/fnins.2012.00024
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85(2):495–522. doi:10.1152/physrev.00012.2004
Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC (2000) Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 119(2):395–405
Yamada W, Kaji T, Onishi S, Nakame K, Yamada K, Kawano T, Mukai M, Souda M, Yoshioka T, Tanimoto A, Ieiri S (2016) Ghrelin Improves Intestinal Mucosal Atrophy During Parenteral Nutrition: An Experimental Study. J Pediatr Surg (in press)
Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK (2008) Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 143(3):334–342. doi:10.1016/j.surg.2007.09.039
Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D (2010) Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59(9):2145–2151. doi:10.2337/db10-0504
Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q (2013) Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 43(2):376–386. doi:10.1007/s12020-012-9761-5
Sax HC, Talamini MA, Brackett K, Fischer JE (1986) Hepatic steatosis in total parenteral nutrition: failure of fatty infiltration to correlate with abnormal serum hepatic enzyme levels. Surgery 100(4):697–704
Zamir O, Nussbaum MS, Bhadra S, Subbiah MT, Rafferty JF, Fischer JE (1994) Effect of enteral feeding on hepatic steatosis induced by total parenteral nutrition. JPEN J Parenter Enteral Nutr 18(1):20–25
Nabeshima A, Yamada S, Guo X, Tanimoto A, Wang KY, Shimajiri S, Kimura S, Tasaki T, Noguchi H, Kitada S, Watanabe T, Fujii J, Kohno K, Sasaguri Y (2013) Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid Redox Signal 19(17):1983–1998. doi:10.1089/ars.2012.4946
Hua Z, Sergi C, Nation PN, Wizzard PR, Ball RO, Pencharz PB, Turner JM, Wales PW (2012) Hepatic ultrastructure in a neonatal piglet model of intestinal failure-associated liver disease (IFALD). J Electron Microsc (Tokyo) 61(3):179–186. doi:10.1093/jmicro/dfs035
El Kasmi KC, Anderson AL, Devereaux MW, Fillon SA, Harris JK, Lovell MA, Finegold MJ, Sokol RJ (2012) Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 55(5):1518–1528. doi:10.1002/hep.25500
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120(2):337–345
Gura KM, Parsons SK, Bechard LJ, Henderson T, Dorsey M, Phipatanakul W, Duggan C, Puder M, Lenders C (2005) Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin Nutr 24(5):839–847. doi:10.1016/j.clnu.2005.05.020
Levy JR, Clore JN, Stevens W (2004) Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology 39(3):608–616. doi:10.1002/hep.20093
Harmon RC, Tiniakos DG, Argo CK (2011) Inflammation in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 5(2):189–200. doi:10.1586/egh.11.21
Koek GH, Liedorp PR, Bast A (2011) The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta 412(15–16):1297–1305. doi:10.1016/j.cca.2011.04.013
Acknowledgments
We thank Mr. Brian Quinn for comments and help with the manuscript. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS, Nos. 25462777, 16K10094, 16K10095, 16K10434, and 16K10466,). This study was supported by the Institute of Laboratory Animal Sciences, Kagoshima University (Frontier Science Research Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Rights and permissions
About this article
Cite this article
Onishi, S., Kaji, T., Yamada, W. et al. The administration of ghrelin improved hepatocellular injury following parenteral feeding in a rat model of short bowel syndrome. Pediatr Surg Int 32, 1165–1171 (2016). https://doi.org/10.1007/s00383-016-3975-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-3975-1