Abstract
Choledochal cysts are rare congenital disorders first described by Vater and Ezler in 1723. Their exact etiology remains incompletely understood; however, an anomalous pancreaticobiliary union (APBDU) and subsequent reflux of biliary contents into the biliary tree are thought to play a role. Accordingly, APBDU-associated choledochal cyst patients are significantly more likely to have evidence of hepatitis, cholangitis or pancreatitis and pathologically confirmed inflammation. In 1977, Todani and colleagues modified the original Alonso-Lej classification to include five types of CC. Type I and IV are the most common and most likely to be associated with malignancy. The majority of choledochal cysts are diagnosed in childhood. Clinical presentation varies and most often consists of nonspecific abdominal pain. Diagnosis is typically accomplished using multimodality imaging techniques including computed tomography, magnetic resonance imaging, ultrasound and MRCP. The use of diagnostic PTC and ERCP in CC has been largely replaced by MRCP. Appropriate management consists of prompt, complete cyst excision followed by restoration of biliary enteric continuity when necessary. Minimally invasive CC resection in the pediatric population has demonstrated acceptable outcomes. Prognosis is generally excellent; however, malignancy risk remains higher than the general population even after complete surgical excision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Choledochal cysts (CC) are congenital dilatations of the biliary tree. Although they are diagnosed in patients of all ages, CC are primarily seen in children, particularly in Asian populations. CC can be associated with severe complications such as cholangitis, perforation, liver failure and malignancy. Prompt complete surgical excision is the mainstay of treatment when possible. Resection is typically deemed necessary to prevent further complications and long-term sequelae. We present an evidence-based review of CC disease with a particular emphasis on accurate diagnosis and proper management in the pediatric population. A search of the available English literature, including MEDLINE/Pubmed, was utilized.
Incidence and etiology
Choledochal cysts (CC) are extremely rare congenital disorders manifested by intra- and/or extrahepatic biliary dilatation. CC were first described by Vater and Ezler in 1723 [1] and are more common in Asian populations with an incidence of 1 in 13,000 versus 1 in 100,000 in Western populations [2]. Females are at higher risk for the disease with a nearly 4:1 female preponderance compared with males [2–4]. Nearly 80% of CC are diagnosed in early infancy [2, 5]. Appropriate management is critical to avoid long-term possible complications such as liver failure and malignancy.
While CC are thought to be congenital, the exact etiology of CC remains incompletely understood. In 1969, Babitt [6] described an anomalous pancreaticobiliary union (APBDU) in 3 children with CC and hypothesized this as a possible etiology. APBDU is defined as union of the pancreatic and biliary ducts outside of the duodenal wall and proximal to the Ampulla of Vater [7]. Embryologically, abnormalities during early development of the hepatic diverticulum are thought to be responsible for APBDU [8]. Ando and colleagues [9] postulated that the embryogenesis of pancreaticobiliary maljunction can be inferred from the development of duodenal atresia. Specifically, the impairment of vacuolation is common to both processes. With regard to APBDU, the impairment of vacuolation is thought to create a stenosis where the pancreatic duct inserts into the upper and lower bile duct, leading to common bile duct dilatation [9].
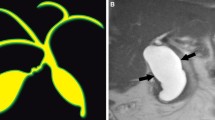
Patients with APBDU are more likely to have an ectopic distal location of the papilla of Vater [10]. Accordingly, as the ampulla of Vater is found more distally, the longer the common channel becomes [10]. A common channel greater than 15 mm is considered abnormal [11] (Fig. 1). The APBDU leads to reflux of pancreatic juice into the common bile duct resulting in chronic inflammation, bile duct wall damage and cystic changes. Animal models of murine APBDU have demonstrated this mechanism [12, 13]. APBDU in the absence of choledochal cyst disease has been reported and its management remains controversial. However, most agree that early intervention is prudent to avoid long-term complications [7, 14–16]. APBDU is seen in up to 90% of patients with CC [17, 18] and this seems to have important clinical implications. In a comparison of APBDU-associated CC versus non-APBDU-associated CC, APBDU-associated CC patients were significantly more likely to have evidence of pathologically confirmed inflammation including hepatitis, cholangitis and pancreatitis [19, 20]. In a retrospective review of 80 pediatric patients who underwent choledochal cyst excision, Jung et al. [21] found that patients with high biliary amylase levels were significantly more likely to be diagnosed later (median age 48 versus 4 months), present with abdominal pain, and had predominantly portal inflammation on histological examination after excision.
Common channel in a 7-year-old female with type I choledochal cyst. Coronal MRCP (a) shows a long common channel (arrow) in a patient with anomalous pancreaticobiliary duct union (APBDU) and type I Choledochal cyst. Notice diffuse dilatation of the extrahepatic common duct. ERCP (b) confirms the diagnosis
The presence of CC in the absence of APBDU likely relates to other underlying pathophysiologies including weak bile duct wall, distal biliary obstruction or sphincter of Oddi dysfunction. Embryologic and motility disorders have also been postulated after some patients with biliary cysts were noted to have fewer ganglion cells than expected [18, 22].
The association of CC with congenital anomalies remains ambiguous. Previous reports have demonstrated an association of pediatric CC and congenital cardiac anomalies. In an analysis of 1646 patients with choledochal cysts in the United States, cardiac anomalies were detected in 44.9% of infants younger than 12 months old who were diagnosed with CC, thereby suggesting that screening for cardiac anomalies may be prudent in this population [23]. Other reports have postulated an association of CC with duodenal atresia, colonic atresia, gastroschisis, annular pancreas and pancreatic cysts [2, 24–29].
Classification
Alonso-Lej and colleagues proposed the first classification system of CC in 1959 [30]. The original classification identified 4 types of biliary cysts (type I–IV). In 1977, Todani and colleagues [31] modified this classification and added a fifth category of CC, Type V biliary cysts or Caroli disease [2]. Type I CC are fusiform or spherical dilatations of the extrahepatic biliary tree (Fig. 2). Importantly, the intrahepatic biliary tree is sometimes dilated secondarily due to biliary stasis. Type I CC are the most commonly encountered CC (80–90% of all CC) (Fig. 3 ). Radiographically, this type of CC appears as anechoic cystic lesion that communicates with the biliary tree [2]. Type I CC, along with type IV cysts, have the highest risk of malignancy [2]. This is not surprising given that both types of these CCs have extrahepatic involvement and are typically associated with APBDU [18]. Type I cysts can be further subdivided into Type IA, IB and IC cysts [2, 32]. Type IA CC have the gallbladder arising directly from the CC with a dilated extrahepatic biliary tree and a non-dilated intrahepatic tree. Type IB CC contain no evidence of APBDU and a focal segment of the common bile duct is dilated [32, 33]. Finally, type IC CC are represented by a fusiform dilatation of the common hepatic duct and common bile duct in the presence of APBDU [2, 32, 33].
Choledochal cyst classification. Used with permission from Rozel et al. [32]
Type II CC consist of a diverticular dilatation of the extrahepatic bile duct system. This type of CC is rare (2% of all CC); of note, this type of CC is considered a true diverticula. Cholangiography will demonstrate opacification of the diverticulum from the common bile duct, which can sometimes be confused with gallbladder duplication [2] (Fig. 4).
Type II choledochal cyst in a 13-month-old male. Doppler ultrasound (a) shows a cystic structure (arrow) near the common duct (arrowheads). Coronal MRCP image (b) shows diverticular dilatation of the distal common bile duct (arrow). Debris is noted within the diverticulum (small arrows). The communication with the distal common duct is well demonstrated (arrowhead). The intra- and extrahepatic ducts are normal
Type III CC (4% of all CC), or choledochoceles, are located within the duodenal wall at the pancreaticobiliary junction [18] (Fig. 5). Unlike other types of CC, type III CC tend to be evenly distributed between the sexes and have a much lower incidence of malignant transformation [2, 34]. APBDU is also rare. These characteristics have lead authors to suggest that type III CC should not be classified as a type of CC [18, 34, 35].
Type IV CC are multiple cysts which can involve both the intrahepatic and extrahepatic biliary tree. Type IV CC can be further subdivided into Type IVa and IVb cysts depending on intrahepatic involvement. Type IVa CC refers to extrahepatic biliary dilatation with at least one intrahepatic cystic dilatation (Fig. 6). Type IVb refers to multiple extrahepatic biliary cysts without intrahepatic involvement [18]. Type IV CC are the second most common CC representing 15–20% of all reported CC.
Type IV choledochal cyst in an 18-year-old female. Doppler ultrasound (a) shows diffuse dilatation of the common duct (arrows). Coronal CT image in the portal venous phase (b) shows dilatation of the common duct (arrow). More anterior coronal view (c) shows cystic dilatation of the left hepatic duct (arrow). The intrahepatic ducts are also dilated (small arrows). Coronal MRCP image (d) shows diffuse dilatation of the common duct (arrow) and cystic dilatation of the left hepatic duct (arrowhead). The gallbladder is also seen (small arrows)
Finally, Todani’s modification added type V CCs, or Caroli disease, which appear as intrahepatic cystic dilatation without evidence of extrahepatic dilatation (Fig. 7). Cancer is seen in up to 8% of patients with Caroli disease with most malignancies presenting in adulthood [18, 36, 37]. Caroli disease-associated malignancy remains rare in the pediatric population [37].
Caroli disease in a 40-year-old male. Axial CT (a) shows intrahepatic cystic ductal dilatation (arrows). There are mild splenomegaly and retroperitoneal varices (arrowhead). Axial (b) and coronal (c) T2 images from MRCP show diffuse intrahepatic cystic ductal dilatation, with sparing of the extrahepatic common duct. d Percutaneous transhepatic cholangiography shows similar findings, with cystic dilatation of the intrahepatic ducts (arrows)
Clinical presentation
The majority (80%) of CC are diagnosed in childhood [2]. Clinical presentation varies and most often consists of nonspecific abdominal pain. The classic triad of jaundice, abdominal pain and right upper quadrant mass is rare and seen mainly in the pediatric population [2, 17, 38]. Jaundice, cholangitis, pancreatitis, portal hypertension, liver function abnormalities and coagulopathy are also seen [2, 17, 39, 40]. Jaundice mainly occurs in type I and IV CC where APBDU allows for the reflux of biliary and pancreatic juices leading to protein plugs and stone formation [39, 41]. Biliary amylase levels may be elevated and correlate with clinical severity [21, 42]. Type V CC typically presents with cholangitis and stone formation [17]. In 1–2% of cases, CC may present with rupture and biliary peritonitis prompting emergency biliary drainage [3, 43–45]. This presentation is typically seen in neonates and infants. Incidental identification is rare in the pediatric population, but seen in nearly one-third of adult CC patients [46].
Presentation in infancy (<1 year old) compared with the classical pediatric group (1–18 years old) is different. Specifically, infants are more likely to present with jaundice, clay colored stools whereas the classic pediatric group is more likely to present with abdominal pain [47]. Less commonly seen presentations include duodenal obstruction and perforation [29]. Compared with adults, the pediatric CC patient is more likely to present with an abdominal mass and an APBDU [48].
Irwin and Morrison reported the first CC-associated malignancy in 1944 [49]. The mechanism by which CC develop malignant change remains unclear; however, pancreatic reflux, biliary stasis and formation of mutagenic secondary biliary acids are thought to play a role [50]. Although a malignancy may develop anywhere within the biliary tree, over 50% of tumors develop within the cyst itself [50]. Pediatric CC are less likely to be associated with malignant transformation [48, 51]. In a review of 5780 CC cases in the literature, Sastry et al. [51] reported that 7.5% of patients had cancer [cholangiocarcinoma (70.4%) and gallbladder cancer (23.5%)]. The incidence of malignancy before the age of 18 was 0.42 versus 11.4% in adults [51].
Diagnostic evaluation
The diagnosis of CC is typically first accomplished using transcorporeal ultrasound (US). Ultimately, multimodality imaging techniques are often utilized including computed tomography (CT), magnetic resonance imaging (MRI), and/or endoscopic retrograde cholangiopancreatography (ERCP) to confirm the extent of ductal involvement or the presence of extrahepatic disease [18]. In the absence of intrahepatic biliary dilation, US alone may be sufficient [52, 53]. Otherwise, the presence of intrahepatic biliary dilatation is an indication for further imaging to differentiate type I CC from type IVa. The presence of a right upper quadrant cyst separate from the gallbladder or a direct commination between the biliary tree and the cyst should be considered CC until proven otherwise [2].
Although highly sensitive, percutaneous transhepatic cholangiography (PTC) or ERCP are utilized less frequently given their invasiveness and associated risks including cholangitis, bleeding, pancreatitis and perforation [54]. Moreover, PTC and ERCP can be technically challenging and require general anesthesia in the pediatric population. Magnetic resonance cholangiopancreatography (MRCP) has also significantly decreased the use of diagnostic ERCP and PTC in CC disease [2, 55–57]. However, as referenced earlier, many CC patients initially present with obstructive jaundice due to biliary stones or proteinaceous plugs. Accordingly, there is still a role for PTC and ERCP in CC disease for the management of complications such as cholangitis or obstructing biliary stones to stabilize these patients and prepare them for definitive surgical resection [11, 58, 59].
MRCP is noninvasive and highly sensitive (70–100%) and specific (90–100%) in the diagnosis of CC disease [56, 60–62]. Additionally, there is no irradiation and modern scanners have alleviated the need for protracted breath holds making it more amenable to the pediatric population [2, 63]. Both ultrasound and CT are highly sensitive and specific in the diagnosis of CC; however, MRCP is better able to delineate CC subtype and associated abnormalities [61]. For example, ultrasound is unable to accurately identify APBDU whereas MRCP can readily define the pancreato-biliary ductal anatomy [61, 64, 65]. Endoscopic ultrasound and ERCP are also able to detect a long common channel; however, their invasive nature and inherent risks make MRCP the preferred diagnostic modality in the pediatric population [61, 62]. MRCP has been shown to be as effective as intraoperative cholangiography for operative planning [66]. Moreover, MRCP reliably detects CC-associated cholangiocarcinoma, choledocholithiasis and is associated with lower cost and morbidity compared to other imaging/diagnostic modalities [53, 61].
The differential diagnosis among patients presenting with suspected CC is broad, including biliary atresia, infectious hepatitis, embryonal hepatic rhabdomyosarcoma, biliary lithiasis, pancreatitis, biliary hamartoma, among others [2]. Neonatal obstructive jaundice is typically explained by either biliary atresia or CC. Cystic biliary atresia (CBA), a subtype of biliary atresia, is particularly difficult to differentiate from CC disease. The management of CBA is entirely different and, therefore, prompt accurate diagnosis is critical [67]. Cystic biliary atresia (CBA) patients typically present earlier (<3 months of age) and their cysts appear smaller with less dilatation of the intrahepatic biliary system [2, 68]. An atretic gallbladder with irregular and hypoplastic biliary radicles is typical of CBA on ultrasound and cholangiography [69–71]. Conversely, infantile CC demonstrates a communication of the cyst with a dilated gallbladder in addition to a dilated intrahepatic biliary tree [69].
Biliary rhabdomyosarcoma is a rare soft tissue tumor affecting 1% of children [72]. The differentiation of biliary rhabdomyosarcoma and CC is difficult yet important given the therapeutic implications [72–74]. Pediatric cases where obstructive jaundice is associated with a mass or intraductal growth make the diagnosis of CC less likely and should prompt evaluation for rhabdomyosarcoma [73, 75].
Differentiating obstructive common bile duct dilatation and congenital common bile duct dilatation can be difficult. When evaluating the cholangiographic characteristics of 85 consecutive children with common bile duct dilatation, Oh and colleagues [76] noted that the children with congenital common bile duct dilatation did not differ significantly in clinical characteristics compared with children who had obstructive CBD dilatation. It is, therefore, prudent and essential to rule out a distal biliary obstruction and secondary biliary dilatation in the pediatric population with dilated biliary trees.
As noted above, type I choledochal cysts may present with intrahepatic biliary dilatation secondary to biliary stasis, thus resembling a type IVa CC. This distinction is critical given the therapeutic implications and the need to include hepatic resection (in the case of type IVa CC) in addition to extrahepatic biliary tree excision.
Pathology
Grossly, CC appear as diffuse dilatations of the bile ducts. Histologic evaluation demonstrates epithelial hyperplasia with round cell infiltration and as well as bile duct wall thickening and fibrosis [77, 78]. Additionally, inflammation is present in nearly 80% of CC, particularly those associated with APBDU [19, 79]. Increased cell proliferative activity in the presence of APBDU can be seen anywhere in the biliary tract, including the gallbladder [78]. Histologic hepatic changes are seen in most patients with CC with varying degrees of severity [79]. Importantly, the presence of APBDU, more severe symptoms, type IVa CC, and younger age correlates with higher degree of liver damage [69, 79–81]. On follow-up liver biopsies, most of these changes resolve after surgical excision; however, preoperative portal fibrosis and central venous distension may remain stable or increase in severity [79].
As noted above, carcinogenesis is most frequently seen in type I and IV CC; however, this is extremely rare in the pediatric population [50]. Malignant transformation typically leads to cholangiocarcinoma or gallbladder cancer. Carcinogenesis is more commonly seen in APBDU-associated CC. Although the exact pathogenesis to carcinoma remains unknown, the reflux of pancreatic contents into the biliary tree in combination with the presence of mutagenic secondary biliary acids due to biliary stasis is thought to play a role. Carcinogenesis most likely occurs as a multistep process with Kras and p53 mutations commonly seen [2, 82].
Management
Cyst excision is the definitive treatment for CC. This has become the preferred management strategy over internal drainage procedures (choledochocystoduodenostomy or choledochocystojejunostomy) which were historically attempted but had high morbidity, likely due to a deficiency of drainage procedures to relieve biliary stasis [83, 84]. Furthermore, the risk of malignant degeneration is only fully mitigated by complete resection: a critical point in the pediatric population with a large number of expectant life years. The specific approach is largely a function of the type of cyst, but generally aims to fully excise the cyst and restore biliary enteric drainage, either primarily into the duodenum or via Roux-en-Y hepaticojejunostomy (RYHJ). Surgical intervention should be elective and patients should be medically optimized prior to operative intervention. Cholangitis or pancreatitis should be adequately treated with broad-spectrum intravenous antibiotics and biliary decompression if needed.
Treatment of type I CC, the most common type, comprises resection of the extrahepatic biliary tree and cholecystectomy with hepaticoenterostomy. After exposure of the portal structures, the common bile duct is skeletonized and transected as distally as possible with the intent to resect the entirety of the cyst without injuring the pancreaticobiliary ductal junction. If the duct is dilated at the distal margin, the mucosa of any remaining residual lumen can be stripped. The cyst itself is then elevated anteriorly off the portal vein with care taken to identify aberrant or variant biliary or vascular anatomy. Proximal transection is typically performed at the hepatic bifurcation, which is carefully examined for stricture or inflammation prior to anastomosis. If these are noted, more proximal transection should be considered.
Type II CC are saccular, non-fusiform diverticula of the common bile duct which are not as likely to be associated with pancreaticobiliary malunion [20] and are typically managed with diverticulectomy or simple cyst excision. Closure can be performed primarily or over a T-tube, and occasionally RYHJ reconstruction is required if there is significant luminal narrowing.
Endoscopic sphincterotomy has been used to manage pediatric patients with type III CC, or choledochoceles, without excising the cyst. Various reports denote adequate symptom control with this approach [85, 86]; however, long-term follow-up is lacking. Cysts not amenable to endoscopic intervention may benefit from lateral duodenotomy with sphincteroplasty and unroofing or marsupialization of the cavity.
Type IVb cysts are treated in the same fashion as type I. Management for IVa disease differs due to the presence of intra- as well as extrahepatic involvement and is dictated by the extent and pattern of the intrahepatic involvement, as well as the presence of functional liver disease. Of foremost importance is the characterization of actual type IVa as opposed to type 1 with upstream ductal dilatation due to stasis and functional obstruction [33]. If the dilatation is anatomic and isolated, partial hepatectomy may be warranted due to the ongoing risk of malignant transformation in the intrahepatic biliary system [87]. If the pattern is more diffuse or imaging is inconclusive, treatment in a type I paradigm with close postoperative surveillance to follow intrahepatic ducts has been utilized [88]. In some reported cases, the intrahepatic component has actually resolved in 3–6 months following adequate drainage [89]. Preoperative percutaneous biliary drainage to decompress the intrahepatic biliary ductal system to differentiate type IVa from type I has been advocated in adults [90] but not reported in children, likely due to the difficulty maintaining external tubes in the pediatric population. Intrahepatic cystojejunostomy in addition to hepaticojejunostomy has also been described as a way to avoid liver resection of type IVa cysts [91], though long-term results are unknown. In the presence of bilobar unresectable intrahepatic cysts, complete extrahepatic excision with hepaticoenterostomy and either internal or external drainage of the remaining cysts should adequately ameliorate biliary stasis.
Intrahepatic cysts in Caroli disease vary from limited disease restricted to a single segment or lobe to diffuse disease involving the entire intrahepatic biliary tree. Unilobar cystic disease in the absence of cirrhosis and portal hypertension should be treated with anatomic hepatectomy and biliary enteric bypass. For bilobar disease, nonoperative management including litholytic agents such as ursodiol should be considered in addition to symptom-directed treatment including antibiotics and percutaneous drainage as appropriate. Aggressive surveillance for malignant transformation is indicated. Orthotopic liver transplantation is not indicated prophylactically, but should be considered the treatment of choice in patients with diffuse symptomatic disease with cirrhosis or portal hypertension [92].
Surgery for CC disease can be performed open or laparoscopically based on patient characteristics and surgeon preference. Laparoscopic cyst excision with reconstruction was first described in 1995 [93] and has been demonstrated in children as young as 3 months [94] and as small as 6 kg [95]. Laparoscopy is associated with longer operative time and shorter hospital stay [96–99] with otherwise comparable outcomes to open approaches, and is rendered most feasible when there is a lack of cholangitis or pancreatitis (Table 1) [100]. Either four or five ports are typically employed [94, 101, 102] in a conventional laparoscopic approach. Single site laparoscopy [103] as well as use of a robotic surgical system [104] has been reported. Routine postoperative drainage has been shown to be unnecessary in a prospective randomized study of 121 children undergoing laparoscopic cyst excision with RYHJ [105].
Hepaticoduodenostomy and RYHJ are the two most commonly utilized techniques of reconstruction, although other replacement conduits such as appendix have been reported [109]. Hepaticoduodenostomy has been favored by some groups [110, 111] but most series suggest significantly more bile reflux compared with RYHJ [112], which is currently the most commonly utilized reconstruction. When RYHJ is employed, an end-to-end anastomosis of the jejunum to the common bile duct is recommended if technically possible to avoid the elongation of a blind pouch as the child grows [113]. If the bile duct is too small or an end-to-side anastomosis is required, it should be as close as possible to the closed end of the jejunal limb. The length of the Roux limb is not predetermined but should be appropriate to the child’s overall bowel length considering future growth.
Choledochal cysts can be diagnosed at any age and can be symptomatic or incidentally discovered. Furthermore, antenatal diagnosis of cysts is increasingly common with modern improvements in ultrasound screening and technology. Symptomatic disease is an indication for surgery at any age, though the timing of repair in asymptomatic newborns is controversial [114]. Classic recommendations were to undergo repair by age 6 months [115], though there is some evidence for repair as early as the first month of life in asymptomatic neonates. In a randomized trial of 36 infants, the incidence of hepatic fibrosis was significantly increased when surgery was postponed to after 1 month of age [116]. Moreover, type IVa CC have a propensity to cause irreversible liver damage in the infantile period [80]. In the presence of concomitant pancreatitis or cholangitis, operative timing should consider the dangers of surgery during active inflammatory response as well as the desire to limit subsequent flares [117]. The risk of malignancy in the first decade of life is less than 1%; however, this increases to over 10% after the age of 30 [11, 50].
Prognosis
Resection of pediatric CC is generally well tolerated. In the postoperative period, early complications can include anastomotic leak, postoperative bleeding, wound infection, acute pancreatitis, and pancreatic or biliary fistula [39, 118]. However, most series are without early mortality and report rates of acute complications including wound infections from 0 to 17%, without significant difference between infants and children [17, 47, 96, 119, 120] (Table 1). Late complications include anastomotic stricture, cholangitis, hepatolithiasis, cirrhosis, and malignancy. Benign anastomotic stricture with recurrent cholangitis is less common than in adults but is still seen in as many as 10–25% of patients and can be associated with both intrahepatic and bile duct stone formation [83, 107, 121, 122].
Rigorous long-term follow-up after pediatric CC resection is limited, but the risk of biliary carcinoma, most often cholangiocarcinoma, clearly remains elevated even after CC excision compared to the general population [50, 123]. Malignant disease has been noted in up to 14% of patients after CC resection as a child [17, 121, 122]. In fact, cancer is the most frequent cause of late mortality in pediatric CC series. Continued surveillance is, therefore, strongly recommended, though it is not known whether there are risk factors such as retained portion of cyst that predispose to malignancy after excision.
References
Vater A, Ezler C (1723) Dissertatio de Scirrhis viserum occasione sections viri tymponite defunte. Wittenb Pam 4 (881):22
Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, Pawlik TM (2014) Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg 219(6):1167–1180. doi:10.1016/j.jamcollsurg.2014.04.023
Yamaguchi M (1980) Congenital choledochal cyst. Analysis of 1433 patients in the Japanese literature. Am J Surg 140(5):653–657
Lipsett PA, Pitt HA (2003) Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg 10(5):352–359. doi:10.1007/s00534-002-0797-4
Wiseman K, Buczkowski AK, Chung SW, Francoeur J, Schaeffer D, Scudamore CH (2005) Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg 189(5):527–531. doi:10.1016/j.amjsurg.2005.01.025 (discussion 531)
Babbitt DP (1969) [Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb]. Ann de Radiol 12(3):231–240
Ono S, Fumino S, Iwai N (2011) Diagnosis and treatment of pancreaticobiliary maljunction in children. Surg Today 41(5):601–605. doi:10.1007/s00595-010-4492-9
Li L, Yamataka A, Wang YX, Wang DY, Wang K, Li ZX, Shimizu T, Yamashiro Y, Zhang JZ, Lane GJ, Miyano T (2003) Anomalous pancreatic duct anatomy, ectopic distal location of the papilla of Vater and congenital biliary dilatation: a new developmental triad? Pediatr Surg Int 19(3):180–185. doi:10.1007/s00383-002-0914-0
Ando H, Kaneko K, Ito F, Seo T, Harada T, Watanabe Y (1999) Embryogenesis of pancreaticobiliary maljunction inferred from development of duodenal atresia. J Hepatobiliary Pancreat Surg 6(1):50–54
Li L, Yamataka A, Yian-Xia W, Da-Yong W, Segawa O, Lane GJ, Kun W, Jin-Zhe Z, Miyano T (2001) Ectopic distal location of the papilla of vater in congenital biliary dilatation: implications for pathogenesis. J Pediatr Surg 36(11):1617–1622. doi:10.1053/jpsu.2001.27932
Liu QY, Nguyen V (2013) Endoscopic approach to the patient with congenital anomalies of the biliary tract. Gastrointest Endosc Clin N Am 23(2):505–518. doi:10.1016/j.giec.2012.12.004
Yamashiro Y, Miyano T, Suruga K, Shimomura H, Suda K, Matsumoto M, Nittono H (1984) Experimental study of the pathogenesis of choledochal cyst and pancreatitis, with special reference to the role of bile acids and pancreatic enzymes in the anomalous choledocho-pancreatico ductal junction. J Pediatr Gastroenterol Nutr 3(5):721–727
Miyano T, Suruga K, Suda K (1981) “The choledocho-pancreatic long common channel disorders” in relation to the etiology of congenital biliary dilatation and other biliary tract disease. Ann Acad Med Singap 10(4):419–426
Ono Y, Kaneko K, Tainaka T, Sumida W, Ando H (2008) Pancreaticobiliary maljunction without bile duct dilatation in children: distinction from choledochal cyst. J Pediatr Gastroenterol Nutr 46(5):555–560. doi:10.1097/MPG.0b013e3181623291
Ando H, Ito T, Nagaya M, Watanabe Y, Seo T, Kaneko K (1995) Pancreaticobiliary maljunction without choledochal cysts in infants and children: clinical features and surgical therapy. J Pediatr Surg 30(12):1658–1662
Fumino S, Ono S, Shimadera S, Kimura O, Iwai N (2010) Impact of age at diagnosis on clinical features in children with anomalous arrangement of the pancreaticobiliary duct. Eur J Pediatr Surg 20(5):325–329. doi:10.1055/s-0030-1255097
de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, Bosma A, Heij HA, Gouma DJ, van Gulik TM (2002) Choledochal cysts: age of presentation, symptoms, and late complications related to Todani’s classification. J Pediatr Surg 37(11):1568–1573
Martin RF (2014) Biliary cysts: a review and simplified classification scheme. Surg Clin North Am 94(2):219–232. doi:10.1016/j.suc.2014.01.011
Park SW, Koh H, Oh JT, Han SJ, Kim S (2014) Relationship between anomalous pancreaticobiliary ductal union and pathologic inflammation of bile duct in choledochal cyst. Pediatr Gastroenterol Hepatol Nutr 17(3):170–177. doi:10.5223/pghn.2014.17.3.170
Song HK, Kim MH, Myung SJ, Lee SK, Kim HJ, Yoo KS, Seo DW, Lee HJ, Lim BC, Min YI (1999) Choledochal cyst associated the with anomalous union of pancreaticobiliary duct (AUPBD) has a more grave clinical course than choledochal cyst alone. Korean J Intern Med 14(2):1–8
Jung SM, Seo JM, Lee SK (2012) The relationship between biliary amylase and the clinical features of choledochal cysts in pediatric patients. World J Surg 36(9):2098–2101. doi:10.1007/s00268-012-1619-8
Tyler KL, Sokol RJ, Oberhaus SM, Le M, Karrer FM, Narkewicz MR, Tyson RW, Murphy JR, Low R, Brown WR (1998) Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology (Baltimore Md) 27(6):1475–1482. doi:10.1002/hep.510270603
Murphy AJ, Axt JR, Lovvorn HN 3rd (2012) Associations between pediatric choledochal cysts, biliary atresia, and congenital cardiac anomalies. J Surg Res 177(2):e59–e63. doi:10.1016/j.jss.2012.04.018
Iwai A, Hamada Y, Takada K, Inagaki N, Nakatake R, Yanai H, Miki H, Araki Y, Sato M, Ono S, Iwai N, Kwon AH (2009) Choledochal cyst associated with duodenal atresia: case report and review of the literature. Pediatr Surg Int 25(11):995–998. doi:10.1007/s00383-009-2462-3
Shih HS, Ko SF, Chaung JH (2005) Is there an association between duodenal atresia and choledochal cyst? J Pediatr Gastroenterol Nutr 40(3):378–381
Nijagal A, Ozgediz D, Feldstein VA, Lee H, Harrison MR (2009) Colonic atresia and choledochal cyst: a rare combination. Pediatr Surg Int 25(1):113–115. doi:10.1007/s00383-008-2280-z
Komuro H, Takahashi MI, Matoba K, Hori T, Hirai M, Gotoh C, Kaneko M (2006) Rare association of severe hypoplasia of the abdominal aorta with imperforate anus, colonic atresia, and choledochal cyst. Pediatr Surg Int 22(3):289–292. doi:10.1007/s00383-005-1604-5
Serber J, Stranzinger E, Geiger JD, Teitelbaum DH (2009) Association of gastroschisis and choledochal cyst. J Pediatr Surg 44(3):e23–e26. doi:10.1016/j.jpedsurg.2008.12.005
Komuro H, Makino S, Tahara K (2000) Choledochal cyst associated with duodenal obstruction. J Pediatr Surg 35(8):1259–1262
Alonso-Lej F, Rever WB Jr, Pessagno DJ (1959) Congenital choledochal cyst, with a report of 2, and an analysis of 94, cases. Int Abstr Surg 108(1):1–30
Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K (1977) Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 134(2):263–269
Rozel C, Garel L, Rypens F, Viremouneix L, Lapierre C, Decarie JC, Dubois J (2011) Imaging of biliary disorders in children. Pediatr Radiol 41(2):208–220. doi:10.1007/s00247-010-1829-x
Todani T, Watanabe Y, Toki A, Morotomi Y (2003) Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg 10(5):340–344. doi:10.1007/s00534-002-0733-7
Ziegler KM, Pitt HA, Zyromski NJ, Chauhan A, Sherman S, Moffatt D, Lehman GA, Lillemoe KD, Rescorla FJ, West KW, Grosfeld JL (2010) Choledochoceles: are they choledochal cysts? Ann Surg 252(4):683–690. doi:10.1097/SLA.0b013e3181f6931f
Ziegler KM, Zyromski NJ (2011) Choledochoceles: are they choledochal cysts? Adv Surg 45:211–224
Bloustein PA (1977) Association of carcinoma with congenital cystic conditions of the liver and bile ducts. Am J Gastroenterol 67(1):40–46
Moslim MA, Gunasekaran G, Vogt D, Cruise M, Morris-Stiff G (2015) Surgical management of caroli’s disease: single center experience and review of the literature. J Gastrointest Surg 19(11):2019–2027. doi:10.1007/s11605-015-2918-9
Stringer MD, Dhawan A, Davenport M, Mieli-Vergani G, Mowat AP, Howard ER (1995) Choledochal cysts: lessons from a 20 year experience. Arch Dis Child 73(6):528–531
Fujishiro J, Masumoto K, Urita Y, Shinkai T, Gotoh C (2013) Pancreatic complications in pediatric choledochal cysts. J Pediatr Surg 48(9):1897–1902. doi:10.1016/j.jpedsurg.2012.12.038
Diao M, Li L, Cheng W (2014) Coagulopathy in a subtype of choledochal cyst and management strategy. World J Gastroenterol 20(30):10606–10612. doi:10.3748/wjg.v20.i30.10606
Kaneko K, Ono Y, Tainaka T, Sumida W, Ando H (2008) Fatty acid calcium stones in patients with pancreaticobiliary maljunction/choledochal cyst as another cause of obstructive symptoms besides protein plugs. J Pediatr Surg 43(3):564–567. doi:10.1016/j.jpedsurg.2007.11.004
Verma A, Bhatnagar V, Prakash S, Srivastava AK (2014) Analysis of bile in various hepatobiliary disease states: a pilot study. J Indian Assoc Pediatr Surg 19(3):151–155. doi:10.4103/0971-9261.136470
Yamoto M, Urushihara N, Fukumoto K, Miyano G, Nouso H, Morita K, Miyake H, Kaneshiro M, Koyama M (2015) Usefulness of laparoscopic cholecystostomy in children with complicated choledochal cyst. Asian J Endosc Surg 8(2):153–157. doi:10.1111/ases.12170
Ahmed I, Sharma A, Gupta A, Chandra N, Rawat J, Singh S (2011) Management of rupture of choledochal cyst. Indian J Gastroenterol 30(2):94–96. doi:10.1007/s12664-011-0098-4
Moss RL, Musemeche CA (1997) Successful management of ruptured choledochal cyst by primary cyst excision and biliary reconstruction. J Pediatr Surg 32(10):1490–1491
Senthilnathan P, Patel ND, Nair AS, Nalankilli VP, Vijay A, Palanivelu C (2015) Laparoscopic management of choledochal cyst-technical modifications and outcome analysis. World J Surg 39(10):2550–2556. doi:10.1007/s00268-015-3111-8
Hung MH, Lin LH, Chen DF, Huang CS (2011) Choledochal cysts in infants and children: experiences over a 20-year period at a single institution. Eur J Pediatr 170(9):1179–1185. doi:10.1007/s00431-011-1429-2
Huang CS, Huang CC, Chen DF (2010) Choledochal cysts: differences between pediatric and adult patients. J Gastroint Surg 14(7):1105–1110. doi:10.1007/s11605-010-1209-8
Irwin ST, Morison JE (1944) Congenital cyst of the common bile duct containing stones and undergoing cancerous change. Br J Surg 32:319–321
Benjamin IS (2003) Biliary cystic disease: the risk of cancer. J Hepatobiliary Pancreat Surg 10(5):335–339. doi:10.1007/s00534-002-0696-8
Sastry AV, Abbadessa B, Wayne MG, Steele JG, Cooperman AM (2015) What is the incidence of biliary carcinoma in choledochal cysts, when do they develop, and how should it affect management? World J Surg 39(2):487–492. doi:10.1007/s00268-014-2831-5
Haliloglu M, Akata D, Gurel S, Ozmen MN, Akhan O (2003) Choledochal cysts in children: evaluation with three-dimensional sonography. J Clin Ultrasound JCU 31(9):478–480. doi:10.1002/jcu.10206
Murphy AJ, Axt JR, Crapp SJ, Martin CA, Crane GL, Lovvorn HN 3rd (2012) Concordance of imaging modalities and cost minimization in the diagnosis of pediatric choledochal cysts. Pediatr Surg Int 28(6):615–621. doi:10.1007/s00383-012-3089-3
Saito T, Terui K, Mitsunaga T, Nakata M, Kuriyama Y, Higashimoto Y, Kouchi K, Onuma N, Takahashi H, Yoshida H (2014) Role of pediatric endoscopic retrograde cholangiopancreatography in an era stressing less-invasive imaging modalities. J Pediatr Gastroenterol Nutr 59(2):204–209. doi:10.1097/mpg.0000000000000399
Kim SH, Lim JH, Yoon HK, Han BK, Lee SK, Kim YI (2000) Choledochal cyst: comparison of MR and conventional cholangiography. Clin Radiol 55(5):378–383. doi:10.1053/crad.2000.0438
Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, Seo DW, Won HJ, Kim MY (2005) Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc 62(3):360–366. doi:10.1016/j.gie.2005.04.026
Lam WW, Lam TP, Saing H, Chan FL, Chan KL (1999) MR cholangiography and CT cholangiography of pediatric patients with choledochal cysts. AJR Am J Roentgenol 173(2):401–405. doi:10.2214/ajr.173.2.10430145
De Angelis P, Foschia F, Romeo E, Caldaro T, Rea F, di Abriola GF, Caccamo R, Santi MR, Torroni F, Monti L, Dall’Oglio L (2012) Role of endoscopic retrograde cholangiopancreatography in diagnosis and management of congenital choledochal cysts: 28 pediatric cases. J Pediatr Surg 47(5):885–888. doi:10.1016/j.jpedsurg.2012.01.040
Otto AK, Neal MD, Slivka AN, Kane TD (2011) An appraisal of endoscopic retrograde cholangiopancreatography (ERCP) for pancreaticobiliary disease in children: our institutional experience in 231 cases. Surg Endosc 25(8):2536–2540. doi:10.1007/s00464-011-1582-8
Huang CT, Lee HC, Chen WT, Jiang CB, Shih SL, Yeung CY (2011) Usefulness of magnetic resonance cholangiopancreatography in pancreatobiliary abnormalities in pediatric patients. Pediatr Neonatol 52(6):332–336. doi:10.1016/j.pedneo.2011.08.006
Sacher VY, Davis JS, Sleeman D, Casillas J (2013) Role of magnetic resonance cholangiopancreatography in diagnosing choledochal cysts: case series and review. World J Radiol 5(8):304–312. doi:10.4329/wjr.v5.i8.304
Tipnis NA, Werlin SL (2007) The use of magnetic resonance cholangiopancreatography in children. Curr Gastroenterol Rep 9(3):225–229
Suzuki M, Shimizu T, Kudo T, Suzuki R, Ohtsuka Y, Yamashiro Y, Shimotakahara A, Yamataka A (2006) Usefulness of nonbreath-hold 1-shot magnetic resonance cholangiopancreatography for the evaluation of choledochal cyst in children. J Pediatr Gastroenterol Nutr 42(5):539–544. doi:10.1097/01.mpg.0000221894.44124.8e
Guo WL, Huang SG, Wang J, Sheng M, Fang L (2012) Imaging findings in 75 pediatric patients with pancreaticobiliary maljunction: a retrospective case study. Pediatr Surg Int 28(10):983–988. doi:10.1007/s00383-012-3159-6
Kim MJ, Han SJ, Yoon CS, Kim JH, Oh JT, Chung KS, Yoo HS (2002) Using MR cholangiopancreatography to reveal anomalous pancreaticobiliary ductal union in infants and children with choledochal cysts. AJR Am J Roentgenol 179(1):209–214. doi:10.2214/ajr.179.1.1790209
Saito T, Hishiki T, Terui K, Sato Y, Mitsunaga T, Terui E, Nakata M, Takenouchi A, Matsuura G, Yahata E, Ohno S, Sato H, Yanagawa N, Masuda Y, Yoshida H (2011) Use of preoperative, 3-dimensional magnetic resonance cholangiopancreatography in pediatric choledochal cysts. Surgery 149(4):569–575. doi:10.1016/j.surg.2010.11.004
Hill SJ, Clifton MS, Derderian SC, Wulkan ML, Ricketts RR (2013) Cystic biliary atresia: a wolf in sheep’s clothing. Am Surg 79(9):870–872
Zhou LY, Guan BY, Li L, Xu ZF, Dai CP, Wang W, Xia HM, Xie XY (2012) Objective differential characteristics of cystic biliary atresia and choledochal cysts in neonates and young infants: sonographic findings. J Ultrasound Med 31(6):833–841
Vijayaraghavan P, Lal R, Sikora SS, Poddar U, Yachha SK (2006) Experience with choledochal cysts in infants. Pediatr Surg Int 22(10):803–807. doi:10.1007/s00383-006-1771-z
Kim WS, Kim IO, Yeon KM, Park KW, Seo JK, Kim CJ (1998) Choledochal cyst with or without biliary atresia in neonates and young infants: US differentiation. Radiology 209(2):465–469. doi:10.1148/radiology.209.2.9807575
Lee HC, Yeung CY, Chang PY, Sheu JC, Wang NL (2000) Dilatation of the biliary tree in children: sonographic diagnosis and its clinical significance. J Ultrasound Med 19(3):177–182 (quiz 183–174)
Nakib G, Calcaterra V, Goruppi I, Romano P, Raffaele A, Schleef J, Pelizzo G (2014) Robotic-assisted surgery approach in a biliary rhabdomyosarcoma misdiagnosed as choledochal cyst. Rare Tumors 6(1):5173. doi:10.4081/rt.2014.5173
Elwahab MA, Hamed H, Shehta A, Ali M, Zalata K (2014) Hepatobiliary rhabdomyosarcoma mimicking choledochal cyst: lessons learned. Int J Surg Case Rep 5(4):196–199. doi:10.1016/j.ijscr.2014.01.020
Tireli GA, Sander S, Dervisoglu S, Demirali O, Unal M (2005) Embryonal rhabdomyosarcoma of the common bile duct mimicking choledochal cyst. J Hepatobiliary Pancreat Surg 12(3):263–265. doi:10.1007/s00534-004-0959-7
Sanz N, de Mingo L, Florez F, Rollan V (1997) Rhabdomyosarcoma of the biliary tree. Pediatr Surg Int 12(2–3):200–201
Oh SH, Chang SH, Kim HJ, Cho JM, Hwang JH, Namgoong JM, Kim DY, Cho YA, Yoon CH, Kim KM (2015) Cholangiographic characteristics of common bile duct dilatation in children. World J Gastroenterol 21(20):6229–6235. doi:10.3748/wjg.v21.i20.6229
Oguchi Y, Okada A, Nakamura T, Okumura K, Miyata M, Nakao K, Kawashima Y (1988) Histopathologic studies of congenital dilatation of the bile duct as related to an anomalous junction of the pancreaticobiliary ductal system: clinical and experimental studies. Surgery 103(2):168–173
Tokiwa K, Ono S, Iwai N (1999) Mucosal cell proliferation activity of the gallbladder in children with anomalous arrangement of the pancreaticobiliary duct. J Hepatobiliary Pancreat Surg 6(3):213–217
Sugandhi N, Agarwala S, Bhatnagar V, Singh MK, Sharma R (2014) Liver histology in choledochal cyst- pathological changes and response to surgery: the overlooked aspect? Pediatr Surg Int 30(2):205–211. doi:10.1007/s00383-013-3453-y
Fumino S, Higuchi K, Aoi S, Furukawa T, Kimura O, Tajiri T (2013) Clinical analysis of liver fibrosis in choledochal cyst. Pediatr Surg Int 29(11):1097–1102. doi:10.1007/s00383-013-3368-7
Fujishiro J, Urita Y, Shinkai T, Gotoh C, Hoshino N, Ono K, Komuro H (2011) Clinical characteristics of liver fibrosis in patients with choledochal cysts. J Pediatr Surg 46(12):2296–2300. doi:10.1016/j.jpedsurg.2011.09.017
Shimotake T, Aoi S, Tomiyama H, Iwai N (2003) DPC-4 (Smad-4) and K-ras gene mutations in biliary tract epithelium in children with anomalous pancreaticobiliary ductal union. J Pediatr Surg 38(5):694–697. doi:10.1016/jpsu.2003.50185
Schier F, Clausen M, Kouki M, Gdanietz K, Waldschmidt J (1994) Late results in the management of choledochal cysts. Eur J Pediatr Surg 4(3):141–144. doi:10.1055/s-2008-1066088
Todani T, Watanabe Y, Toki A, Urushihara N, Sato Y (1988) Reoperation for congenital choledochal cyst. Ann Surg 207(2):142–147
Saeki I, Takahashi Y, Matsuura T, Takahata S, Tanaka M, Taguchi T (2009) Successful endoscopic unroofing for a pediatric choledochocele. J Pediatr Surg 44(8):1643–1645. doi:10.1016/j.jpedsurg.2009.03.042
Dohmoto M, Kamiya T, Hunerbein M, Valdez H, Ibanegaray J, Prado J (1996) Endoscopic treatment of a choledochocele in a 2-year-old child. Surg Endosc 10(10):1016–1018
He XD, Wang L, Liu W, Liu Q, Qu Q, Li BL, Hong T (2014) The risk of carcinogenesis in congenital choledochal cyst patients: an analysis of 214 cases. Ann Hepatol 13(6):819–826
Acker SN, Bruny JL, Narkewicz MR, Roach JP, Rogers A, Karrer FM (2013) Preoperative imaging does not predict intrahepatic involvement in choledochal cysts. J Pediatr Surg 48(12):2378–2382. doi:10.1016/j.jpedsurg.2013.08.008
Joseph VT (1990) Surgical techniques and long-term results in the treatment of choledochal cyst. J Pediatr Surg 25(7):782–787
Savader SJ, Venbrux AC, Benenati JF, Mitchell SE, Widlus DM, Cameron JL, Osterman FA Jr (1991) Choledochal cysts: role of noninvasive imaging, percutaneous transhepatic cholangiography, and percutaneous biliary drainage in diagnosis and treatment. J Vasc Interv Radiol JVIR 2(3):379–385
Urushihara N, Fukumoto K, Fukuzawa H, Tani M, Matsuoka T, Suzuki K, Kawashima S, Hasegawa S (2007) Hepaticojejunostomy and intrahepatic cystojejunostomy for type IV-A choledochal cyst. J Pediatr Surg 42(10):1753–1756. doi:10.1016/j.jpedsurg.2007.06.012
Shi LB, Peng SY, Meng XK, Peng CH, Liu YB, Chen XP, Ji ZL, Yang DT, Chen HR (2001) Diagnosis and treatment of congenital choledochal cyst: 20 years’ experience in China. World J Gastroenterol WJG 7(5):732–734
Farello GA, Cerofolini A, Rebonato M, Bergamaschi G, Ferrari C, Chiappetta A (1995) Congenital choledochal cyst: video-guided laparoscopic treatment. Surg Laparosc Endosc 5(5):354–358
Le DM, Woo RK, Sylvester K, Krummel TM, Albanese CT (2006) Laparoscopic resection of type 1 choledochal cysts in pediatric patients. Surg Endosc 20(2):249–251. doi:10.1007/s00464-005-0151-4
Lee JH, Kim SH, Kim HY, Choi YH, Jung SE, Park KW (2013) Early experience of laparoscopic choledochal cyst excision in children. J Korean Surg Soc 85(5):225–229. doi:10.4174/jkss.2013.85.5.225
Liuming H, Hongwu Z, Gang L, Jun J, Wenying H, Wong KK, Miao X, Qizhi Y, Jun Z, Shuli L, Li L (2011) The effect of laparoscopic excision vs open excision in children with choledochal cyst: a midterm follow-up study. J Pediatr Surg 46(4):662–665. doi:10.1016/j.jpedsurg.2010.10.012
Diao M, Li L, Cheng W (2011) Laparoscopic versus open Roux-en-Y hepatojejunostomy for children with choledochal cysts: intermediate-term follow-up results. Surg Endosc 25(5):1567–1573. doi:10.1007/s00464-010-1435-x
Zhen C, Xia Z, Long L, Lishuang M, Pu Y, Wenjuan Z, Xiaofan L (2015) Laparoscopic excision versus open excision for the treatment of choledochal cysts: a systematic review and meta-analysis. Int Surg 100(1):115–122. doi:10.9738/intsurg-d-14-00165.1
Nguyen Thanh L, Hien PD, Dung le A, Son TN (2010) Laparoscopic repair for choledochal cyst: lessons learned from 190 cases. J Pediatr Surg 45(3):540–544. doi:10.1016/j.jpedsurg.2009.08.013
Ure BM, Nustede R, Becker H (2005) Laparoscopic resection of congenital choledochal cyst, hepaticojejunostomy, and externally made Roux-en-Y anastomosis. J Pediatr Surg 40(4):728–730. doi:10.1016/j.jpedsurg.2005.01.013
Margonis GA, Spolverato G, Kim Y, Marques H, Poultsides G, Maithel S, Aldrighetti L, Bauer TW, Jabbour N, Gamblin TC, Soares K, Pawlik TM (2015) Minimally invasive resection of choledochal cyst: a feasible and safe surgical option. J Gastrointest Surg 19(5):858–865. doi:10.1007/s11605-014-2722-y
Qiao G, Li L, Li S, Tang S, Wang B, Xi H, Gao Z, Sun Q (2015) Laparoscopic cyst excision and Roux-Y hepaticojejunostomy for children with choledochal cysts in China: a multicenter study. Surg Endosc 29(1):140–144. doi:10.1007/s00464-014-3667-7
Diao M, Li L, Li Q, Ye M, Cheng W (2013) Single-incision versus conventional laparoscopic cyst excision and Roux-Y hepaticojejunostomy for children with choledochal cysts: a case-control study. World J Surg 37(7):1707–1713. doi:10.1007/s00268-013-2012-y
Chang EY, Hong YJ, Chang HK, Oh JT, Han SJ (2012) Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech Part A 22(6):609–614. doi:10.1089/lap.2011.0503
Diao M, Li L, Cheng W (2012) To drain or not to drain in Roux-en-Y hepatojejunostomy for children with choledochal cysts in the laparoscopic era: a prospective randomized study. J Pediatr Surg 47(8):1485–1489. doi:10.1016/j.jpedsurg.2011.10.066
She WH, Chung HY, Lan LC, Wong KK, Saing H, Tam PK (2009) Management of choledochal cyst: 30 years of experience and results in a single center. J Pediatr Surg 44(12):2307–2311. doi:10.1016/j.jpedsurg.2009.07.071
Miyano T, Yamataka A, Kato Y, Segawa O, Lane G, Takamizawa S, Kohno S, Fujiwara T (1996) Hepaticoenterostomy after excision of choledochal cyst in children: a 30-year experience with 180 cases. J Pediatr Surg 31(10):1417–1421
Yamataka A, Ohshiro K, Okada Y, Hosoda Y, Fujiwara T, Kohno S, Sunagawa M, Futagawa S, Sakakibara N, Miyano T (1997) Complications after cyst excision with hepaticoenterostomy for choledochal cysts and their surgical management in children versus adults. J Pediatr Surg 32(7):1097–1102
Wei MF, Qi BQ, Xia GL, Yuan JY, Wang G, Weng YZ, Xu ZY, Yang XJ, Zhou XF, Tong EC (1998) Use of the appendix to replace the choledochus. Pediatr Surg Int 13(7):494–496
Schimpl G, Aigner R, Sorantin E, Mayr J, Sauer H (1997) Comparison of hepaticoantrostomy and hepaticojejunostomy for biliary reconstruction after resection of a choledochal cyst. Pediatr Surg Int 12(4):271–275
Yeung F, Chung PH, Wong KK, Tam PK (2015) Biliary-enteric reconstruction with hepaticoduodenostomy following laparoscopic excision of choledochal cyst is associated with better postoperative outcomes: a single-centre experience. Pediatr Surg Int 31(2):149–153. doi:10.1007/s00383-014-3648-x
Shimotakahara A, Yamataka A, Yanai T, Kobayashi H, Okazaki T, Lane GJ, Miyano T (2005) Roux-en-Y hepaticojejunostomy or hepaticoduodenostomy for biliary reconstruction during the surgical treatment of choledochal cyst: which is better? Pediatr Surg Int 21(1):5–7. doi:10.1007/s00383-004-1252-1
Miyano T (2006) Choledochal cysts. In: Stringer MD, Oldham KT, Mouriquand PDE (eds) Pediatric surgery and urology: long-term outcomes. Cambridge University Press, Cambridge, pp 465–479
Lee SC, Kim HY, Jung SE, Park KW, Kim WK (2006) Is excision of a choledochal cyst in the neonatal period necessary? J Pediatr Surg 41(12):1984–1986. doi:10.1016/j.jpedsurg.2006.08.023
Okada T, Sasaki F, Ueki S, Hirokata G, Okuyama K, Cho K, Todo S (2004) Postnatal management for prenatally diagnosed choledochal cysts. J Pediatr Surg 39(7):1055–1058
Diao M, Li L, Cheng W (2012) Timing of surgery for prenatally diagnosed asymptomatic choledochal cysts: a prospective randomized study. J Pediatr Surg 47(3):506–512. doi:10.1016/j.jpedsurg.2011.09.056
Cho MJ, Kim DY, Kim SC, Kim TH, Kim IK (2011) Early vs. delayed surgery for choledochal cyst with acute pancreatitis in children. Hepato-gastroenterology 58 (107–108):709–712
Ono S, Maeda K, Baba K, Usui Y, Tsuji Y, Yano T, Hatanaka W, Yamamoto H (2013) The efficacy of double-balloon enteroscopy for intrahepatic bile duct stones after Roux-en-Y hepaticojejunostomy for choledochal cysts. Pediatr Surg Int 29(11):1103–1107. doi:10.1007/s00383-013-3376-7
Liu SL, Li L, Hou WY, Zhang J, Huang LM, Li X, Xie HW, Cheng W (2009) Laparoscopic excision of choledochal cyst and Roux-en-Y hepaticojejunostomy in symptomatic neonates. J Pediatr Surg 44(3):508–511. doi:10.1016/j.jpedsurg.2008.08.006
Hong L, Wu Y, Yan Z, Xu M, Chu J, Chen QM (2008) Laparoscopic surgery for choledochal cyst in children: a case review of 31 patients. Eur J Pediatr Surg 18(2):67–71. doi:10.1055/s-2008-1038486
Todani T, Watanabe Y, Urushihara N, Noda T, Morotomi Y (1995) Biliary complications after excisional procedure for choledochal cyst. J Pediatr Surg 30(3):478–481
Ono S, Fumino S, Shimadera S, Iwai N (2010) Long-term outcomes after hepaticojejunostomy for choledochal cyst: a 10- to 27-year follow-up. J Pediatr Surg 45(2):376–378. doi:10.1016/j.jpedsurg.2009.10.078
Soares KC, Kim Y, Spolverato G, Maithel S, Bauer TW, Marques H, Sobral M, Knoblich M, Tran T, Aldrighetti L, Jabbour N, Poultsides GA, Gamblin TC, Pawlik TM (2015) Presentation and clinical outcomes of choledochal cysts in children and adults: a multi-institutional analysis. JAMA Surg 150 (6):577–584. doi:10.1001/jamasurg.2015.0226
Author information
Authors and Affiliations
Corresponding author
Additional information
Kevin C. Soares and Seth D. Goldstein contributed equally to this work.
Rights and permissions
About this article
Cite this article
Soares, K.C., Goldstein, S.D., Ghaseb, M.A. et al. Pediatric choledochal cysts: diagnosis and current management. Pediatr Surg Int 33, 637–650 (2017). https://doi.org/10.1007/s00383-017-4083-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-017-4083-6