Abstract
Background
Blockade of the renin–angiotensin system (RAS) has been shown to alleviate inflammatory processes in the gastrointestinal tract. The aim of this study was to determine if blockade of the RAS would be effective in an immunologically relevant colitis model, and to compare outcome with an acute colitis model.
Methods
A losartan analog, CCG-203025 (C23H26ClN3O5S) containing a highly polar sulfonic acid moiety that we expected would allow localized mucosal antagonism with minimal systemic absorption was selected as an angiotensin II type 1a receptor antagonist (AT1aR-A). Two colitis models were studied: (1) Acute colitis was induced in 8- to 10-week-old C57BL/6J mice by 2.5 % dextran sodium sulfate (DSS, in drinking water) for 7 days. (2) IL10-/-colitis Piroxicam (200 ppm) was administered orally in feed to 5-week-old IL-10-/-mice (C57BL/6J background) for 14 days followed by enalaprilat (ACE-I), CCG-203025 or PBS administered transanally for 14 days.
Results
In the DSS model, weight loss and histologic score for CCG-203025 were better than with placebo. In the IL10-/-model, ACE-I suppressed histologic damage better than CCG-203025. Both ACE-I and CCG-203025 reduced pro-inflammatory cytokines and chemokines.
Conclusions
This study demonstrated the therapeutic efficacy of both ACE-I and AT1aR-A for preventing the development of both acute and immunologically relevant colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The renin–angiotensin system (RAS) is well known to have various physiologic roles [1–4]. Angiotensin II (ATII) type 1a receptor (AT1aR) blockade has been previously shown to alleviate gastrointestinal inflammation. We previously reported that high dose, enteral angiotensin converting enzyme-inhibitor (ACE-I) and AT1aR antagonist (AT1aR-A) were highly effective for preventing histologic changes, decreasing TNF-α expression and epithelial cell (EC) apoptosis in a mouse dextran sodium sulfate (DSS) model of colitis induced by chemical stimulation [5, 6]. AT1aR predominates and appears to be the critical mediator of pro-inflammatory and pro-apoptotic signaling [3, 7–9]. However, this activity was mainly studied in acute chemically destructive models of colitis. Immunologically driven models of colitis have been in demand for understanding the mechanism of RAS in inflammatory bowel disease (IBD) and use of angiotensinogen knockout mice, AT1aR knockout mice or use of AT1aR-A has led to a marked improvement in the severity of a dextran sodium sulfate (DSS) and trinitrobenzene sulphonic acid-induced colitis [10, 11].
Studies using various animal models of colitis have also shown the importance of intestinal inflammation. The spontaneous development of colitis in the proximal colon will occur in IL-10-deficient mice only sporadically after several months. Since the severity of the disease in this model depends on environmental conditions, animals developing colitis at different times will not develop the same disease. To address these problems, a novel model of a non-steroidal anti-inflammatory drug (NSAID)-induced colitis was reported. The colitis that develops in NSAID-treated IL-10 KO mice is similar to the spontaneous colitis that can develop in IL-10 KO mice. A short course of NSAID treatment rapidly activates intestinal inflammation in this strain of mice as a model of colitis [12–14].
The aim of this study was to determine if blockade of the RAS would be effective in an immunologically relevant colitis model, and to compare this with an acute chemical model.
Materials and methods
Animals
Specific pathogen free, 8- to 10-week-old, 20–22 g C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were kept in a 12-h day–night rhythm at 23 °C and relative humidity of 40–60 % and healthy 5-week-old (35–41 days after birth, body weight around 14–18 g) IL-10-/-mice with a C57BL/6 background (originally obtained from Jackson Laboratories, but now bred at University of Michigan) were used for this study. Animals were fed standard rodent feed (LabDiet 5001 Rodent Diet, PMI Nutrition International, LLC, Brentwood, MO) and water ad libitum. All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan (UCUCA protocol 08773-3).
Induction of colitis
Renin–angiotensin system treatment in acute colitis
Colitis was induced in 8- to 10-week-old C57BL/6J mice using 2.5 % (W/V) reagent-grade dextran sulfate sodium (DSS; Molecular weight; 36,000–50,000, ICN Biomedicals, Inc, Aurora, OH) dissolved in drinking water which was ingested ad libitum for 7 days. Mice were distributed into three study groups (N = 8). Daily transanal doses (0.3 ml) of AT1aR-A, CCG-203025 (100 mg/kg/day) or placebo, phosphate-buffered saline (PBS), were started at the same time as DSS. Body weight was measured daily. Mice were killed on day 7 and colon lesions were assessed macro- and microscopically.
Immunologically relevant colitis
200 ppm of Piroxicam (non-steroidal anti-inflammatory drug: NSAID; Sigma-Aldrich, St Louis, MO) was added to feeds given to 5-week-old IL-10-/-mice (C57BL/6J background) for 14 days (N = 8/group). The NSAID was mixed with rodent powdered feed (NIH-31 M) using geometric dilution to ensure uniform distribution of the NSAID in feed [13]. From week 6 of life, Enalaprilat (12.5 mg/kg, BID), CCG-203025 (50 mg/kg, BID) or PBS were administered transanally for 14 days. After treatment, mice were killed to assess the colon macro- and microscopically. Mucosal mRNA expression of pro-inflammatory cytokines and chemokines were examined using RT-PCR.
Angiotensin II type I receptor antagonist (CCG-203025)
Experimental and Spectroscopic Data
Instrumentation NMR spectra were recorded on a Varian 400 or 500 MHz spectrometer. Chemical shifts were reported in δ (parts per million) by reference to the hydrogenated residues of deuterated solvent as internal standard CDCL3: δ = 7.28 (1H NMR). Mass spectra were recorded on a Micromass LCT time-of-flight instrument utilizing the electrospray ionization mode. Melting points were measured on a MEL-TEMP melting point apparatus and are uncorrected. The purity of compounds was assessed via analytical rpHPLC with a gradient of 10 B to 90 % B over 6 min (solvent A H2O, solvent B acetonitrile, C18 column, 3.5 µm, 4.6 × 100 mm, 254 nm µ). 2-((4-((2-butyl-4-chloro-5-(methoxymethyl)-1H-imidazol-1-yl)methyl)phenyl)-carbamoyl)benzenesulfonic acid HPLC (t R = 4.79 min). 1H NMR (400 MHz, DMSO-d6) δ 11.45 (s, 1H), 7.83–7.85 (m, 1H), 7.74–7.76 (m, 1H), 7.67–7.69 (m, 2H), 7.52–7.55 (m, 2H), 7.13–7.19 (m, 2H), 5.33 (s, 2H), 4.35 (s, 2H), 3.28 (s, 3H), 2.54–2.55 (t, 2H), 1.64–1.67 (m, 2H), 1.33–1.38 (m, 2H), 0.87–0.90 (m, 3H). ESI–MS m/z 489.9 (M–H+) [5] (Fig 1).
Harvesting
Mice were killed 7 days after DSS and 21 days in the immunologically relevant colitis group by carbon dioxide asphyxiation. A 0.5-cm segment was excised from the proximal colon and placed in 10 % formaldehyde. Formalin-preserved sections of distal colon were preserved in paraffin, sectioned transversely (5 μm) and stained with hematoxylin and eosin (HE). The remaining colon was immediately processed for mucosal cell isolation as described elsewhere [5, 6, 15].
Histologic score
Harvested colon specimens were fixed in 10 % phosphate-buffered formalin. Specimens were paraffin-embedded, sectioned, and stained with HIM. Histologic grading of colitis was performed in a masked fashion (investigator blinded to the study group) according to techniques described elsewhere [15]:
-
1.
For DSS colitis mice, crypt shortening and distortion with thickening of the lamina propria by inflammatory infiltrate was assigned a score of 0 (normal) through 4 (complete loss of crypts, ulceration, or severe thickening of the lamina propria). Colitis scores (0–4) from 4 points of a left-sided colon segment were summed to give a maximum score of 16 per segment and a minimum score of 0. The mean of at least 2 segments was the histologic score for each mouse.
-
2.
For IL-10-/-mice, histologic scores ranged from 0 to 10. The maximum score was calculated by summing scores for the following 4 parameters; mucosal ulceration: 0–3 (0 = normal; 1 = surface epithelial inflammation; 2 = erosions; 3 = ulcerations); epithelial hyperplasia: 0–3 (0 = normal; 1 = mild; 2 = moderate; 3 = pseudo polyps); lamina propria mononuclear infiltrate: 0–2 (0 = normal; 1 = slightly increased; 2 = markedly increased); lamina propria neutrophil infiltrate: 0–2 (0 = normal; 1 = slightly increased; 2 = markedly increased) as previously described [14].
Mucosal cell isolation and purification
Isolation of mucosal cells was performed using a protocol previously described [16]. Colon, excluding the cecum, was placed in RPMI cell culture medium on ice, and fecal contents were gently flushed out. Colonic epithelium was isolated for RNA as described [15].
Real-time polymerase chain reaction (RT-PCR)
Mucosal scrapings were placed in Tirol (Invitrogen), homogenized, RNA extracted and purified as described [15]. All primers for selected gene sequences were designed using proprietary software (Laser gene, DNA star Inc, Madison, WI). Real-time PCR (RT-PCR) was performed using a Rotor-Gene 6000 (Corbett Life Science, Sydney, Australia) and β-actin was used as an internal control for normalization. Fold changes of target genes were calculated using comparative quantification to β-actin.
Immunoblot analysis
Techniques employed were similar to those previously described [6, 17]. We used mouse anti–TNF-α (1:200 Santa Cruz Biotechnology, Inc, Santa Cruz, CA), mouse anti-AT1aR (1:200 Santa Cruz Biotechnology, Inc), mouse anti-phosphor-ΙκΒα (1:1000: Cell Signaling Technology Inc, Danvers, MA), mouse anti-ΙκΒα (1:1000 Cell Signaling Technology Inc), rabbit anti-phospho-NFκΒ (1:1000 Cell Signaling Technology Inc) and rabbit anti-NFκΒ (1:2000 Cell Signaling Technology Inc), respectively. Membranes were then washed and incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000: Zymed Laboratories, San Francisco, CA) and goat anti-mouse IgG (1:2000: Santa Cruz Biotechnology, Inc). Results were expressed as the ratio of target density to the density of β-actin expression.
Immunohistochemistry
Immunohistochemical staining was used to determine the expression of AT1aR in each treatment group using avidin–biotin–peroxidase complex (ABC) staining system (Santa Cruz Biotechnology, Inc, Santa Cruz, CA). Primary antibodies (dilution) anti-rabbit AT1aR (1/50) were applied to prepared segments of distal bowel. Slide examination was performed independently in a blind manner using a Nikon A1 microscope (Nikon Instruments Inc, Tokyo, Japan). Staining was scored by counting the number of AT1aR-positive cells per 100 counted cells in the lamina propria under high-power (×200) magnification.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Results were analyzed using the t test for comparison of two means, and a one-way analysis of variance (ANOVA) for comparison of multiple groups. A post hoc Bonferroni test was used to assess statistical difference between groups. The Chi-square test was used for categorical data (Prism software; GraphPad Software, Inc., San Diego, CA). A value of P < 0.05 was considered to be statistically significant.
Results
Effect of AT1aR-A on DSS colitis
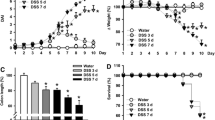
After DSS administration, mice developed colitis, which was manifested by loose stools, intestinal bleeding, and weight loss. Weight changes (reported as percentage change from baseline body weight on day 1) are shown in Fig. 2a. As previously reported [6], significant weight loss occurred toward the end of 1 week of DSS. The difference between the Naïve and the DSS-treated groups became significant after day 5 of DSS (P < 0.01). After 1 week, weight loss was severe in the placebo group (−6.8 ± 3.3 % weight loss), but significantly attenuated by AT1aR-A (CCG-203025: −3.6 ± 2.9 %, P < 0.05 versus placebo).
To evaluate if transanal treatment with AT1aR-A was associated with a reduction in the severity of colitis, a blinded histological score at day 7 was assessed. In the DSS + PBS group, mice consistently developed severe ulcerative lesions in the distal colon. These histologic changes were significantly attenuated in the AT1aR-A-treated mice (P < 0.001). Histopathology and histologic scores are presented in Fig. 2b. The colon of all ATa1R-A-treated mice showed nearly normal mucosal architecture.
Effect of AT1aR-A on pro-Inflammatory cytokine expression
Expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IFNγ) were investigated based on previous work which showed a reduction in the expression of these cytokines after ACE-I and AT1aR-A treatment [5, 15]. These cytokines are also known to be upregulated in the DSS colitis model, and may be responsible for acute tissue injury. Figure 3 shows how mRNA expression of TNF-α and IL-1β was significantly lower in AT1aR-A-treated groups compared with the DSS + PBS group. DSS + placebo mice showed significantly increased levels of all pro-inflammatory cytokines. However, AT1aR-A compound significantly decreased the mRNA expression of these cytokines. Additionally, IL-10 expression was examined as IL-10 can downregulate or completely inhibit the expression of several pro-inflammatory cytokines. Although there were no significant differences in IL-10 expression between DSS + placebo and AT1aR-A groups, there was a trend to high expression in the AT1aR-A-treated group.
Mechanism of AT1aR-A effectiveness
AT1a receptor signaling has been shown to be an important transactivation pathway for effective TNF-α signaling [5]. Therefore, we hypothesized that blockade of AT1aR would downregulate the TNF-α signaling pathway with the expression of mucosal AT1aR, TNF-α downstream (ΙκΒ, NFκΒ) as detected by Western blot techniques. Results were expressed as the ratio of b-actin for immunoblots. Note that AT1aR and TNF precursor were increased in the DSS model, and significantly decreased after AT1aR-A. Administration of AT1aR-A in the DSS model significantly decreased phosphorylated ΙκΒ expression. Furthermore, AT1aR-A significantly prevented phosphorylation of NFκΒ p65 (Fig 4).
Expression of mucosal AT1aR, TNF-α downstream (IκΒ, NFκΒ) as detected by Western blot. Results are expressed the ratio of β-actin for immunoblots. Note that AT1aR and TNF precursor were increased in the DSS model, and significantly decreased with AT1aR-A. Administration of AT1aR antagonist in the DSS model significantly decreased phosphorylated IκΒ expression. AT1aR-A also significantly prevented phosphorylation of NFκΒ p65
Effect of ACE-I and AT1aR-A on immunologically relevant colitis
200 ppm Piroxicam was administered with feed given for the first 14 days and treatments, twice a day via the transanal route, were started after 7 days and continued after piroxicam for a total of 14 days. Weight loss started to decline on day 7 after piroxicam and continued for the remainder of the study. However, after day 14, weight loss was significantly reduced in both treatment groups (Placebo: −20.8 ± 4.3 %, ACE-I: −11.9 ± 7.3, AT1aR-A: −7.0 ± 9.2) (Fig. 5a). Representative histologic sections of mid-colon are shown after HE staining. Microscopically, marked infiltrations of mononuclear cells and polymorphonuclear cells were observed in colon mucosa of control IL-10KO piroxicam-treated mice. Histologic scores were significantly reduced in ACE-I-treated mice (Fig. 5b). Macroscopic findings revealed that the shortening and thickening of the colon were improved in the AT1aR-A and ACE-I groups (Fig. 5c).
a 200 ppm piroxicam was administered by mixing in mouse feed for the first 14 days and treatments, twice a day, administered transanally starting after 7 days and continuing after piroxicam was ceased for a total of 14 days. b Representative HE-stained sections of mid-colon (10× magnification). Microscopically marked infiltrations of mononuclear cells and polymorphonuclear cells are present in colon mucosa from control IL-10KO piroxicam-treated mice. Histologic scores were reduced significantly in ACE-I-treated mice. c Macroscopic findings revealed that the shortening and thickening of the colon were improved in the AT1aR-A and ACE-I groups
Effect of AT1aR-A on pro-inflammatory cytokines and chemokine expression
Inflammatory cytokines were measured as markers of the pro-inflammatory response [18]. Treatments led to a reduction in the abundance of several pro-inflammatory cytokines and chemokines. Expression of mucosal cytokines TNF-α, IL-6, IL-1β, and IFNγ were detected by real-time PCR and these cytokines were significantly decreased in both AT1aR-A and ACE-I treatment groups compared with the placebo group (Fig. 6). Chemokines were also reduced by treatment with AT1aR-A or ACE-I compared with the placebo group (Fig. 7).
Expression of mucosal cytokines TNF-α, IL-6, IL-1β, and IFNγ as detected by real-time PCR. These cytokines were significantly decreased in both the AT1aR-A and ACE-I treatment groups. Results are mean ± SD for a minimum of 6 samples from each group. Comparisons made using ANOVA with post hoc Bonferroni test
Expression of AT1aR in IL-10KO mice confirmed by immunohistochemical staining
AT1aR expression was visualized in IL10 KO mice using immunohistochemical staining. Interestingly, AT1aR was expressed on epithelial cells. A marked increase in AT1aR was noted in the piroxicam-treated IL-10 mice. However, blockade of this receptor with AT1aR-A and ACE-I, prevented an increase in AT1aR expression predominately in the lamina propria layer. A summary of mean AT1aR-positive cell rates per 100 counted cells in the lamina propria showed a significantly decreased number of positive cells in both treatment groups (Fig. 8).
Discussion
In a previous study, we demonstrated that ACE-I and AT1aR-A markedly decreased pro-inflammatory cytokines in a DSS model, and cytokine levels measured in this study were almost consistent with those results. Here, we used an AT1aR-A compound and ACE-I to determine the role of AT1aR in a DSS-induced colitis model and immunologically relevant colitis model. We demonstrated the efficacy of high-dose RAS treatment for preventing histologic changes, colonic apoptosis, and increased pro-inflammatory cytokine response in these colitis models.
However, there are several differences between the DSS and IL-10 KO models used in this study. The DSS model is a chemical-induced acute colitis; whereas, the IL-10 KO model is an immunologic model. Thus, cytokine levels in the immunologic model may be subjected to different controls when presented with ACE-I and AT1aR-A treatments. In addition, mice in the DSS model were 8–10 weeks old and in the IL10-KO model were 5 weeks old. This age difference could also account for differences in cytokine response. In addition, being an immunologic model means that the response of the host immune system may not be natural irrespective of the presence of colitis.
Several authors have used a DSS colitis model to investigate RAS blockade in colitis [3, 5, 6, 19]. In the present study, we utilized an IL10KO colitis immune-based model and demonstrated that ACE-I and AT1aR-A treatments were as good, or superior, to placebos. AT1aR expression was markedly increased in the DSS model. We examined the expression of this receptor in IL10 KO mice using immunohistochemistry staining. Interestingly, AT1aR expression was identified on epithelial cells with a marked increase in AT1aR noted in piroxicam-fed IL-10 mice. However, blockade of this receptor with AT1aR-A and ACE-I prevented the increase in AT1aR expression predominately in the lamina propria layer.
There are two major ATII receptors, the AT1R (including the 1a and 1b sub-types) and AT2R. AT1aR is best known to mediate vasoconstriction, and is, therefore, used for treating hypertension. In addition to its anti-hypertensive actions, AT1aR-A has been shown to strongly suppress the generation of reactive oxygen species induced by ATII in activated leukocytes [8, 20–22] and reduce production of pro-inflammatory cytokines and adhesion molecules induced by ATII. These RAS actions may also be critical for mediation of ADAM (a disintegrin and metalloproteinase) function as signaling by AT1aR is needed for transactivation of TNF-α signaling in many cell types [23]. Interestingly, pharmacologic blockade of ADAM is becoming a potential clinical approach of blocking TNF-α signaling [24].
The mechanisms by which blockade of the RAS are not fully understood. Initial interest in the RAS in the gastrointestinal tract is based on the fact that several components of the RAS are expressed strongly in the small and large intestine of rodents and humans [3, 6, 15, 17, 25–27]. ATII activation of NFκB has been shown in human monocytes [28] and in cultured vascular smooth muscle cells [29]. Based on this, the NFκΒ activation pathway has emerged as an extremely attractive target for the development of anti-inflammatory drugs [30–33]. Although the function of ATII in the intestine is not well understood, our laboratory has previously shown that ACE is expressed in the intestinal epithelium, increases in a rodent colitis model, and is critically important in promoting the development of intestinal epithelial cell apoptosis [3, 15, 17, 19]. However, ACE-I and AT1aR-A have been shown to have a variety of anti-inflammatory effects on a series of pro-inflammatory and growth signaling actions that occur after ACE-mediated conversion of angiotensin I to angiotensin II. Downstream signals from this receptor include an upregulation of MAdCAM-1 under colonic inflammatory conditions via NFκΒ translocation into the nucleus and subsequent translocation. As TNF-α is a key cytokine mediating inflammation in colitis, we examined mucosal regulators of TNF activation. In general, the conversion of inactive TNF into an active form requires the cleavage of the transmembrane TNF-α precursor to generate the soluble active form of TNF-α. This action is performed by TNF-α converting enzyme ADAM17, a transmembrane metalloprotease. Conversely, TIMP3 (tissue inhibitor of metalloproteinase-3) is a secreted protein that negatively controls ADAM17 activity. As a result, a balance between ADAM17 and TIMP3 expression may be important in timely regulation of the inflammatory response. As both TNF-α and AT1a receptor signaling have a common downstream activation of NFκΒ, we examined for the expression of NFκΒ using immunoblot analysis. Not surprisingly, we showed that AT1aR and TNF precursors were increased in the DSS model, and that they were significantly decreased after AT1aR-A. Administration of AT1aR-A in the DSS model significantly decreased phosphorylated ΙκΒ expression and significantly prevented phosphorylation of NFκΒ p65.
Although the AT1aR-A used in this study, CCG-203025, is a Deschloro-losartan (DCL) analog that is poorly absorbed orally [5], it produced significant clinical and histological improvements in both acute and immunologically relevant colitis models; it is important to note that both the pro-inflammatory cytokine response and histopathology were not treated by AT1aR-A treatment. Thus, other mechanisms not affected by ACE-I and AT1aR-A are likely to be responsible for inflammation and a more comprehensive study using various dosages may be needed to explore the full efficacy of this class of drugs.
In conclusion, this study demonstrates the therapeutic efficacy of both ACE-I and AT1aR-A for preventing the development of both acute and immunologically relevant colitis. The differential expression of ACE and AT1aR in the bowel may account for the different actions of the two compounds in the two models. Thus, specially designed AT1R-A compounds with poor oral absorption, such as CCG-203025, may have potential as new therapeutic agents that block RAS for safely treating inflammatory bowel disease. Future work will be needed to optimize ACE-I and AT1aR-A dosage and route of administration to understand the mechanisms that lead to their anti-inflammatory activity to prevent ischemic colitis and hypotension.
References
Paul M, Poyan Mehr A, Kreutz R (2006) Physiology of local renin-angiotensin systems. Physiol Rev 86(3):747–803
Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M (2005) Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation 111(19):2509–2517
Koga H, Yang H, Haxhija EQ, Teitelbaum DH (2008) The role of angiotensin II type 1a receptor on intestinal epithelial cells following small bowel resection in a mouse model. Pediatr Surg Int 24(12):1279–1286
Marchesi C, Paradis P, Schiffrin EL (2008) Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29(7):367–374
Okawada M, Koga H, Larsen SD, Showalter HD, Turbiak AJ, Jin X, Lucas PC, Lipka E, Hillfinger J, Kim JS, Teitelbaum DH (2011) Use of enterally delivered angiotensin II type Ia receptor antagonists to reduce the severity of colitis. Dig Dis Sci 56(9):2553–2565
Koga H, Yang H, Adler J, Zimmermann E, Haxhijia E, Teitelbaum D (2008) Angiotensin converting enzyme inhibitor prevents colonic fibrosis in a mouse colitis model: development of a unique mode of treatment. Surgery 144(2):259–268
Li X, Rayford H, Uhal BD (2003) Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol 163(6):2523–2530
Okuda T, Yoshida N, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y, Yoshikawa T (2008) CV-11974, angiotensin II type I receptor antagonist, reduces the severity of indomethacin-induced rat enteritis. Dig Dis Sci 53(3):657–663
Santiago O, Rivera E, Ferder L, Appleyard C (2008) An angiotensin II receptor antagonist reduces inflammatory parameters in two models of colitis. Regul Pept 146(1–3):250–259
Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S (2005) Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut 54(3):349–356
Sasaki M, Wada T, Mizushima T, Ogasawara N, Kubota E, Mori Y, Shimura T, Mizoshita T, Okamoto Y, Kataoka H, Kamiya T, Joh T (2007) Amelioration of dextran sulfate sodium induced colitis in Angiotensin II Type 1 receptor knockout mice. Gastroenterology 131:M1671
Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG (2002) Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 123(5):1527–1542
Narushima S, DiMeo D, Tian J, Zhang J, Liu D, Berg DJ (2008) 5-Lipoxygenase-derived lipid mediators are not required for the development of NSAID-induced inflammatory bowel disease in IL-10-/-mice. Am J Physiol Gastrointest Liver Physiol 294(2):G477–G488
Kamada N, Inoue N, Hisamatsu T, Okamoto S, Matsuoka K, Sato T, Chinen H, Hong K, Yamada T, Suzuki Y, Suzuki T, Watanabe N, Tsuchimoto K, Hibi T (2005) Nonpathogenic Escherichia coli strain Nissle 1917 prevents murine acute and chronic colitis. Inflamm Bowel Dis 11(5):455–463
Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH (2007) Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci 52(4):1060–1070
Yang H, Antony PA, Wildhaber BE, Teitelbaum DH (2004) Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172(7):4151–4158
Wildhaber BE, Yang H, Haxhija EQ, Spencer AU, Teitelbaum DH (2005) Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis 10(6):1305–1315
Sueyoshi R, Ignatoski KM, Daignault S, Okawada M, Teitelbaum DH (2013) Angiotensin converting enzyme-inhibitor reduces colitis severity in an IL-10 knockout model. Dig Dis Sci 58(11):3165–3177
Haxhija E, Yang H, Spencer A, Koga H, Sun X, Teitelbaum D (2008) Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin converting enzyme. Amer J Physiol Gastrointestinal Liver Physiol 295(1):G88–G98
El Bekay R, Alvarez M, Monteseirin J, Alba G, Chacon P, Vega A, Martin-Nieto J, Jimenez J, Pintado E, Bedoya FJ, Sobrino F (2003) Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood 102(2):662–671
Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, Mohanty P, Tripathy D, Garg R (2003) Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metabo 88(9):4496–4501
Raiden S, Nahmod K, Nahmod V, Semeniuk G, Pereira Y, Alvarez C, Giordano M, Geffner JR (2002) Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J Pharmacol Exp Ther 303(1):45–51
Ohtsu H, Dempsey P, Eguchi S (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 291:C1–C10
Moss M, Sklair-Tavron L, Nudelman R (2008) Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol 4(6):300–309
Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, Camilleri JP, Bariety J (1986) Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry 85(1):73–80
Danilov SM, Faerman AI, Printseva O, Martynov AV, Sakharov I, Trakht IN (1987) Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry 87(5):487–490
Wildhaber B, Teitelbaum D (2003) Intestinal intraepithelial lymphocytes express angiotensin converting enzyme—a role for controlling epithelial cell apoptosis. Gastroenterology 124(4):A597
Kranzhofer R, Browatzki M, Schmidt J, Kubler W (1999) Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun 257(3):826–828
Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J (2000) Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res 86(12):1266–1272
May MJ, Marienfeld RB, Ghosh S (2002) Characterization of the Ikappa B-kinase NEMO binding domain. J Biol Chem 277(48):45992–46000
Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F (2002) Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int 61(6):1986–1995
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336(15):1066–1071
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107(1):7–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okawada, M., Wilson, M.W., Larsen, S.D. et al. Blockade of the renin–angiotensin system prevents acute and immunologically relevant colitis in murine models. Pediatr Surg Int 32, 1103–1114 (2016). https://doi.org/10.1007/s00383-016-3965-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-3965-3