Abstract
Background
Careful examination of motor-evoked potential (MEP) findings is critical to the safety of intraoperative neuromonitoring during neurosurgery. We reviewed the intraoperative MEP findings in a pediatric patient who had undergone hemispherotomy for refractory epilepsy.
Case description
The patient was a 4-year-and-2-month-old boy with extensive right cerebral hemisphere, drug-resistant epilepsy, left upper and lower extremity paralysis, and cognitive impairment. We examined intraoperative MEP results both before and after hemispherotomy. Post-hemispherotomy and MEPs were successfully elicited through transcranial electrical stimulation (TES) but not via direct cortical stimulation on the right side. Furthermore, TES on the right side, following hemispherotomy, led to a reduction in the MEP amplification effect resulting from tetanic stimulation of the left unilateral median and tibial nerves. Conversely, we observed the effects of MEP amplification during TES on the left side after tetanic stimulation of these nerves. Postoperatively, the patient underwent magnetic resonance imaging and electroencephalogram examinations, confirming the anatomical and electrophysiological completeness of the dissection. Notably, the seizures disappeared, and no apparent complications were observed.
Conclusion
Collectively, our findings suggest that TES can still activate deep structures and elicit MEPs, even in cases where the corticospinal connections to the posterior limb of the internal capsule are entirely severed. Thalamo-cortical interactions may affect the MEP amplification, observed during tetanic stimulation. Injury to the corticospinal tracts of the white matter may be obscured on conventional MEP findings; however, it may be identified by MEP changes in tetanic stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motor-evoked potentials (MEPs) can be employed to monitor motor nerves safely, even under general anesthesia, owing to recent advancements in anesthesia and testing techniques. At our institution, we are exploring the use of the MEP amplification effect obtained through tetanic stimulation of the unilateral median and tibial nerves (mt-MEP) for more effective intraoperative MEP monitoring when conventional MEPs generated without tetanic stimulation (c-MEPs) [1] fail to yield sufficient amplitude. We recently reported that MEPs induced following tetanic stimulation of the pudendal nerve (p-MEPs) during pediatric craniotomy can provide an additional MEP amplification effect [2]. However, false negatives and positives can arise owing to various factors, and recorded MEP waveforms may not always accurately reflect motor function [3]. The mechanism behind the MEP amplification effect of tetanic stimulation also remains unclear and warrants cautious interpretation. In this study, we present noteworthy intraoperative MEP findings in a case of hemispherotomy.
Case presentation
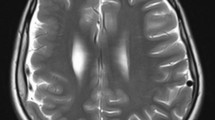
The patient was a 4-year-and 3-month-old boy who experienced status epilepticus at the age of 1 year and 8 months, leading to an emergency visit to a local doctor. Head magnetic resonance imaging (MRI) revealed extensive cortical dysplasia in the right cerebral hemisphere, and focal epilepsy treatment was initiated (Fig. 1A–B). The seizures persisted despite trying various antiseizure medications, and developmental regression occurred. Subsequently, the patient was referred to our department for surgical intervention. Physical examination revealed a manual muscle testing grade 2, with left-sided hemiparesis, especially in the left hand, and restricted isolated movement of an unknown degree. Seizure semiology included daily convulsions on the left side of his body with left conjugate deviation of the eyes. EEG revealed a right frontal predominant spike and wave complex (Fig. 1C). We suspected an extensive epileptogenic zone in the right cerebral hemisphere and opted for hemispherotomy. Detailed methods of intraoperative MEP are presented in Supplementary information.
A, B Preoperative cranial T2-weighted magnetic resonance imaging, axial section, revealing wide cortical dysplasia in the right cerebral hemisphere. C Preoperative interictal EEG indicating frequent bilateral synchronous spike and wave activity in the right frontal region. Sampling rate of 500 Hz, high-frequency filter of 60 Hz, and time constant of 0.3 s. D, E Postoperative cranial T2-weighted magnetic resonance imaging, axial and coronal sections, respectively, depicting complete detachment of the right thalamus from the right hemisphere. F Postoperative interictal EEG with lateralized interictal epileptiform discharges in the right hemisphere. Sampling rate of 500 Hz, high-frequency filter of 60 Hz, and time constant of 0.3 s
The suprathreshold stimulation intensity for MEPs was 500 V for TES and 30 mA for DCS. After establishing the baseline of c-MEPs preoperatively (left (Lt.) adductor pollicis brevis (APB): 36.7 µV, Lt. abductor hallucis longus (AH): 15.8 µV, right (Rt.) APB: 21 µV, Rt. AH: 15.7 µV), intraoperative MEP monitoring was initiated. Tibialis anterior and gastrocnemius were excluded from the study owing to the inability to obtain valid MEP waveforms. Detailed results are presented in Table 1. Post-hemispherotomy, MEPs could be measured from the left upper and lower extremities to the right cerebral hemisphere after TES. However, the MEP amplification effect of tetanic stimulation of the right median and tibial nerves was attenuated in both Lt. APB and Lt. AH, with MEPs reaching amplitudes similar to those observed at preoperative baseline (Fig. 2A, B). Both Lt. APB and Lt. AH also exhibited MEP amplification effects with pudendal nerve tetanic stimulation; however, the MEP amplification effect was significantly reduced post-hemispherotomy in Lt. AH (Fig. 2G, H). MEPs were not obtained during DCS to the right cerebral hemisphere, pre- or post-hemispherotomy, with or without tetanic stimulation.
Comparison of mean amplitudes between pre-hemispherotomy (pre-) and post-hemispherotomy (post) in each muscle and side of the tetanic stimulation group. The Mann–Whitney U test was employed for each comparison. A, B Transcranial electrical stimulation (TES) to the right cerebral hemisphere helped measure MEPs in both the left APB and AH, both pre and post. However, post-hemispherotomy, the MEP amplification effect of right peripheral nerve tetanic stimulation was attenuated in both the APB and AH. C, D TES to the left cerebral hemisphere enabled stable MEPs to be obtained throughout the surgery. E, F Notably, left tetanic stimulation resulted in significantly higher right AH and MEP in Post than that in Pre. G–J Overall, the MEP amplification effect was observed in the p-MEP group; however, in the right-side TES, the MEP amplification effect of AH was significantly attenuated in post. *p < 0.05, **p < 0.01. APB, adductor pollicis brevis muscle; AH, abductor hallucis longus muscle; mt, tetanic stimulation of the unilateral median and tibial nerves; MEP, motor-evoked potential; p, tetanic stimulation of the pudendal nerve; TES, transcranial electrical stimulation
TES to the left cerebral hemisphere enabled the acquisition of stable MEPs in the right upper and lower limbs throughout the surgery (Fig. 2C, D; I, J). mt-MEPs and p-MEPs demonstrated similar amplification effects compared with that of c-MEPs. Post-hemispherotomy, tetanic stimulation of the left median and tibial nerves exhibited an amplifying effect on right upper and lower limb MEPs during TES to the left cerebral hemisphere; Rt. AH exhibited significantly higher amplification than pre-hemispherotomy (Fig. 2E, F).
Surgery was completed without any complications. Postoperative MRI and EEG confirmed the anatomical and electrophysiological success of the hemispherotomy (Fig. 1D–F). Postoperatively, the degree of paralysis in the left upper and lower extremities remained unchanged, and the seizures disappeared.
Discussion
We obtained MEPs with TES but not DCS on the hemispherotomy side post-hemispherotomy. In addition, the MEP amplification effect of tetanic stimulation of the unilateral median and tibial nerves was attenuated by TES on the hemispherotomy side post-hemispherotomy. Furthermore, tetanic stimulation of the unilateral median and tibial nerves contralateral to the hemispherotomy side exhibited MEP amplification effects during TES on the non-hemispherotomy side. To the best of our knowledge, this is the first report discussing intraoperative MEP findings during hemispherotomy.
During TES, stimulation deep in the cerebral white matter can result in false negatives beyond the damaged area of the corticospinal tract. Previous reports indicate that high stimulation intensities via the foramen magnum can lead to activation caudal to the pyramidal tracts [4, 5]. In this case, MEPs were recorded even when the corticospinal tract in the posterior limb of the internal capsule was completely disconnected. This suggests that stimuli were propagated to deeper regions via structures other than the brain parenchyma, such as the epidermis and dura mater. Thus, it is considered difficult to assess deep white matter damage using TES alone.
The mechanism underlying the amplifying effect of tetanic stimulation of the peripheral or pudendal nerves on MEPs remains unclear, but several hypotheses exist [6]. In our case, tetanic stimulation of the left median and tibial nerve lost its MEP amplifying effect in the right TES post-hemispherotomy. In contrast, the MEP amplifying effect was preserved in the left TES post-hemispherotomy. These findings suggest that the MEP amplification effect of tetanic stimulation may involve thalamo-cortical interactions, with bilateral thalamic interactions or ipsilateral innervation of sensory nerves playing a role in the transmission of tetanic stimulation (Fig. 3) [7, 8]. This may explain the higher amplification of MEPs during tetanic stimulation of the pudendal nerve compared to the peripheral nerve, possibly owing to the activation of a broader range of regions in the bilateral cerebral hemispheres [9]. The specific-MEP changes of tetanic stimulation may identify white matter damage even in TES.
Tetanic stimulation likely produces MEP amplification effect. A Unilateral peripheral nerve tetanic stimulation ascends the thalamospinal tract (green arrow), causing an MEP amplification effect between the thalamus and cortex, with TES evoking MEPs (black arrow). B Post-hemispherotomy (red line), tetanic stimulation does not proceed above the thalamus, and no MEP amplification effect occurs; TES propagates through non-parenchymal areas (big, dotted line), and MEPs are evoked below the thalamus without the benefit of tetanic stimulation. C MEP amplification effects may also occur in unilateral peripheral nerve tetanic stimulation via the ipsilateral thalamus due to interaction or ipsilateral dominance of sensation from the contralateral thalamus (small, dotted line). MEP, motor-evoked potential; TES, transcranial electrical stimulation
Conclusion
Our findings indicate that TES can elicit MEPs from deep structures even when the corticospinal tracts in the posterior limb of the internal capsule are entirely disconnected. The MEP amplification effect of tetanic stimulation may involve thalamo-cortical interactions. Injury to the corticospinal tracts of the white matter is difficult to detect by c-MEP alone; however, it may be identified by MEP changes in tetanic stimulation.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H (2008) The application of tetanic stimulation of the unilateral tibial nerve before transcranial stimulation can augment the amplitudes of myogenic motor-evoked potentials from the muscles in the bilateral upper and lower limbs. Anesth Analg 107:215–220. https://doi.org/10.1213/ane.0b013e318177082e
Sasaki R, Tamura K, Yamazaki S, Kim TK, Takatani T, Hayashi H, Motoyama Y, Nakagawa I, Park YS, Kawaguchi M, Nakase H (2023) Effects of intraoperative motor-evoked potential amplification following tetanic stimulation of the pudendal nerve in pediatric craniotomy. J Neurosurg Pediatr 31:488–495. https://doi.org/10.3171/2023.1.peds22505
Chung J, Park W, Hong SH, Park JC, Ahn JS, Kwun BD, Lee SA, Kim SH, Jeon JY (2018) Intraoperative use of transcranial motor/sensory evoked potential monitoring in the clipping of intracranial aneurysms: evaluation of false-positive and false-negative cases. J Neurosurg 130:936–948. https://doi.org/10.3171/2017.8.jns17791
Burke D, Hicks RG, Stephen JP (1990) Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. J Physiol 425:283–299
Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M (1994) Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol 481:243–250
Sasaki R, Tamura K, Kim TK, Takatani T, Nakagawa I, Park YS, Nakase H (2023) Tetanic stimulation of the pudendal nerve amplifies intraoperative motor evoked potential in pediatric craniotomy. World Neurosurg 174:227–228. https://doi.org/10.1016/j.wneu.2023.03.071
Nihashi T, Naganawa S, Sato C, Kawai H, Nakamura T, Fukatsu H, Ishigaki T, Aoki I (2005) Contralateral and ipsilateral responses in primary somatosensory cortex following electrical median nerve stimulation–an fMRI study. Clin Neurophysiol 116:842–848. https://doi.org/10.1016/j.clinph.2004.10.011
Hautasaari P, Saloranta H, Savić AM, Korniloff K, Kujala UM, Tarkka IM (2020) Bilateral activations in operculo-insular area show temporal dissociation after peripheral electrical stimulation in healthy adults. Eur J Neurosci 52:4604–4612. https://doi.org/10.1111/ejn.13946
Luijten SPR, Groenendijk IM, Holstege JC, De Zeeuw CI, van der Zwaag W, Blok BFM (2020) Single subject and group whole-brain fMRI mapping of male genital sensation at 7 Tesla. Sci Rep 10:2487. https://doi.org/10.1038/s41598-020-58966-9
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
R.S. designed the study, the main conceptual ideas, and the proof outline. R.S. and T.T. collected the data. R.S. and K.T. aided in interpreting the results and worked on the manuscript. I.N. supervised the project. R.S. wrote the manuscript with support from Y.P. and I.N. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Ethics approval
IRB approval for a case report is waived at the Nara Medical University.
Consent for publication
Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasaki, R., Tamura, K., Takatani, T. et al. Intraoperative motor-evoked potential with tetanic stimulation changes pre- and post-hemispherotomy. Childs Nerv Syst 40, 563–567 (2024). https://doi.org/10.1007/s00381-023-06170-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06170-1