Abstract
Introduction
There have been a few cases where completely thrombosed cavernous carotid artery (CCA) aneurysms have resembled neoplasms based on neuroimaging data, but no reports have been documented in children.
Case report
We describe an unusual pediatric case of a huge cavernous sinus mass mimicking a cystic neoplasm with peripheral rim enhancement on magnetic resonance imaging (MRI), where the surgery and subsequent histopathological investigation revealed that this mass was a completely thrombosed giant aneurysm of the CCA. The patient showed postoperatively no new neurological deficits and discharged a week later after surgery.
Conclusions
In this case report, we describe a pediatric case of a completely thrombosed giant CCA aneurysm with ipsilateral internal carotid artery (ICA) occlusion, which imitates an intra-axial cystic lesion on MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysms of the CCA account for between 3 and 5% of all intracranial aneurysms [1]. Because they are located in extradural space, the clinical manifestation is different from other intradural aneurysms. However, they can grow large in size, and can present with pressure symptoms due to mass effect on cranial nerves, such as ophthalmoplegia and facial pain [2]. Spontaneous intramural thrombosis of giant intracranial aneurysms occurs in between 13 and 20% of cases [3]. We describe an unusual pediatric case of a huge cavernous sinus mass mimicking a cystic neoplasm with peripheral rim enhancement on MRI, where the surgery and subsequent histopathological investigation revealed that this mass was a completely thrombosed giant aneurysm of the CCA. We also searched the literature on thrombosed CCA aneurysms that mimic intracranial neoplasms.
Case report
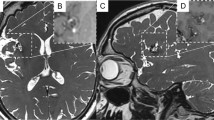
A 2-year-old boy with no previous medical history was admitted to us with progressive oculomotor palsy and seizure for 3 months. The symptoms began insidiously and were not associated with a history suggestive of subarachnoid hemorrhage. A clinical examination revealed left ptosis, oculomotor paresis, and left relative afferent pupillary defect. The patient was evaluated with gadolinium-enhanced MRI that showed a huge cystic lesion adjacent to the left ICA with peripheral rim enhancement on T1-weighted and hypointensity on T2-weighted images that was suspicious for a neoplastic process (Fig. 1). Differential diagnosis included intra-axial tumors such as glioma or ganglioglioma, dermoid cyst, epidermoid cyst, vestibular schwannoma, and ependymoma. Then, we performed MR angiography, which showed a giant left cavernous sinus mass measuring 2.7 × 2.9 × 4 cm and the occlusion of the left ICA, with no filling past the petrous segment (Fig. 2). CTA also verified the findings of the MRA with a lack of flow through the left ICA from the skull base to the suparclinoid segment (Fig. 3). Head CT scan also showed the direct erosion of the cavernous sinus wall and erosion into the sella turcica by the growing mass. His left middle cerebral artery (MCA) and anterior cerebral artery (ACA) were supplied through a patent Willis circuit via a left posterior communicating artery (PCoA) and a patent anterior communicating artery. Reconstitution of flow at the supraclinoid segment with filling of the left MCA, ACA, and PCoA was seen. Based on the T1W MRI overlayed to the TOF-MRI, a vascular origin of the lesion was considered more likely (Fig. 4). Therefore, we performed a subsequent craniotomy for resection of the lesion using a left pterional craniotomy. The lesion exposed was found to be a large completely thrombosed aneurysm originating from the CCA. After cutting the wall of the aneurysm, thrombectomy, aneurysm wall resection, and reconstruction were performed (Fig. 5).
Hystopathologic examination confirmed the diagnosis of thrombosed aneurysm, which revealed hemosiderin deposits, calcifications, and inflammatory infiltrates within the remnants of the vessel wall. The patient showed no new neurological deficits postoperatively was put on aspirin 50 mg daily for stroke prevention and was discharged a week later after admission. At his 1-month follow-up, he was doing well; his ophthalmoparesis and ptosis had improved.
Discussion
Pediatric cerebral aneurysms occur at a frequency between 0.17 and 6.8% among all patients treated for intracranial aneurysms, where the percentage of pediatric cerebral aneurysms that are giant (diameter ≥ 25 mm) range from 14 to 54% [4]. Complete intraluminal thrombosis occurs especially in large and giant cerebral aneurysms in 13–20% of cases [5]. However, there are few reports where completely thrombosed CCA aneurysms have imitated neoplasms based on initial presentation and imaging [6, 7], while no cases have been reported among the pediatric population. They also treated their patient with open surgery and removal of intramural clot via an aneurysmotomy [8].
Most of the patients with large CCA aneurysms presented with symptoms of ophthalmoparesis, facial pain, or hyposthesia due to the mass effect. In our patient, the medial extension of the mass caused the oculomotor paresis, while the seizure activity is probably because of the lateral extension and compression of the insular cortex by an enlarging lesion.
CT angiography provided reliable and informative data regarding the thrombosed aneurysm, the extent of ICA thrombosis, and the distal blood flow provided by collateral circulation through the circle of Willis comparable to DSA with high sensitivity and specificity [9]. Preoperatively, a DSA is always helpful and must be included in the preoperative workup to visualize the parent vessel of the giant intracranial aneurysm and plan the operation. But in the case of a completely thrombosed aneurysm, DSA could be negative [10]. The accepted methods of treatment for giant cavernous aneurysms are occlusion of the ICA, with or without bypass, depending on the cross-flow, as well as results of balloon test occlusion [11]. Endovascular interventions for both the feeding vessel and the aneurysm itself are also done with good results and less morbidity than an extensive open procedure [12]. We also believe there is a role for conservative management in patients who are not acutely and severely symptomatic and fail balloon test occlusion [13]. Further clinical and imagiological follow-up is needed once recanalization of thrombosed lesions, although rare [14], cannot be anticipated [15]. Some authors advocate performing control angiographic studies at 3, 6, and 12 months and subsequently with yearly intervals until at least 3-year follow-up, due to the risk of recurrence and rehemorrhage [16].
Conclusions
In this case report, we describe a pediatric case of a completely thrombosed giant CCA aneurysm with ipsilateral internal carotid artery (ICA) occlusion, which imitates an intra-axial cystic lesion on MRI. We also searched the literature on thrombosed CCA aneurysms that mimic intracranial neoplasms.
References
Mikabe T, Ogihara R, Tomita S, Kin H, Karasawa H, Watanabe S et al (1980) Giant intracranial aneurysm visualized by prolonged injection angiography: case report (author’s transl) No Shinkei Geka. 8:749–53. [PubMed] [Google Scholar]
Hahn CD, Nicolle DA, Lownie SP, Drake CG (2000) Giant cavernous carotid aneurysms: clinical presentation in fifty-seven cases. J Neuroophthalmol 20:253–8. [PubMed] [Google Scholar]
Whittle IR, Williams DB, Halmagyi GM, Besser M (1982) Spontaneous thrombosis of a giant intracranial aneurysm and ipsilateral internal carotid artery. Case report. J Neurosurg 56:287–9. [PubMed] [Google Scholar]
Iplikcioglu AC, Dinc C, Bek S, Bikmaz K (2006) Spontaneous thrombosis and resorption of a giant fusiform A2 aneurysm. Case illustration J Neurosurg 105:788
Whittle IR, Williams DB, Halmagyi GM, Besser M (2018) Completely Thrombosed Giant intracranial aneurysm with spontaneous thrombosis of the parent artery: is it nature’s divine intervention and a self-cure? .World Neurosurg 118:132–8
Lim DH, Jung S, Jung TY, Kim TS (2008) An unusual case of a thrombosed giant distal PICA aneurysm simulating a large cavernous angioma. J Korean Neurosurg Soc 43:155–8. [PMC free article] [PubMed] [Google Scholar]
Spallone A (1982) Giant, completely thrombosed intracranial aneurysm simulating tumor of the foramen magnum. Surg Neurol 18(5):372–376
Sastri SB, Sadasiva N, Pandey P (2013) Giant cavernous carotid aneurysm with spontaneous ipsilateral ICA occlusion: report of 2 cases and review of literature. J Neurosci Rural Pract 4(Suppl 1):S113–S116
Wintermark M, Uske A, Chalaron M, Regli L, Maeder P, Meuli R et al (2003) Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg 98:828–836
Trungu S, Bruzzaniti P, Forcato S, Cimatti M, Raco A (2017) Completely thrombosed distal middle cerebral artery aneurysm mimicking a cavernous angioma: case report and review of the literature. World Neurosurg 103:955.e1–4
Kupersmith MJ, Hurst R, Berenstein A, Choi IS, Jafar J, Ransohoff J (1992) The benign course of cavernous carotid artery aneurysms. J Neurosurg 77:690–3. [PubMed] [Google Scholar]
Perrini P, Bortolotti C, Wang H, Fraser K, Lanzino G (2005) Thrombosed giant intracavernous aneurysm with subsequent spontaneous ipsilateral carotid artery occlusion. Acta Neurochir (Wien) 147:215–7. [PubMed] [Google Scholar]
Vasconcellos LP, Flores JA, Conti ML, Veiga JC, Lancellotti CL (2009) Spontaneous thrombosis of internal carotid artery: a natural history of giant carotid cavernous aneurysms. Arq Neuropsiquiatr 67:278–83. [PubMed] [Google Scholar]
Lee KC, Joo JY, Lee KS, Shin YS (1999) Recanalization of completely thrombosed giant aneurysm: case report. Surgical Neurol 51(1):94–98. [PubMed] [Google Scholar]
Roccatagliata L, Guédin P, Condette-Auliac S et al (2010) Partially thrombosed intracranial aneurysms: symptoms, evolution, and therapeutic management. Acta Neurochirurgica 152(12):2133–2142. [PubMed] [Google Scholar]
Liang J, Bao Y, Zhang H et al (2009) The clinical features and treatment of pediatric intracranial aneurysm. Child’s Nervous Sys 25(3):317–324. [PubMed] [Google Scholar]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was not sought for this case report as it was not required due to the nature of the case report.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Conflict of interest
There were no conflicts of interest during the writing or submission of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malikov, A., Secen, A.E., Daglioglu, E. et al. A pediatric case of completely thrombosed giant cavernous carotid aneurysm with ipsilateral ICA occlusion mimicking an intra-axial cystic lesion: a case report and review of the literature. Childs Nerv Syst 38, 1809–1812 (2022). https://doi.org/10.1007/s00381-022-05481-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05481-z