Abstract
Background
Shunt malfunction is a common complication and often presents with hydrocephalus. While the diagnosis is often supported by radiographic studies, subtle changes in CSF volume may not be detectable on routine evaluation. The purpose of this study was to develop a novel automated volumetric software for evaluation of shunt failure in pediatric patients, especially in patients who may not manifest a significant change in their ventricular size.
Methods
A single-institution retrospective review of shunted patients was conducted. Ventricular volume measurements were performed using manual and automated methods by three independent analysts. Manual measurements were produced using OsiriX software, whereas automated measurements were produced using the proprietary software. A p value < 0.05 was considered statistically significant.
Results
Twenty-two patients met the inclusion criteria (13 males, 9 females). Mean age of the cohort was 4.9 years (range 0.1–18 years). Average measured CSF volume was similar between the manual and automated methods (169.8 mL vs 172.5 mL, p = 0.56). However, the average time to generate results was significantly shorter with the automated algorithm compared to the manual method (2244 s vs 38.3 s, p < 0.01). In 3/5 symptomatic patients whose neuroimaging was interpreted as stable, the novel algorithm detected the otherwise radiographically undetectable CSF volume changes.
Conclusion
The automated software accurately measures the ventricular volumes in pediatric patients with hydrocephalus. The application of this technology is valuable in patients who present clinically without obvious radiographic changes. Future studies with larger cohorts are needed to validate our preliminary findings and further assess the utility of this technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cerebrospinal fluid (CSF) volume is renewed two to three times each day [1], and a disruption of this physiologic process can lead to excess CSF buildup, causing hydrocephalus [2]. This pathology has an incidence of one in every 500 children [3] and accounts for approximately two billion dollars in health expenditures in the USA [4].
The mainstay treatment of hydrocephalus is shunt insertion, which diverts CSF from the ventricular system toward another body cavity, and a ventriculoperitoneal shunt (VPS) is the most common conduit [5]. Although shunts have drastically improved outcomes in children with hydrocephalus, they are still prone to complications [5,6,7]. Shunt malfunction can be due to catheter occlusion, disconnection, and infection [5, 6]. A defective shunt is unable to adequately divert CSF from the ventricular system, thus leading to subsequent hydrocephalus and its myriad of symptoms such as vomiting, irritability, and drowsiness [2, 8, 9]. Failure is a common sequelae of shunts as Stone et al. noted that 84.5% of patients required at least one or more revisions in a cohort of patients with over 19 years of follow-up [6]. Regardless of the etiology, shunt failure is a costly complication that is more common in children [10].
A diagnosis of shunt failure is based on clinical presentation and radiographic findings [11, 12], with computed tomography (CT) often used as the confirmatory test [13]. Although hydrocephalus is generally detectable on neuroimaging, there is a subset of symptomatic patients with meaningful ventricular volume changes whose CT scans may have no detectable changes on routine radiographic evaluation [14,15,16]. Although not easily noticeable, such small volume changes can have severe clinical implications [17,18,19]. Therefore, it is imperative to investigate technological modalities that can aid in earlier detection of shunt failure and recognition of subtle radiographic changes that still have meaningful volumetric changes consistent with shunt malfunction.
Recently, the use of artificial intelligence (AI) has gained momentum in neuroradiology [20]. Prior reports [21, 22] have provided promising data on the utility of such algorithms in earlier detection of hydrocephalus; however, there is still a paucity of literature with respect to this topic. In this study, we present our institution’s preliminary experience with using a novel automated volumetric software for rapid and accurate evaluation of hydrocephalus in pediatric patients and demonstrate its potential to impact clinical therapeutic options.
Methods
Study design
This study was approved by the institutional review board (IRB# 00,006,193), and a total waiver of consent was obtained. A retrospective review of patients evaluated for shunt malfunction at our institution between 2008 and 2013 was performed. We included pediatric patients with CSF shunts who presented to the emergency department with symptoms suggestive of hydrocephalus secondary to shunt malfunction. Patients who had other shunt-related complications without hydrocephalus (e.g., infection) or patients with incomplete medical records and imaging were excluded from analysis. Ventricular volume measurements were analyzed using manual and automated methods. Manual measurements were produced using standard OsiriX software, whereas automated measurements were produced using proprietary software. Variables included patient demographics, clinical outcome, time taken for volume analysis, and inter-rater reliability of measured volume. CSF volume assessments were correlated with patients who eventually underwent shunt revision to determine whether our automated algorithm offered any predictive value of shunt failure and if an earlier diagnosis could have expedited surgical intervention.
Algorithm
Head CT scan pixels can be stacked according to density in a histogram or a tetris plot. In doing so, the pixels fall into two clear distributions corresponding to CSF and brain parenchyma (Fig. 1). Our novel algorithm takes advantage of the differing color densities between the CSF and brain parenchyma pixels. To calculate the CSF volume using the novel software, the user identifies the location of the ventricles in a head CT scan, so that the algorithm displays the pixels of the head CT stacked according to density and shows the range of pixels that match what the user identified as CSF on the CT scan (Fig. 2). The user then confirms the range of CSF densities and provides appropriate definition. Subsequently, the algorithm searches through the image for pixels that have densities within the defined range and calculates the volume in milliliters. The algorithm in its entirety was developed by the second author (M.Q).
A Head CT scan in a color scheme showing the different densities of each pixel. B Surface plot where the Z axis corresponds to the intensity of the CT image. C Tetris plot in which each pixel in the image is stacked according to its intensity. The pixels of CSF (blue) and those of brain parenchyma (green) fall into two distinct distributions
A Presents a conventional color-scaled image. B Tetris plot of the data, each pixel is stacked according to its density. C Manual outlining of the CSF regions. D The regions in the image that fall in the user specified CSF range outlined in the black. The user identifies the ventricles by clicking on them with a mouse (black diamonds indicated by blue arrows)
Statistical analysis
Demographic and outcome descriptive statistics are reported for the overall sample. The average time of CSF volume analysis was recorded using the manual method and automated algorithm. Independent samples t test and Wilcoxon signed-rank test were used for parametric and non-parametric continuous variables, respectively. Chi-square and Fisher’s exact test were used for adequate cell-count and low cell-count categorical outcomes (≥ 25% of cells with expected cell-count ≤ 5), respectively. All statistical analysis was performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC). A p value < 0.05 was considered statistically significant.
Results
Patient population and algorithm accuracy
A total of 22 patients (13 males, 9 females) met the inclusion criteria. Mean age of the cohort was 4.9 years (range 0.1–18 years). CSF volume calculation was performed using the manual method and the automated algorithm on the entire cohort. Three different analysts (two neurosurgeons and the creator of the algorithm) repeated these measurements using both methods of analysis. Average measured CSF volume was similar between the manual and automated methods (169.8 mL vs 172.5 mL, p = 0.56). However, the automated algorithm performed this analysis significantly faster than the manual method (58.5 times faster, 38.3 s vs 2244 s, p < 0.01). The reproducibility between investigators and the two methodologies (manual and automated) was also evaluated. The correlation between the three examiners was > 99% (\(\pm\) 1.43%, range: 99.3 to 99.5%).
Clinical application
The volumetric data were correlated with the clinical setting of each patient. Of the 22 patients, 17 had notable ventricular volume changes that were identified by a neuroradiologist and subsequently underwent shunt revision. However, there were five patients whose CT scans upon initial presentation were interpreted as stable by neuroradiology. In three of these patients, there was a meaningful objective increase in ventricular volume sizes, accurately detected by our novel algorithm, despite not being identified by a neuroradiologist. A detailed clinical summary of these patients is presented as case illustrations.
Case illustrations
Case 1
An 11-month-old male with a history of myelomeningocele repaired shortly after birth and status post a VPS for hydrocephalus presented to the emergency department (ED) for evaluation of increased irritability. A head CT scan was interpreted by neuroradiology as stable compared to the patient’s prior images, and he was discharged home. Three days later, the patient was brought back to the ED with continued episodes of irritability. He remained neurologically intact, and his repeat CT scan was interpreted as continued stability; therefore, the patient was discharged home. Four days later, he returned to the ED for a third time with continued irritability, deviation of the right eye, and lethargy. A CT scan on this admission showed clear interval dilation of his ventricles, and a shunt tap was attempted, yet no proximal flow of CSF was obtained. The patient was diagnosed with hydrocephalus secondary to proximal shunt failure and was taken to the operating room for emergent proximal and distal revision of his VPS. There were no perioperative complications, and the patient was subsequently discharged.
Case 2
A 13-year-old female with a history of a repaired occipital encephalocele, developmental delay, and VPS placement for hydrocephalus presented to the ED for persistent headache and photophobia which had been attributed to her migraines. Initial CT scan was interpreted as normal (unchanged from her baseline), and the patient was discharged home. She returned the following day with similar symptoms and underwent repeat CT evaluation, which was interpreted as unchanged/stable; therefore, the patient was discharged. Fifty-three days later, the patient returned with worsening symptoms and CT evaluation displayed ventriculomegaly. She was taken to the operating room immediately for shunt revision. The remainder of her hospital course was without complications, and she was discharged.
Case 3
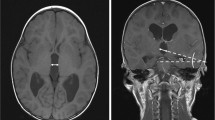
A three-year-old male with a history of premature birth with intraventricular hemorrhage, seizure disorder, and hydrocephalus treated with ventriculoatrial shunt (VAS) presented to the ED with increased seizure activity. The patient was admitted for observation and a CT scan was interpreted as stable. Two days later, a second CT scan was interpreted as possible, but not determinable, enlargement of the right frontal horn (Fig. 3). Given the stable neurological exam, the patient was subsequently discharged. He returned 6 weeks later with altered mental status, emesis, and lethargy. A shunt tap revealed slow proximal flow and a CT displayed an interval increase in size of the right frontal horn. Therefore, the patient underwent emergent proximal revision of the VAS without complications and was subsequently discharged.
Comparison of the CT scans in illustrative Case 3. In both images, the ventricular volume was calculated via the automated volumetric analysis. A This CT scan was taken upon initial presentation and the calculated ventricular volume is 66.0 mL. B This CT scan was taken two days after the initial presentation and the calculated ventricular volume is 73.5 mL. This corresponds to an increase of 11.3%
In all three of the presented cases, the automated volumetric analysis showed that the CSF ventricular volumes had increased since initial admission (41.7%, 43.8%, and 11.3%, respectively) (Table 1). This increase, however, was not definitively detected on routine interpretation by a neuroradiologist. Use of the novel algorithm would have detected these changes, therefore preventing the delay in the patients’ care.
Discussion
Shunt malfunction may lead to inadequate CSF diversion, thus leading to subsequent hydrocephalus [5,6,7]. Early diagnosis is critical, as small changes in CSF volume can have grave consequences [17,18,19]. Although subtle changes in CSF volume may correlate with clinical symptoms, they are not always detectable on routine interpretation of radiographic studies [17,18,19]. Therefore, the development of a technology that accurately and quickly detects minute yet clinically important CSF volume changes can offer significant improvement in the timely diagnosis of shunt failure. In this study, we present our institution’s preliminary experience with a novel automated volumetric software for rapid and accurate evaluation of hydrocephalus in pediatric patients.
The present study is among the few that have evaluated an automated software for CSF volume analysis. Congruent with previous reports in the literature [17, 21, 22], our novel software and its evaluation demonstrated the accuracy of this technology. In a series of 80 CT scans, Klimont et al. reported excellent accuracy of their automated segmentation software to assess ventricular volume changes [18]. The algorithm achieved a mean dice score of 0.92 (\(\pm\) 0.03) on cross-validation, demonstrating its potential for practical applications [18]. In a series of 200 pediatric patients with obstructive hydrocephalus, Quon et al. evaluated an automated deep learning model for ventricular segmentation and volume calculation in magnetic resonance imaging (MRI) [19]. The results demonstrated superb accuracy of the model, while taking only 1.48 s to analyze each scan [19]. Similarly, Shao and colleagues proposed a method in evaluation of ventriculomegaly that took only an average of 120 s [17]. In our study, we demonstrated the accuracy of our automated system, while providing the results 58.5 times faster than the manual method. Such findings further validate the accuracy of this technology and warrant consideration for its incorporation into the armamentarium of neuroradiologists and neurosurgeons.
Shunt malfunction commonly presents with nonspecific symptoms such as irritability, headache, and vomiting, thus frequently making it difficult to clinically distinguish from other more common and benign childhood illnesses [12, 23, 24]. Therefore, physicians routinely rely on neuroimaging as confirmatory studies [11, 12, 25]. Although hydrocephalus is often evident on imaging, there are instances (~ 5%) in which ventricular volumes appear normal or unchanged despite shunt malfunction [15, 16]. In a series of 175 pediatric patients with VPS, McNatt et al. noted that 9% had no evidence of ventricular dilatation despite having symptoms of shunt malfunction [16]. Winston et al. reported similar findings in 12 patients whose neuroimaging was interpreted as stable in the setting of shunt obstruction [15]. In our series, the CT scans of five patients were interpreted as stable; however, three of those patients had meaningful ventricular volume changes that were undetected by neuroradiologists. This corresponds to a remarkable false negative rate of 60%, albeit the subtle nature of these volumetric changes. The automated volumetric analysis demonstrated the ability to detect such subtle changes, which would have led to earlier intervention, and prevented any delay in diagnosis and management of these patients.
In recent years, the interest to integrate AI into medical imaging has grown exponentially, as reflected by the 400% increase in the number of peer-reviewed publications on this topic during a 9-year interval (2010–2019) [26]. The primary emphasis has been on identifying urgent findings such as intracranial hemorrhage [27], large vessel occlusion [28], and traumatic brain injury [29]. AI has also been used to estimate the volume of anatomical structures for Alzheimer disease [30], ventricular size in hydrocephalus [31], and tumor volume for intracranial masses [22]. However, there are still obstacles to integration of AI algorithms into clinical practice: infrastructure needs, appropriate computing, and handling of protected health information are among the many challenges [26]. Additionally, there needs to be a systematic protocol that ensures reliable performance and routinely updates the algorithm. Lack of standardization across AI algorithm is another major limitation that needs to be addressed [26]. Nonetheless, our results, along with many other studies that have demonstrated the clinical application of AI in neuroimaging, guide us closer to incorporating it into routine clinical decision-making. Upon further validation of our preliminary results, this software has the potential to be utilized in evaluating ventricular volume changes in other etiologies of hydrocephalus such as congenital anomalies and brain tumors and also utilized in adult patients.
Limitations
This was a retrospective review which has limitations of its nature. As a single-center evaluation, there were only 22 patients included in the analysis, significantly limiting the statistical power. There is also the potential bias in performance of the algorithm due to the small number of analysts that performed the segmentations tasks, meaning we may have inadvertently committed errors in the segmentation process. Lastly, we only evaluated the efficacy of our algorithm in pediatric patients, even though adults are also affected by hydrocephalus secondary to shunt failure. Therefore, this limits the external validity of our results.
Conclusion
Determining shunt failure can be challenging in patients who do not have overt radiographic changes. We have demonstrated that ventricular volumes of CT scans in pediatric patients can be accurately and rapidly measured with automated software. The application of this technology is valuable for patients who present clinically without any recognizable changes on radiographic evaluation, as the novel software is able to detect subtle ventricular volume changes that may go unnoticed by neuroradiologists and neurosurgeons. This algorithm can also offer insightful information that may prevent delays in treatment and should further be assessed in larger cohorts to validate our findings. However, it is critical to keep in mind that the diagnosis of shunt malfunction requires a comprehensive clinical evaluation, composed of a detailed history, complemented with physical examination and imaging studies. Thus, although imaging advancements enhance our diagnostic capabilities, they should not be the sole criteria for making a diagnosis and should always be interpreted when taking the entire clinical picture into account.
References
Bradley WG (2015) CSF Flow in the brain in the context of normal pressure hydrocephalus. Am J Neuroradiol 36:831–838. https://doi.org/10.3174/ajnr.A4124
Wright Z, Larrew TW, Eskandari R (2016) Pediatric hydrocephalus: current state of diagnosis and treatment. Pediatr Rev 37:478–490. https://doi.org/10.1542/pir.2015-0134
Flannery AM, Mitchell L (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 1: Introduction and methodology. J Neurosurg Pediatr 14(Suppl 1):3–7. https://doi.org/10.3171/2014.7.PEDS14321
Simon TD, Riva-Cambrin J, Srivastava R et al (2008) Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr 1:131–137. https://doi.org/10.3171/PED/2008/1/2/131
Kestle JR (2003) Pediatric hydrocephalus: current management. Neurol Clin 21(883–895):vii. https://doi.org/10.1016/s0733-8619(03)00016-1
Stone JJ, Walker CT, Jacobson M et al (2013) Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr 11:15–19. https://doi.org/10.3171/2012.9.PEDS1298
Agarwal N, Shukla RM, Agarwal D et al (2017) Pediatric ventriculoperitoneal shunts and their complications: an analysis. J Indian Assoc Pediatr Surg 22:155–157. https://doi.org/10.4103/0971-9261.207624
Krishnan P, Raybaud C, Palasamudram S, Shroff M (2019) Neuroimaging in pediatric hydrocephalus. Indian J Pediatr 86:952–960. https://doi.org/10.1007/s12098-019-02962-z
Tully HM, Dobyns WB (2014) Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet 57:359–368. https://doi.org/10.1016/j.ejmg.2014.06.002
Shannon CN, Simon TD, Reed GT et al (2011) The economic impact of ventriculoperitoneal shunt failure: clinical article. J Neurosurg Pediatr 8:593–599. https://doi.org/10.3171/2011.9.PEDS11192
Garton HJ, Kestle JR, Drake JM (2001) Predicting shunt failure on the basis of clinical symptoms and signs in children. J Neurosurg 94:202–210. https://doi.org/10.3171/jns.2001.94.2.0202
Piatt JH, Garton HJL (2008) Clinical diagnosis of ventriculoperitoneal shunt failure among children with hydrocephalus. Pediatr Emerg Care 24:201–210. https://doi.org/10.1097/pec.0b013e31816a8d43
Dinçer A, Özek MM (2011) Radiologic evaluation of pediatric hydrocephalus. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 27:1543–1562. https://doi.org/10.1007/s00381-011-1559-x
Sze RW, Ghioni V, Weinberger E et al (2003) Rapid computed tomography technique to measure ventricular volumes in the child with suspected ventriculoperitoneal shunt failure II. Clinical application J Comput Assist Tomogr 27:668–673. https://doi.org/10.1097/00004728-200309000-00002
Winston KR, Lopez JA, Freeman J (2006) CSF shunt failure with stable normal ventricular size. Pediatr Neurosurg 42:151–155. https://doi.org/10.1159/000091857
McNatt SA, Kim A, Hohuan D et al (2008) Pediatric shunt malfunction without ventricular dilatation. Pediatr Neurosurg 44:128–132. https://doi.org/10.1159/000113115
Shao M, Han S, Carass A et al (2019) Brain ventricle parcellation using a deep neural network: Application to patients with ventriculomegaly. NeuroImage Clin 23:101871. https://doi.org/10.1016/j.nicl.2019.101871
Klimont M, Flieger M, Rzeszutek J et al (2019) Automated ventricular system segmentation in paediatric patients treated for hydrocephalus using deep learning methods. BioMed Res Int 2019:3059170. https://doi.org/10.1155/2019/3059170
Quon JL, Han M, Kim LH et al (2020) Artificial intelligence for automatic cerebral ventricle segmentation and volume calculation: a clinical tool for the evaluation of pediatric hydrocephalus. J Neurosurg Pediatr 1–8. https://doi.org/10.3171/2020.6.PEDS20251
Meshaka R, Pinto Dos Santos D, Arthurs OJ et al (2021) Artificial intelligence reporting guidelines: what the pediatric radiologist needs to know. Pediatr Radiol. https://doi.org/10.1007/s00247-021-05129-1
Goo HW (2021) Hydrocephalus: Ventricular volume quantification using three-dimensional brain CT data and semiautomatic three-dimensional threshold-based segmentation approach. Korean J Radiol 22:435–441. https://doi.org/10.3348/kjr.2020.0671
Irie R, Otsuka Y, Hagiwara A et al (2020) A novel deep learning approach with a 3D convolutional ladder network for differential diagnosis of idiopathic normal pressure hydrocephalus and Alzheimer’s disease. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med 19:351–358. https://doi.org/10.2463/mrms.mp.2019-0106
Kim TY, Stewart G, Voth M et al (2006) Signs and symptoms of cerebrospinal fluid shunt malfunction in the pediatric emergency department. Pediatr Emerg Care 22:28–34. https://doi.org/10.1097/01.pec.0000195764.50565.8c
Lee TT, Uribe J, Ragheb J et al (1999) Unique clinical presentation of pediatric shunt malfunction. Pediatr Neurosurg 30:122–126. https://doi.org/10.1159/000028778
Turhan T, Ersahin Y, Dinc M, Mutluer S (2011) Cerebro-spinal fluid shunt revisions, importance of the symptoms and shunt structure. Turk Neurosurg 21:66–73
Lui YW, Chang PD, Zaharchuk G et al (2020) Artificial intelligence in neuroradiology: current status and future directions. AJNR Am J Neuroradiol 41:E52–E59. https://doi.org/10.3174/ajnr.A6681
Ginat DT (2020) Analysis of head CT scans flagged by deep learning software for acute intracranial hemorrhage. Neuroradiology 62:335–340. https://doi.org/10.1007/s00234-019-02330-w
Murray NM, Unberath M, Hager GD, Hui FK (2020) Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerventional Surg 12:156–164. https://doi.org/10.1136/neurintsurg-2019-015135
Stone JR, Wilde EA, Taylor BA et al (2016) Supervised learning technique for the automated identification of white matter hyperintensities in traumatic brain injury. Brain Inj 30:1458–1468. https://doi.org/10.1080/02699052.2016.1222080
Li F, Liu M, Alzheimer’s Disease Neuroimaging Initiative, (2019) A hybrid convolutional and recurrent neural network for hippocampus analysis in Alzheimer’s disease. J Neurosci Methods 323:108–118. https://doi.org/10.1016/j.jneumeth.2019.05.006
Huff TJ, Ludwig PE, Salazar D, Cramer JA (2019) Fully automated intracranial ventricle segmentation on CT with 2D regional convolutional neural network to estimate ventricular volume. Int J Comput Assist Radiol Surg 14:1923–1932. https://doi.org/10.1007/s11548-019-02038-5
Funding
Khashayar Mozaffari is supported by the Gurtin Skull-Base Research Fellowship. This funding source had no role in the design, execution, or reporting of this study and its results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interests concerning the methods or findings of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jha, T.R., Quigley, M.F., Mozaffari, K. et al. Prediction of shunt failure facilitated by rapid and accurate volumetric analysis: a single institution’s preliminary experience. Childs Nerv Syst 38, 1907–1912 (2022). https://doi.org/10.1007/s00381-022-05552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05552-1