Abstract
Purpose
Hydrocephalus is one of the most common pathologies in pediatric neurosurgery. One of the causes of recurring events of headaches among shunted children is “slit ventricle syndrome” (SVS). Several potential treatments have been proposed, yet SVS often represents a treatment challenge. The goal of the current series is to present our experience with adding a positional shunt-assist (SA) (Miethke, Aesculap) for the treatment of SVS.
Methods
Clinical data was retrospectively collected from all consecutive children with SVS that were treated with SA (Miethke, Aesculap) at our center. Surgical and clinical outcomes as expressed by hospital visits, or need for additional surgery, were evaluated.
Results
Nine cases were included. Hydrocephalus etiology included IVH (6), postinfectious (1), and congenital syndromes (2). Average age at first shunt was 4 months. Primary shunt type was differential-pressure-valve in all. Average age at SVS onset was 4 years. Average age at SA placement was 5.5 years. There were no perioperative complications besides a single stich abscess. A 6-month follow-up period after SA was compared to a 6-month period prior to the SA: average hospital visits decreased from 2.4 to 0.6 per patient (p < 0.0002). 4/9 patients needed an LP or shunt revision before the SA surgery, while no procedure was indicated during the immediate 6-month follow-up. At the last follow-up, there was a significant reduction in the rate of ER visits compared to prior to surgery; however, the number of neurosurgical procedures did not significantly differ.
Conclusion
Using a SA for SVS was associated with a short-term improvement of symptoms in the majority of cases, reduction in hospital visits, and reduced need for SVS-related procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shunt systems are associated with a wide variety of long-term complications, and most children will undergo additional surgeries [1]. About 15% of shunted children have headaches [2].One cause of recurring headaches among shunted children is the “slit ventricle syndrome” (SVS) [3]. The syndrome is a constellation of mostly transient but severe symptoms of increased intracranial pressure, associated with small ventricles. SVS is not clearly and objectively defined [3, 4], and there are several pathophysiological hypotheses regarding its mechanism. The main presumed cause for SVS is shunt over-drainage prior to suture closure, leading to craniocerebral disproportion, and a non-compliant skull and ventricles. This may lead to temporary ventricular catheter obstruction caused by the collapse of the ventricle over the catheter, leading to a significant rise in intracranial pressure. Over-drainage, siphoning, and SVS are related conditions, where siphoning of CSF leads to over-drainage, which is then hypothesized to cause SVS [5]. One of the most recognized risk factors for SVS is shunt implantation during the first months of life. Another risk factor (which may be related to early shunt placement) is the primary hydrocephalic etiology (e.g., posthemorrhagic hydrocephalus of the newborn, or neonatal meningitis). Another risk factor associated with SVS is the valve mechanism (the use of differential pressure valves increases the risk for the development of SVS) [4].
Several techniques have been suggested to prevent and or treat SVS. Using a higher valve drainage setting for differential valves has not been shown to be of proven value [6]. Similarly, using delta systems, with integrated “anti-siphon” components has limited value [7]. Flow-regulated valves have been shown to have a trend towards lesser SVS [6]. During recent years, gravitational shunts have been shown to cause less SVS [8], although no formal comparison between pressure regulated valves and gravitational valves has been performed.
A valve upgrade to a higher pressure setting has been suggested, including using programmable valves, however with limited results [9, 10]. Other surgical techniques such as repositioning of the ventricular catheter or ETV (endoscopic third ventriculostomy) have a higher risk due to the small ventricular size [11, 12]. Cranial expansion and sub-temporal decompression have been described, aiming at increasing the intracranial volume and solving the “craniocerebral disproportion” component of SVS [13, 14]. These surgeries entail large cranial procedures and possibly have limited value when not coupled with a valve upgrade.
Recently, it has been suggested that converting pressure-regulated valves to gravitational valves (reducing CSF drainage during the upright position) reduces the incidence of SVS [8, 15].
Another approach is to add a gravitational device, to overcome the siphoning effect [1]. When the device is horizontal (during recumbent position), the gravitational unit is open and does not add any resistance to the existing valve. When the device is in a vertical position (during standing), it adds a fixed resistance, depending on the predefined setting, reducing the shunt drainage. This mechanism counteracts the siphoning effect during standing.
The objective of the current study was to examine the value of adding a gravitational unit (shunt assist, SA) in children with SVS.
Methods
Following institutional review board (IRB) approval, we retrospectively collected data on consecutive cases from Dana Children’s Hospital Tel Aviv Medical Center (2010–2022).
The inclusion criteria were children diagnosed with SVS and treated with SA. The diagnosis of SVS was based on clinical manifestation suggestive of increased intracranial pressure (headaches, somnolence, vomiting, and bradycardia) and slit ventricles on imaging (CT or MRI). The exclusion criteria were patients over 18 years old during SA surgery and patients who refused to be part of a research.
A SA was offered to all cases with recurrent SVS events. We did not have strict criteria, and often, parents elected to continue to follow and only after subsequent SVS events, elected to undergo surgery.
Shunt assist (SA, by Miethke, Aesculap, Germany) placement technique was standardized in all patients. Under general anesthesia, a 3-cm longitudinal incision was performed in the mid-chest height over the distal catheter. The SA was placed in a vertical orientation under the superficial chest muscle fascia for additive tissue layer coverage. There are several SA pressure levels, ranging from 10 to 35cmH2O. As recommended by the manufacturer, SA of 20–25cmH2O was used in children.
Collected data included demographics, hydrocephalus etiology, type of first shunt and valve, age at first shunt, and shunt revision surgeries. Details about SVS are as follows: date of diagnosis, need for a surgery after SVS diagnosis, type of SA and date of surgery, and surgical complications. Details about emergency room (ER) visits and neurosurgical procedures were collected for different periods, comparing the periods between SVS diagnosis and SA surgery and between SA surgery and the last follow-up and comparing 6 months prior to the SA surgery to 6 months following the SA surgery. Surgical and clinical outcomes as expressed by ER and hospital visits, or need for additional procedures, were evaluated.
Data analysis
Significant differences were assessed using an independent sample t-test, and the comparison of paired groups was done using the Wilcoxon test (SPSS Inc., Chicago, IL, USA).
Results
Nine patients were included in this study, 8 males (90%) and 1 female (10%); 6 were born preterm. Hydrocephalus etiology included IVH (6), postinfectious (1), and congenital of unknown etiology (2). All patients had their first shunt placement surgery during the first 8 months of life, with an average age of 4 months. Primary valve was a differential-pressure-valve in all. The average age for SVS onset was 4 years. Six patients underwent shunt revisions prior to the SA placement, of which 2 needed additional procedures (1 cranial expansion and 1 bilateral sub-temporal expansion + cranial expansion + foramen magnum decompression + re-cranial decompression and another shunt revision). The mean age at SA placement was 5.5 years, and it was placed at a mean of 17 ± 36.7 months (range 3 weeks–116 months) after SVS onset. A shunt assist of 20cmH2O was used in 8 patients, and a 25cmH2O was used in one patient. There were no perioperative complications besides a single stich abscess treated conservatively.
By looking at the timeline between SVS diagnosis to SA surgery, 6 patients had the SA surgery during the first 3 months of SVS onset, 2 patient had the SA surgery 16 months after SVS onset, and one patient had the SA surgery 115 months after the SVS onset. Eight patients visited the ER between the SVS onset and SA surgery: 3 patients visited once, 2 patients visited twice, one visited 4, one visited 6, and one visited 21 times. The average number of visits to the ER was 4.2 (range 1–21). Four patients underwent a neurosurgical intervention during this timeline: 3 underwent an LP, and one patient underwent 5 different surgeries.
The mean follow-up period after the SA surgery was 24 ± 10.4 months (range 4.5–43 months). Following the SA surgery and until the last follow-up, 6 patients visited the ER, 5 of them more than once. The average number of visits to the ER was 3.1 (range 1–9). Three patients needed neurosurgical interventions: one needed 8 surgeries, one need 2 (cranial expansion and LP), and one needed cranial expansion and foramen magnum decompression with cervical laminectomy for secondary Chiari.
Comparing the pre- and postoperative ER visit rate—from SVS diagnosis to SA to the period between SA and the last follow-up (using the Wilcoxon test), there was a reduction from a mean of 1.7 ± 2.5 visits per month, to 0.17 ± 0.17 visits per month (p = 0.028). The number of neurosurgical procedures reduced from 0.55 ± 0.98 to 0.05 ± 0.11, yet the reduction was not statistically significant (p = 0.25).
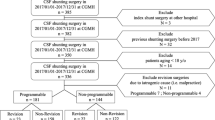
A 6-month follow-up period after SA was compared to a 6-month period prior to the SA: average hospital visits decreased from 2.4 to 0.6 per patient (p < 0.0002) (Fig. 1). 4/9 patients needed an LP or shunt revision before the SA surgery, while no procedure was indicated in the immediate 6-month follow-up (Fig. 2). There were no perioperative complications besides a single stich abscess treated locally.
This diagram shows the neurosurgical events (surgery/LP) that each patient had, from the day of SVS diagnosis until the last follow-up. 4/9 needed NS procedure (LP/surgery) during 6-m preop. No procedure was indicated in the immediate 6-month post SA surgery. 3/9 had a NS procedure until their last follow-up. PRE OP: preoperation. POST OP: postoperation. FU: follow-up. SVS: slit ventricle syndrome. DX: diagnosis. ER: emergency room
By comparing the ventricle size on CT before and after the SA surgery, we found that 4 had no change in ventricle size, 4 had smaller ventricles, and one had larger ventricle. There was no correlation between the ventricular size trend and the need of future neurosurgical procedures.
Discussion
In the current study, we have shown the positive immediate effect of SA on the course of SVS in shunted children. There was an immediate and significant improvement in the rate of ER visits, as well as the need for additional neurosurgical procedures. At last follow-up, the number of ER visits remained significantly low, yet the number of needed neurosurgical procedures was not significantly different compared to prior to surgery (probably reflecting the low rate all along). We have also shown that the complication rate of SA surgery is extremely low, making this an effective and safe treatment for SVS.
SVS is a debilitating condition following early shunt surgery in children. The rate of SVS is estimated at 4–37% [1]. It has been shown to affect especially those with a history of IVH: especially when they have a less extent of brain injury and thus are children with a better functional status.
Despite many shunt systems, there is no clearly preferred valve system that avoids SVS, although gravitational systems, and flow regulated valves have been associated with a trend for lower rates of SVS.
SVS is often a self-limiting and conservative treatment, including good hydration and various medications may suffice [16]. If these initial measures fail, a lumbar puncture may be of value [17]. For refractory SVS, several surgical options are described [18]. These include shunt revision (proximal catheter repositioning), as well as valve upgrades, or increasing the cranial vault volume: via a cranial expansion or sub-temporal decompression (STD) surgeries. The use of adding a SA has not been described. The advantages of this rather minimal and simple procedure are the avoidance of a more extensive surgery (i.e., cranial expansion or STD), or requiring a more extensive exposure (i.e. valve replacement), or risking shunt catheter mal-positioning (in the context of very small ventricles). Adding the SA in the chest wall is fast, simple, safe, and effective. Although not specifically studied, the impact on SVS-related symptoms as evaluated by ER visits and need for additional procedures is expected to reflect an improvement in the quality of life.
We had some limitations in our study. This is a retrospective study on a limited number of children. There is no control group, and each patient served as its own control (comparing 6 months prior to 6 months after the SA surgery).
Clinical evaluation was not objective or defined, and we used a general measure: as ER visits (due to SVS related symptoms), or need for any neurosurgical procedure, as an outcome measure. Potentially, patients may have elected not to present to the ER despite similar symptoms.
The vast majority of patients were male, possibly biasing the results. Also, the follow-up period was short, especially looking at the first postoperative 6 months (for standardization), possibly missing later failures.
Due to the small patient group, we were unable to evaluate the effect of timing of SA placement from SVS diagnosis, as well as other factors (e.g. age, etiology, and anatomical considerations) on the outcome.
It is also unknown if there is any advantage for early SA surgery shortly after SVS onset, or even as a prophylactic measure. Another question that arises is if to change the valve to an adjustable differential pressure valve with gravitational unit. The strategy of valve exchange to prevent chronic over-drainage is well tolerated and seems to improve a patient’s clinical outcome in terms of ventricular width, symptom relieve, and revision rate [19].
Despite these limitations and unknowns, we suggest the following algorithm for treating SVS (Fig. 3): primary treatment of SVS is conservative: fluids, steroids, and possibly a lumbar puncture. For failed cases during the acute attack, either a proximal shunt revision (or rarely, and ETV), sub-temporal decompression (STD), or cranial expansion is a valid surgical treatment. Whether or not to couple a cranial expansion with a SA is an open question, although there is a logic to do so, to avoid settling of the bones in the original position. In cases with recurrent SVS, in between attacks, we recommend placing a SA.
Conclusions
In this small group, SA was found to be a simple, safe, and effective procedure for reducing SVS related symptoms and need for neurosurgical procedures during the first 6 months after surgery. The number of ER visits remained significantly low at the last follow-up. Further evaluation among large study groups is needed to evaluate the long-term outcomes, the role of timing of SA surgery, as well as other variables on the outcome.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
References
Panagopoulos D, Stranjalis G, Gavra M, Boviatsis E, Korfias S (2022) Shunt over-drainage, slit ventricle syndrome, programmable valves and anti-siphon devices. A narrative review of a multifactorial and intractable problem. J Integr Neurosci 21(3):84. https://doi.org/10.31083/j.jin2103084
Stellman-Ward G, Bannister C, Lewis M, Shaw J (1997) The incidence of chronic headache in children with shunted hydrocephalus. Euro J Pediat Surg 7(S1):12–14. https://doi.org/10.1055/s-2008-1071201
Panagopoulos D, Karydakis P, Themistocleous M (2021) Slit ventricle syndrome: historical considerations, diagnosis, pathophysiology, and treatment review. Brain Circ 7(3):167. https://doi.org/10.4103/bc.bc_29_21
Kraemer MR, Sandoval-Garcia C, Bragg T, Iskandar BJ (2017) Shunt-dependent hydrocephalus: management style among members of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr 20(3):216–224. https://doi.org/10.3171/2017.2.PEDS16265
Beez T, Munoz-Bendix C, Ahmadi SA, Messing-Jünger M, Steiger HJ, Röhrig A (2019) Conservative and operative management of iatrogenic craniocerebral disproportion—a case-based review. Child’s Nervous System 35(1):19–27. https://doi.org/10.1007/s00381-018-3981-9
Jain H, Sgouros S, Walsh AR, Hockley AD (2000) The treatment of infantile hydrocephalus: “differential-pressure” or ”flow-control” valves. Child’s Nervous System 16(4):242–246. https://doi.org/10.1007/s003810050505
Davis SE, Levy ML, McComb JG, Sposto R (2000) The delta valve: how does its clinical performance compare with two other pressure differential valves without antisiphon control? Pediatr Neurosurg 33(2):58–63. https://doi.org/10.1159/000028988
Freimann FB, Sprung C (2012) Shunting with gravitational valves—can adjustments end the era of revisions for overdrainage-related events? J Neurosurg 117(6):1197–1204. https://doi.org/10.3171/2012.8.JNS1233
Park SW, Yoon SH, Cho KH, Shin YS (2007) Valve pressure upgrade may produce progressive deterioration of vision in children with slit ventricle syndrome. Pediatr Neurosurg 43(5):428–432. https://doi.org/10.1159/000106398
Rohde V, Mayfrank L, Ramakers VT, Gilsbach JM (1998) Four-year experience with the routine use of the programmable Hakim valve in the management of children with hydrocephalus. Acta Neurochir (Wien) 140(11):1127–1134. https://doi.org/10.1007/s007010050226
Chernov MF, Kamikawa S, Yamane F, Ishihara S, Hori T (2005) Neurofiberscope-guided management of slit-ventricle syndrome due to shunt placement. J Neurosurg Pediatr 102(3):260–267. https://doi.org/10.3171/ped.2005.102.3.0260
Benabarre A, Ibáñez J, Boget T, Obiols J, Martínez-Aran A, Vieta E (2001) Neuropsychological and psychiatric complications in endoscopic third ventriculostomy: a clinical case report. J Neurol Neurosurg Psychiatry 71(2):268–271. https://doi.org/10.1136/jnnp.71.2.268
Roth J, Biyani N, Udayakumaran S et al (2011) Modified bilateral subtemporal decompression for resistant slit ventricle syndrome. Child’s Nervous System 27(1):101–110. https://doi.org/10.1007/s00381-010-1220-0
Rekate HL (2004) The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg 40(6):259–263. https://doi.org/10.1159/000083737
Gutowski P, Gölz L, Rot S, Lemcke J, Thomale UW (2020) Gravitational shunt valves in hydrocephalus to challenge the sequelae of over-drainage. Expert Rev Med Devices 17(11):1155–1168. https://doi.org/10.1080/17434440.2020.1837622
Fattal-Valevski A, Beni-Adani L, Constantini S (2005) Short-term dexamethasone treatment for symptomatic slit ventricle syndrome. Child’s Nervous System 21(11):981–984. https://doi.org/10.1007/s00381-004-1132-y
Ros B, Iglesias S, Martín Á, Carrasco A, Ibáñez G, Arráez MA (2018) Shunt overdrainage syndrome: review of the literature. Neurosurg Rev 41(4):969–981. https://doi.org/10.1007/s10143-017-0849-5
Rekate HL (2008) Shunt-related headaches: the slit ventricle syndromes. Child’s Nervous System 24(4):423–430. https://doi.org/10.1007/s00381-008-0579-7
Alavi S, Schulz M, Schaumann A, Schwarz K, Thomale UW (2017) Valve exchange towards an adjustable differential pressure valve with gravitational unit, clinical outcome of a single-center study. Child’s Nervous System 33(5):759–765. https://doi.org/10.1007/s00381-017-3387-0
Acknowledgements
We thank Mrs. Adina Sherer for editorial assistance and Moran Artzi and Tomer Ziv-Baran for their contribution to the statistical analysis.
Author information
Authors and Affiliations
Contributions
Conception and design: Roth and Constantini S. Acquisition of data: Azolai and Constantini L. Analysis and interpretation of data: Azolai and Roth. Drafting the article: Azolai and Roth. Critically revising the article: all authors. Review of the submitted version of the manuscript: all authors. Approval of the final version of the manuscript on behalf of all authors: Roth. Study supervision: Roth.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by Helsinki Committee (0198–22-TLV).
Conflict of interest
The authors report no competing of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azolai, L., Constantini, S., Constantini, L. et al. Positional shunt assist for slit ventricle syndrome. Childs Nerv Syst 40, 109–114 (2024). https://doi.org/10.1007/s00381-023-06145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06145-2