Abstract
Purpose
Pulmonary hypoplasia (PH), characterized by alveolar immaturity, is one of the leading causes of respiratory insufficiency in newborns with congenital diaphragmatic hernia (CDH). Leptin (Lep) and its receptor (Lep-R) play an important role in fetal lung growth by stimulating alveolar differentiation and maturation. Lep and Lep-R are strongly expressed by alveolar cells during the saccular stage of fetal lung development. Lep-deficient mice exhibit decreased alveolarization with reduced pulmonary surfactant phospholipid synthesis, similar to human and nitrofen-induced PH. Prenatal administration of all-trans retinoic acid (ATRA) has been shown to stimulate alveolarization in nitrofen-induced PH. Recent studies have demonstrated that Lep and Lep-R expression in developing lungs is regulated by ATRA. We hypothesized that prenatal treatment with ATRA increases pulmonary Lep and Lep-R expression in the nitrofen model of CDH-associated PH.
Methods
Time-mated rats received either 100 mg nitrofen or vehicle via oral-gastric lavage on embryonic day 9.5 (E9.5). Control and nitrofen-exposed dams were randomly assigned to either intraperitoneal ATRA (5 mg/kg/d) or placebo administration on E18.5, E19.5 and E20.5. Fetal lungs were harvested on E21.5, and divided into Control+Placebo, Control+ATRA, Nitrofen+Placebo and Nitrofen+ATRA. Alveolarization was assessed using stereo- and morphometric analysis techniques. Surfactant phospholipid synthesis was analyzed by labeling for surfactant protein B (SP-B). Pulmonary gene expression levels of Lep and Lep-R were determined using quantitative real-time polymerase chain reaction. Immunohistochemical staining for Lep and Lep-R was performed to evaluate alveolar protein expression and localization.
Results
In vivo administration of ATRA resulted in significantly increased lung-to-body weight ratio with enhanced radial alveolar count and decreased mean linear intercept compared to placebo treatment. Immunofluorescence analysis demonstrated markedly increased pulmonary SP-B expression in Nitrofen+ATRA compared to Nitrofen+Placebo. Relative mRNA expression of Lep and Lep-R was significantly increased in Nitrofen+ATRA compared to Nitrofen+Placebo. Lep and Lep-R immunoreactivity was markedly increased in interstitial and alveolar epithelial cells of Nitrofen+ATRA compared to Nitrofen+Placebo.
Conclusion
Increased Lep and Lep-R expression after prenatal administration of ATRA in nitrofen-induced PH suggests that ATRA may have therapeutic potential in attenuating CDH-associated PH by stimulating alveolarization and de novo surfactant production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) is a relatively common embryological defect in the integrity of the developing diaphragm, which affects approximately 2.5 per 10.000 live births each year [1, 2]. Although numerous chromosomal aberrations and gene mutations have been linked to CDH, the etiology of the diaphragmatic defect is identified in less than 50 % of patients [3]. Some newborns with CDH have relatively mild disease, whereas others suffer from life-threatening respiratory insufficiency, which affects both lungs and results from a combination of severe pulmonary hypoplasia (PH) and persistent pulmonary hypertension [4]. Despite improvements in postnatal resuscitation and lung-protective treatment, CDH remains one of the major therapeutic challenges in neonatal intensive care, causing high mortality and long-term morbidity for survivors [5, 6].
The majority of our current knowledge about the pathogenesis of CDH has originated from experimental animal studies [7]. Administration of the herbicide nitrofen (2,4-dichlorophenyl-p-nitrophenyl ether) to pregnant rodents during midgestation has been shown to result in CDH in approximately 70 % and PH in 100 % of the offspring [8]. This teratogenic model is widely used in order to investigate the fundamental developmental and physiological defects of CDH to understand their effects on diaphragm and lung morphogenesis, as timing of the diaphragmatic insult and bilateral PH is remarkably similar to the human condition [9].
One of the most biologically active metabolites of vitamin A, all-trans retinoic acid (ATRA), is an essential component of the complex gene network that regulates fetal lung morphogenesis and diaphragmatic development [10]. Recent findings from animal research have indicated that disruption of retinoid signaling contributes to the formation of CDH and associated lung hypoplasia [11]. Furthermore, it has been demonstrated that administration of ATRA during late gestation upregulates pulmonary expression of several genes involved in the retinoic acid signaling pathway [12]. It has also been shown that ATRA reduces the severity of PH in nitrofen-induced hypoplastic lung explants [13]. Additional evidence that prenatal administration of ATRA stimulates alveolarization in hypoplastic lungs has been provided by in vivo studies in rats with nitrofen-induced CDH [14], suggesting that ATRA may have a therapeutic potential in attenuating CDH-associated PH. However, further research is needed to establish the exact molecular and cellular effects of ATRA treatment on fetal alveolar development.
Leptin (Lep), a 16-kDa peptide product of the ob gene and its receptor (Lep-R) play an important role in fetal lung growth by stimulating alveolar maturation and de novo synthesis of surfactant phospholipids [15]. In rats, Lep and Lep-R are strongly expressed by distal alveolar cells during the saccular stage of fetal lung development with a 7- to 10-fold increase prior to term (day 22) [15, 16]. Lep-deficient mice exhibit decreased alveolarization with reduced pulmonary surfactant phospholipid synthesis [17], similar to human and nitrofen-induced PH. Previous studies have demonstrated that Lep and Lep-R expression in developing lungs is regulated by retinoid signaling [18].
We designed this study to investigate the hypothesis that prenatal administration of ATRA increases Lep and Lep-R expression in hypoplastic lungs of nitrofen-induced CDH.
Materials and methods
Animal model, drugs and experimental design
After obtaining ethical approval (Ref. REC668b) from the local research ethics committee, pathogen-free Sprague–Dawley® rats (Harlan Laboratories, Shardlow, UK) were kept in a well-controlled environment (50–55 % humidity, 19–21 °C, 12-h light period, food and water ad libitum). Following acclimatization, animals were mated overnight and females were checked daily for presence of a vaginal plug. The day of plugging was defined as embryonic day 0.5 (E0.5) and timed-pregnant subjects were randomly divided into two experimental groups (“Nitrofen” and “Control”). On E9.5, dams were briefly anesthetized with 2 % volatile isoflurane (Piramal Healthcare Ltd, Morpeth, UK) and 100 mg nitrofen (2,4-dichlorophenyl-p-nitrophenyl ether) (Wako Chemicals GmbH, Neuss, Germany) was administered in 1 ml olive oil via oral-gastric lavage, whereas control animals received vehicle alone. Five milligram/kg ATRA (Sigma Aldrich, Saint Louis, USA) was dissolved in 1 ml cottonseed oil and injected intraperitoneally under short anesthesia on E18.5, E19.5 and E20.5, whereas control rats received solvent alone. On the selected end point E21.5 (alveolar phase), fetuses were delivered via caesarean section, weighed and killed by decapitation. After laparotomy, fetuses were inspected under a Leica S8AP0 stereomicroscope (Leica Microsystems AG, Heerbrugg, Switzerland) for diaphragmatic defects and whole lungs were microdissected under sterile conditions. All explants were weighed before fixation. Specimens for morphometric and immunohistochemical/-fluorescence analysis were fixed in 4 % paraformaldehyde (PFA) (Santa Cruz Biotechnology Inc, Santa Cruz, USA) for 24 h, whereas specimens for RNA isolation and subsequent quantitative real-time polymerase chain reaction (qRT-PCR) were snap-frozen in liquid nitrogen (and stored at −80 °C). To obtain a representative number of samples, fetuses in each experimental group originated from at least three different dams (4–6 /l). In total, 64 fetal lungs were used for this study, which can be divided into Control+Placebo (n = 16), Control+ATRA (n = 16), Nitrofen+Placebo (n = 16) and Nitrofen+ATRA (n = 16).
All animal procedures were performed following current guidelines for management and welfare of laboratory animals and were approved by the Department of Health and Children (Ref. B100/4378) under the Cruelty to Animals Act, 1876 (as amended by European Communities Regulations 2002 and 2005).
Preparation of lung specimens for morphometric and immunohistochemical/-fluorescence analysis
The PFA-fixed lung specimens were washed overnight in ice-cold phosphate buffered saline (PBS) (Oxide Ltd, Basingstoke, UK) to remove exterior debris, embedded in O.C.T. compound mounting medium (VWR International Ltd, Dublin, Ireland) and snap-frozen in liquid nitrogen. Frozen blocks were stored at −80 °C until cryosectioning was performed. All lung specimens were cut transversely at a thickness of 10 μm using a CM1900 cryostat (Leica Microsystems GmbH, Nussloch, Germany) at −20 °C and serial sections were mounted on SuperFrost® Plus microscopy glass slides (VWR International Ltd, Dublin, Ireland).
Assessment of lung morphometry
Two independent-blind investigators unaware of the experimental group performed lung morphometry, which was objectively assessed by determining radial alveolar count (RAC) and mean linear intercept (MLI) on hematoxylin- and eosin-stained (Sigma Aldrich, Saint Louis, USA) sections. Fifty randomly selected, non-overlapping fields from serial sections were investigated under a Leica DM LB research microscope (Leica Microsystems GmbH, Wetzlar, Germany). Each field was viewed at 40-fold magnification, and the image was digitized and projected on a computer screen using a Leica DC300F digital camera (Leica Microsystems AG, Heerbrugg, Switzerland). For each field, the number of alveoli was counted visually and RAC was performed by identifying respiratory bronchioles, as described by Randell et al. [19]. Briefly, the number of distal air sacs that were transacted by a line drawn from a terminal respiratory bronchiole to the nearest pleural surface was counted. No counts were made if the respiratory bronchiole was nearer to the edge of the slide than to the nearest connective tissue septum. The MLI represents the average alveolar diameter, alveolar septal thickness and tissue density, which is the proportion of the field occupied by tissue (area occupied by tissue/area occupied by lung tissue + alveoli). All images were analyzed with ImageJ 1.47a (National Institute of Health, Bethesda, USA), a public domain, Java™-based image processing and analysis software program.
Determination of surfactant phospholipid synthesis
Surfactant phospholipid synthesis was determined by labelling for surfactant protein B (SP-B), which plays an essential role in alveolar stability and thus contributes to the biophysiological function of the lung. Thawed frozen sections were incubated with PBS containing 1.0 % Triton X-100 (Sigma Aldrich Ltd, Arklow, Ireland) for 20 min to improve cell permeabilization. To prevent nonspecific absorption, sections were blocked with 10 % normal goat serum (Sigma Aldrich, Saint Louis, USA) for 30 min, followed by incubation with affinity-purified rabbit polyclonal anti-SP-B antibodies (sc-7702-R, 1:100) (Santa Cruz Biotechnology Inc, Santa Cruz, USA) at 4 °C overnight. On the next day, sections were washed in PBS+0.05 % Tween and incubated with Alexa Fluor® 647 goat anti-rabbit secondary antibodies (A21244, 1:200) (Bio-Sciences Ltd, Dun Laoghaire, Ireland) at room temperature for 30 min. The sections were counterstained with DAPI (10236276001, 1:1000) (Roche Diagnostics GmbH, Mannheim, Germany) for 10 min to visualize double-stranded DNA. Following coverslipping with fluorescent mounting medium (DAKO Ltd, Cambridgeshire, UK), two investigators independently evaluated the sections with a LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany). All images were analyzed with ZEN (Carl Zeiss MicroImaging GmbH, Jena, Germany), an image processing and analysis software program.
Total RNA isolation and complementary DNA synthesis
Total RNA was isolated from snap-frozen lung specimens with the acid guanidinium thiocyanate-phenol-chloroform extraction method using a TRIzol® reagent (Invitrogen™ by Life Technologies™, Carlsbad, USA) according to the manufacturer’s protocol. Concentration and purity of total RNAs were determined spectrophotometrically using a NanoDrop ND-1000 UV-vis® system (Thermo Scientific Fisher, Wilmington, USA). Reverse transcription of 1 µg total RNA was carried out using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol.
Quantitative real-time polymerase chain reaction
Lep and Lep-R mRNA expression was quantified with a LightCyler® 480 system using a SYBR® Green I Master Mix (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. Primer sequences for target and reference genes were designed with the online tool Primer3 (http://primer3.ut.ee) using rat nucleotide sequences from the GenBank® database (http://www.ncbi.nlm.nih.gov/genbank). A genomic service provider (Eurofins MWG Operon, Ebersberg, Germany) synthesized all selected primers. Gene-specific primer sequences are listed in Table 1. qRT-PCR conditions were 95 °C for 5 min, followed by 55 amplification cycles at 95 °C for 10 s, 60 °C for 15 s and 72 °C for 10 s. Relative mRNA expression levels were determined using the comparative cycle threshold method and results were normalized to the expression of our housekeeping gene β-actin. All qRT-PCR experiments were run in duplicate for each sample and primer pair.
Immunohistochemical staining
The distribution of alveolar Lep and Lep-R proteins was evaluated by specific immunohistochemical staining to localize their exact cellular expression. Thawed frozen sections were incubated with PBS containing 1.0 % Triton X-100 (Sigma Aldrich, Saint Louis, USA) for 20 min to improve cell permeabilization. To avoid masking of antigenic sites, sections were immersed in heated Target Retrieval Solution® (DAKO Ltd, Cambridgeshire, UK) in a microwave oven at 750 W for 15 min. Endogenous peroxidase activity was blocked using Peroxidase Block® (DAKO Ltd, Cambridgeshire, UK) according to the manufacturer’s protocol for 5 min. To prevent nonspecific absorption, sections were blocked with 10 % normal goat serum (Sigma Aldrich, Saint Louis, USA) for 30 min, followed by incubation with affinity-purified rabbit polyclonal anti-Lep (ab3583, 1:100) and anti-Lep-R (ab5593, 1:250) antibodies (Abcam plc, Cambridge, UK) at 4 °C overnight. On the next day, sections were washed in PBS + 0.05 % Tween and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies (K4011, 1:100) (DAKO Ltd, Cambridgeshire, UK) at room temperature for 30 min. The antibody-antigen complexes were then visualized by staining with diaminobenzidine (DAB) + Substrate Buffer® and DAB + Chromogen® (DAKO Ltd, Cambridgeshire, UK) for 30 s. After counterstaining with hematoxylin (Sigma Aldrich, Saint Louis, USA) for 10 s, sections were coverslipped using DPX Mountant for histology (Sigma Aldrich, Saint Louis, USA). All sections were independently evaluated by two investigators with a Leica DM LB research microscope (Leica Microsystems GmbH, Wetzlar, Germany) using the image processing and analysis software program Leica IM50 version 1.20 (Leica Microsystems AG, Heerbrugg, Switzerland).
Statistical analysis
Data was analyzed using GraphPad Prism 5 (GraphPad Software Inc, La Jolla, USA) and tested for Gaussian distribution with a Kolmogorov–Smirnov test. All results are presented as mean ± SEM. To determine any statistical differences between the four experimental groups, one-way ANOVA with Tukey’s test for post-test analysis was performed. A P value < 0.05 was considered as statistically significant.
Results
Effect of in vivo treatment with ATRA on lung-to-body weight ratio
Since body and lung weight is a reflection of overall development, the in vivo effect of ATRA on body and lung weight was analyzed in E21.5 fetuses. There was a significant increase in lung-to-body weight ratio (2.14 ± 0.03 % vs. 1.78 ± 0.05 %; P < 0.05) after prenatal administration of ATRA in Nitrofen+ATRA compared to Nitrofen+Placebo group.
Effect of in vivo treatment with ATRA on lung morphometry
Morphometric analysis of fetal lungs on E21.5 revealed a significant advance in alveolar development after prenatal administration of ATRA. Nitrofen-exposed fetuses that received ATRA application shortly before birth showed an enhancement of alveolarization, which was expressed in a significant increase in RAC (6.66 ± 1.3 vs. 5.70 ± 1.2 per mm2; P < 0.0001) and decrease in MLI (42.44 ± 1.5 vs. 45.06 ± 1.3 μm; P < 0.0001) compared to Nitrofen+Placebo-treated fetuses, whereas there was no significant difference between Control+Placebo and Control+ATRA group (Fig. 1).
Effect of in vivo treatment with ATRA on lung morphometry. Prenatal administration of ATRA resulted in nitrofen-exposed fetuses in a significantly increased radial alveolar count (a) and decreased mean linear intercept (b) on E21.5 compared to placebo-treated lungs (***P < 0.0001, vs. Nitrofen+Placebo)
Effect of in vivo treatment with ATRA on surfactant phospholipid synthesis
Immunoflurescence staining with SP-B was performed to evaluate surfactant phospholipid synthesis in fetal lungs on E21.5. Confocal laser scanning microscopy demonstrated markedly increased alveolar SP-B protein expression after prenatal administration of ATRA in Nitrofen+ATRA compared to Nitrofen+Placebo group (Fig. 2).
Effect of in vivo treatment with ATRA on surfactant phospholipid synthesis. Prenatal administration of ATRA resulted in nitrofen-exposed fetuses in a markedly increased alveolar SP-B staining on E21.5, as determined by specific labelling for the surfactant phospholipid marker SP-B. Representative cryostat sections of fixed lung tissue stained with SP-B antibodies (red staining) and DAPI (blue staining) are shown (Magnification ×40)
Effect of in vivo treatment with ATRA on pulmonary gene expression levels of Lep and Lep-R
As Lep and its receptor Lep-R are both molecular markers characterizing alveolar maturation, pulmonary gene expression levels of Lep and Lep-R were analyzed by qRT-PCR. Nitrofen-exposed lungs of fetuses that received ATRA application shortly before birth exhibited on E21.5 a significantly increased relative mRNA expression of Lep (4.65 ± 0.67 vs. 2.38 ± 0.67; P < 0.05) and Lep-R (1.73 ± 0.10 vs. 0.83 ± 0.12; P < 0.05) compared to Nitrofen+Placebo group.
Effect of in vivo treatment with ATRA on alveolar protein expression of Lep and Lep-R
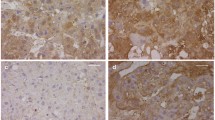
To determine whether the increased amounts of pulmonary Lep and Lep-R transcripts after prenatal administration of ATRA were also translated to the protein level, alveolar Lep and Lep-R protein expression was evaluated in E21.5 lungs by labeling with specific Lep and Lep-R antibodies. Light microscopy confirmed the qRT-PCR results showing markedly increased Lep and Lep-R immunoreactivity in interstitial and alveolar epithelial cells of Nitrofen+ATRA compared to Nitrofen+Placebo group (Fig. 3).
Effect of in vivo treatment with ATRA on alveolar protein expression of Lep and Lep-R. Prenatal administration of ATRA resulted in nitrofen-exposed fetuses in a markedly increased immunoreactivity in interstitial and alveolar cells compared to placebo-treated lungs. Representative cryostat sections of fixed lung tissue stained with Lep or Lep-R antibodys (brown staining) and hematoxylin (blue staining) are shown (Magnification ×40)
Discussion
Formation of primordial alveolar air sacs and maturation of distal airspaces are spatiotemporally orchestrated by a myriad of molecular and cellular interactions, which in turn stimulate fetal alveolar growth [20]. Based on findings from experimental animal studies, it has been suggested that decreased alveolarization and reduced surfactant phospholipids synthesis contribute to the development of PH in CDH [21, 22]. The retinoid signaling pathway has been shown to play a central role during this critical period of fetal lung development [23], and it is widely accepted that its disruption results in pulmonary immaturity and diaphragmatic defects [10]. Furthermore, it has been demonstrated that prenatal administration of ATRA, a biologically active metabolite within the retinoid signaling pathway, accelerates the proliferation of alveolar cells and thus attenuates PH in nitrofen-induced CDH [24]. In this study, we observed that in vivo treatment with ATRA resulted in a significantly increased lung-to-body weight ratio with enhanced radial alveolar count and decreased mean linear intercept compared to placebo treatment. These results are consistent with previous studies from our laboratory, demonstrating that prenatal administration of ATRA stimulates alveolarization in nitrofen-induced hypoplastic lungs [13, 14]. However, the exact molecular and cellular effects of ATRA treatment during fetal alveolar growth remain poorly understood.
In recent years, Lep has been found to have a central role in the stimulation of pulmonary development [25]. The lungs are one of the few organs in the fetus that express Lep and its functional receptor Lep-R [26], which are mutually expressed by lipid-containing interstitial fibroblasts (accounting for approximately 50 % of resident alveolar wall cells in immature lungs) and alveolar epithelial cells type II (AECII) [15]. The expression of Lep and Lep-R has been shown to arise just before the onset of AECII maturation, beginning during the late canalicular stage of fetal lung development with a significant increase over the last few days of gestation [15, 16]. In a recent study, it has been demonstrated that Lep upregulates the intracellular expression and extracellular secretion of surfactant proteins in AECII [27]. Additionally, Lep-deficient mice exhibit decreased alveolarization with reduced pulmonary surfactant phospholipid synthesis [17]. Kirwin et al. [28] have reported that Lep increases the maturation of AECII and expression of surfactant protein B, which is accompanied by an increase in fetal lung weight. These findings highlight the important role of Lep and Lep-R during prenatal lung growth in regulating alveolar differentiation and de novo surfactant production, suggesting these as potential physiological markers of fetal lung maturity [27, 28]. Previous studies have indicated that Lep and Lep-R expression in developing lungs is regulated by retinoid signaling [18]. In the present study, we found significantly increased pulmonary mRNA levels of Lep and Lep-R after in vivo treatment with ATRA compared to Nitrofen+Placebo group, which was accompanied by a strikingly increased SP-B expression in AECII. Our study also demonstrated that Lep and Lep-R immunoreactivity was markedly increased in interstitial and alveolar epithelial cells of Nitrofen+ATRA compared to Nitrofen+Placebo group. These results confirmed that the quantitative increases in Lep and Lep-R mRNA transcripts were also translated to the protein level, indicating that ATRA upregulates Lep signaling in hypoplastic rat lungs. In immature lungs, ATRA is utilized by alveolar cells for regulation of many retinoid-responsive genes, thus contributing to the formation of alveolar septa [29] and surfactant protein synthesis in AECIIs [30]. As normal functioning alveolar cells are able to synthesize ATRA [18], it is not surprising that we did not find differences between Control+ATRA and Control+Placebo group.
The use of retinoids during pregnancy is controversial and currently restricted by the Food and Drug Administration because of its teratogenic side effects on neuronal and cardiovascular development of the embryo [31]. However, it has been reported that pregnant women with acute leukemia have been successfully treated with ATRA during the third trimester of pregnancy with no adverse effects on the newborn [32]. This allows a possible time window for its use in pregnant women during late gestation when alveolarization of fetal lungs begins.
In conclusion, we have provided for the first time convincing evidence that prenatal administration of ATRA upregulates Lep signaling in nitrofen-induced hypoplastic rat lungs, which in turn stimulates surfactant phospholipid synthesis. These findings suggest that ATRA may have a therapeutic potential in attenuating CDH-associated PH by stimulating alveolarization and de novo surfactant production. However, further studies in translational research are required to improve our understanding of the molecular and cellular effects of ATRA treatment on fetal alveolar development.
References
Gallot D, Boda C, Ughetto S et al (2007) Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 29(3):276–283
Loane M, Dolk H, Kelly A et al (2011) Paper 4: EUROCAT statistical monitoring: identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91(Suppl 1):S31–S43
Slavotinek AM (2014) The genetics of common disorders—Congenital diaphragmatic hernia. Eur J Med Genet 57(8):418–423
Keijzer R, Puri P (2010) Congenital diaphragmatic hernia. Semin Pediatr Surg 19(3):180–185
Peetsold MG, Heij HA, Kneepkens CM et al (2009) The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatr Surg Int 25(1):1–17
Rocha G, Azevedo I, Pinto JC et al (2012) Follow-up of the survivors of congenital diaphragmatic hernia. Early Hum Dev 88(4):255–258
Chiu PP (2014) New insights into congenital diaphragmatic hernia—a surgeon’s introduction to CDH animal models. Front Pediatr 2:36
Noble BR, Babiuk RP, Clugston RD et al (2007) Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am J Physiol Lung Cell Mol Physiol 293(4):L1079–L1087
van Loenhout RB, Tibboel D, Post M et al (2009) Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology 96(3):137–149
Montedonico S, Nakazawa N, Puri P (2008) Congenital diaphragmatic hernia and retinoids: searching for an etiology. Pediatr Surg Int 24(7):755–761
Clugston RD, Zhang W, Alvarez S et al (2010) Understanding abnormal retinoid signaling as a causative mechanism in congenital diaphragmatic hernia. Am J Respir Cell Mol Biol 42(3):276–285
Doi T, Sugimoto K, Puri P (2009) Up-regulation of COUP-TFII gene expression in the nitrofen-induced hypoplastic lung. J Pediatr Surg 44(2):321–324
Montedonico S, Nakazawa N, Puri P (2006) Retinoic acid rescues lung hypoplasia in nitrofen-induced hypoplastic foetal rat lung explants. Pediatr Surg Int 22(1):2–8
Montedonico S, Sugimoto K, Felle P et al (2008) Prenatal treatment with retinoic acid promotes pulmonary alveologenesis in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg 43(3):500–507
Torday JS, Sun H, Wang L et al (2002) Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol Lung Cell Mol Physiol 282(3):L405–L410
Henson MC, Swan KF, Edwards DE et al (2004) Leptin receptor expression in fetal lung increases in late gestation in the baboon: a model for human pregnancy. Reproduction 127(1):87–94
Tankersley C, Kleeberger S, Russ B et al (1996) Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81(2):716–723
McGowan SE, Harvey CS, Jackson SK (1995) Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Physiol 269(4 Pt 1):L463–L472
Randell SH, Mercer RR, Young SL (1989) Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat 186(1):55–68
Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141(3):502–513
Alfanso LF, Arnaiz A, Alvarez FJ et al (1996) Lung hypoplasia and surfactant system immaturity induced in the fetal rat by prenatal exposure to nitrofen. Biol Neonatol 69(2):94–100
Utsuki T, Hashizume K, Iwamori M (2001) Impaired spreading of surfactant phospholipids in the lungs of newborn rats with pulmonary hypoplasia as a model of congenital diaphragmatic hernia induced by nitrofen. Biochim Biophys Acta 1531(1–2):90–98
Simon DM, Mariani TJ (2007) Role of PPARs and retinoid X receptors in the regulation of lung maturation and development. PPAR Res 2007:91240
Sugimoto K, Takayasu H, Nakazawa N et al (2008) Prenatal treatment with retinoic acid accelerates type 1 alveolar cell proliferation of the hypoplastic lung in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg 43(2):367–372
Huang K, Rabold R, Abston E et al (2008) Effects of leptin deficiency on postnatal lung development in mice. J Appl Physiol 105(1):249–259
Bergen HT, Cherlet TC, Manuel P et al (2002) Identification of leptin receptors in lung and isolated fetal type II cells. Am J Respir Cell Mol Biol 27(1):71–77
Chen H, Zhang JP, Huang H et al (2013) Leptin promotes fetal lung maturity and upregulates SP-A expression in pulmonary alveoli type-II epithelial cells involving TTF-1 activation. PLoS One 8(7):e69297
Kirwin SM, Bhandari V, Dimatteo D et al (2006) Leptin enhances lung maturity in the fetal rat. Pediatr Res 60(2):200–204
Massaro D, Massaro GD (2010) Lung development, lung function, and retinoids. N Engl J Med 362(19):1829–1831
Chytil F (1996) Retinoids in lung development. FASEB J 10(9):986–992
Desai A, Kartono F, Del Rosso JQ (2007) Systemic retinoid therapy: a status report on optimal use and safety of long-term therapy. Dermatol Clin 25(2):185–193
Valappil S, Kurkar M, Howell R (2007) Outcome of pregnancy in women treated with all-trans retinoic acid; a case report and review of literature. Hematology 12(5):415–418
Acknowledgments
This research was supported by the National Children’s Research Centre and the Children’s Medical and Research Foundation.
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedmacher, F., Hofmann, A.D., Takahashi, T. et al. Prenatal administration of all-trans retinoic acid upregulates leptin signaling in hypoplastic rat lungs with experimental congenital diaphragmatic hernia. Pediatr Surg Int 30, 1183–1190 (2014). https://doi.org/10.1007/s00383-014-3605-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3605-8