Abstract
Introduction
Premature neonates have a high risk of intraventricular hemorrhage (IVH) at birth, the blood products of which activate inflammatory cascades that can cause hydrocephalus and long-term neurological morbidities and sequelae. However, there is no consensus for one treatment strategy. While the mainstay of treatment involves CSF diversion to reduce intracranial pressure, a number of interventions focus on blood product removal at various stages including extraventricular drains (EVD), intra-ventricular thrombolytics, drainage-irrigation-fibrinolytic therapy (DRIFT), and neuroendoscopic lavage (NEL).
Methods
We performed a systematic review and meta-analysis to compare the risks and benefits commonly associated with active blood product removal treatment strategies. We searched MEDLINE, Embase, Scopus, Cochrane Library, and CINAHL databases through Dec 2020 for articles reporting on outcomes of EVDs, thrombolytics, DRIFT, and NEL. Outcomes of interest were rate of conversion to ventriculoperitoneal shunt (VPS), infection, mortality, secondary hemorrhage, and cognitive disability.
Results
Of the 10,398 articles identified in the search, 23 full-text articles representing 22 cohorts and 530 patients were included for meta-analysis. These articles included retrospective, prospective, and randomized controlled studies on the use of EVDs (n = 7), thrombolytics (n = 8), DRIFT therapy (n = 3), and NEL (n = 5). Pooled rates of reported outcomes for EVD, thrombolytics, DRIFT, and NEL for ventriculoperitoneal shunt (VPS) placement were 51.1%, 43.3%, 34.3%, and 54.8%; for infection, 15.4%, 12.5%, 4.7%, and 11.0%; for mortality, 20.0%, 11.6%, 6.0%, and 4.9%; for secondary hemorrhage, 5.8%, 7.8%, 20.0%, and 6.9%; for cognitive impairment, 52.6%, 50.0%, 53.7%, and 50.9%. Meta-regression using type of treatment as a categorical covariate showed no effect of treatment modality on rate of VPS conversion or cognitive disability.
Conclusion
There was a significant effect of treatment modality on secondary hemorrhage and mortality; however, mortality was no longer significant after adjusting for year of publication. Re-hemorrhage rate was significantly higher for DRIFT (p < 0.001) but did not differ among the other modalities. NEL also had lower mortality relative to EVD (p < 0.001) and thrombolytics (p = 0.013), which was no longer significant after adjusting for year of publication. Thus, NEL appears to be safer than DRIFT in terms of risk of hemorrhage, and not different than other blood-product removal strategies in terms of mortality. Outcomes–in terms of shunting and cognitive impairment–did not differ. Later year of publication was predictive of lower rates of mortality, but not the other outcome variables. Further prospective and randomized studies will be necessary to directly compare NEL with other temporizing procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraventricular hemorrhage (IVH) with progressive posthemorrhagic hydrocephalus (PHH) remains a significant problem in preterm births and may portend long-term neurologic sequelae [1]. These include, but are not limited to, cognitive delay, visual impairment, motor impairment, epilepsy, cerebral palsy, neurodevelopmental delay, and long-standing hydrocephalus [2, 3]. Multifactorial in nature, IVH is dependent on the intrinsic fragility of the germinal matrix, fluctuations in cerebral blood flow, and coagulation disorders [4]. Together, these result in germinal matrix hemorrhage, typically within the first three postpartum days, which can then progress to IVH. Subsequently, the buildup of intraventricular blood products obstructs the arachnoid villi, blocking the main site of cerebrospinal fluid (CSF) reabsorption. This can cause the release of TGF-ß1 into the CSF resulting in the accumulation of extracellular matrix proteins and glial fibrillary acidic proteins, ultimately leading to hydrocephalus and irreversible damage to the brain [5].
Theoretically, methods that involve direct removal of blood products may lessen the inflammatory cycle of blood breakdown products and address the pathogenesis underpinning of PHH. Unfortunately, intra-ventricular thrombolytics followed by drainage and the one clinical trial that employed drainage-irrigation-fibrinolytic (DRIFT) infusion did not improve short-term outcomes and were associated with bleeding risks [3, 6]. However, 2- and 10-year neurodevelopmental outcomes were significantly improved for patients who underwent DRIFT compared to controls treated with traditional CSF diversion, suggesting a positive long-term effect after the initial risks of the procedure [6, 7]. More recently, several groups have started using neuroendoscopic lavage (NEL), which involves direct access and aspiration of intraventricular blood products, with fewer bleeding complications and comparable 2-year neurodevelopmental outcomes to DRIFT [8]. Therefore, we sought to investigate the effectiveness of NEL compared to other treatment methods involving the removal of blood products that result in PHH.
We aimed to conduct a systematic review of the literature with meta-analysis to evaluate and compare the different methods involving blood product removal for the treatment of PHH. The aims were to estimate and compare rates of surgical and developmental outcomes between PHH treatment methods that involve blood product removal: EVD, thrombolytics, DRIFT, and NEL. The findings of this study will provide an estimate of how NEL performs relative to previous attempts at blood product removal. While EVD does not involve the active breakdown of blood products, we elected to include EVD because there is continuous drainage of blood breakdown products and inflammatory substances from the CSF while it is in use.
Methods
Search strategy
A systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9] was conducted to investigate treatments involving blood clot removal for IVH of prematurity. PubMed MEDLINE, Embase, Cochrane Library, Scopus, and CINHAL databases were searched from their inception through Dec 2020. Search terms pertained to premature infants with IVH, interventions including NEL, EVD, thrombolytics, and DRIFT, and outcomes including incidence of VPS placement, infection, mortality, and cognitive disability. Detailed search strategies are delineated in Appendix 1.
Selection criteria
Once articles were identified through the search, duplicates were removed and non-full text English language journal articles including abstracts, conference presentations, editorials, and case reports were excluded. All remaining articles were screened based on title and abstract. After title and abstract exclusion, the remaining articles underwent full-text review. Articles were selected by two authors independently (V.K. and L.M.M.) based on the inclusion and exclusion criteria listed in Table 1. Disagreements were resolved between the same two authors.
Data extraction
The following information was extracted from studies that met all inclusion criteria: number of infants included in each study who underwent the specified intervention, mean gestational age (GA), mean birth weight (BW), grade of IVH, study design, study duration, treatment method (EVD, thrombolytics, DRIFT, and/or NEL), outcomes, and complications. The primary outcome of interest was conversion to VPS. Secondary outcomes of interest included rates of infection, morality, secondary hemorrhages, and any severe cognitive disability as defined by the particular study. A summary of all outcomes of interest is detailed in Table 2.
Quality assessment
The quality of evidence was evaluated according to the Cochrane ROBINS-I guidelines [10]. The quality score for each included study is indicated in Table 2. Egger’s test was used to assess publication bias [11].
Statistical analysis
Meta-analyses were performed using Comprehensive Meta-Analysis v3 (Biostat, Inc.) and other statistical analyses performed using R, version 3.5.1. We calculated pooled proportions of rates for the five outcomes of interest: rate of conversion to VPS, infection, mortality, secondary hemorrhage, and cognitive disability stratified by treatment modality subgroup (EVD, thrombolytics, DRIFT, and NEL). A random-effects model with 95% confidence intervals (CI) was used. Weights were calculated using the DerSimonian and Laird method. Between studies, heterogeneity was measured by the I2 statistic and Tau2 value. I2 values greater than 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively. To determine the effect of treatment modality on outcomes, a subgroup analysis was done using meta-regression with treatment as a categorical moderator. Meta-regression was run for each outcome measure with and without adjusting for publication year as a potential confounding factor. In cases where there was a significant effect, the regression was run with each intervention as the designated reference group to compare each intervention to the others.
Standard statistical comparisons were performed to compare differences between year of publication, GA, and BWs across studies for each modality. P values equal to or less than 0.05 were considered to be statistically significant. Means and standard deviations (SD) are reported as mean ± SD.
Results
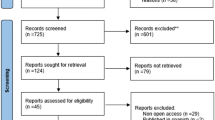
Of 114 resultant articles, 23 met inclusion criteria (Fig. 1). Three articles reported on the same cohort enrolled in the DRIFT randomized controlled trial: the first in 2003 [12] after the initial end of the trial, the second in 2010 [6] at 2-year follow-up, and the last in 2019 [7] at 10-year follow-up. Data from the initial report and 2-year follow-up were combined and considered as one instance. We excluded the 10-year follow-up study in this meta-analysis due to the fact that developmental outcomes at 2 years were more comparable to outcomes reported within a similar follow-up period among the rest of the studies. Thus, a total of 22 cohorts from 23 articles were included in the meta-analysis.
Of the included studies, two were of low quality, 17 were of moderate quality, and four were of high quality. Table 2 lists each study, quality assessments, and outcomes.
There was significant bias for outcomes of infection (T = 4.66, p < 0.001), mortality (T = 3.78, p = 0.001), and secondary hemorrhage (T = 4.65, p < 0.001).
IVH treatment modalities
Among the 23 included articles, six reported on EVD [13,14,15,16,17,18], eight on thrombolytics [1, 19,20,21,22,23,24,25], one on both EVD and EVD + thrombolytics [26], five on NEL [8, 27,28,29,30], and three (representing 2 cohorts) on outcomes of the DRIFT trial [6, 12, 31]. Fifteen studies only included patients with grade III or IV IVH, and eight studies did not report on grade. The average year of publication was 1995 ± 13 for EVD, 2000 ± 9 for thrombolytics, 2018 ± 2 for NEL, and 2005 ± 3 for DRIFT (only included the initial publication for each cohort). Average GA was 30.7 ± 4.4 for EVD, 27.8 ± 1.1 for thrombolytics, 27.8 ± 1.5 for NEL, and 27.5 ± 0.7 for DRIFT. Average BW was 1280 ± 157 for EVD, 1116 ± 217 for thrombolytics, 1350 ± 297 for NEL, and 1100 ± 71 for DRIFT.
There was a significant effect of average year of publication among treatment modalities (p = 0.016). Studies on NEL were published significantly later than the studies on EVD (p = 0.033), DRIFT (p = 0.035), and thrombolytics (p = 0.022). There were no significant differences between treatment modalities for GA (p = 0.35) or for BW (p = 0.21).
Extraventricular drain (EVD)
Seven articles reported on the use of EVDs. All were retrospective observational studies. Pooled rates of outcome were 51% (95% CI 42.3–59.6) for conversion to VPS, 15.4% (95% CI 10.4–22.1) for infection, 20% (95% CI 14.1–27.5) for mortality, 5.8% (95% CI 3.0–10.8) for secondary hemorrhage, and 52.6% (95% CI 31.8–72.5) for cognitive disability. There was significant heterogeneity in cognitive disability outcomes (Q = 15.05, I2 73.4%, p = 0.005, Tau2 = 0.69).
Thrombolytics
Eight articles reported on the use of thrombolytics. All were retrospective cohorts except one [21] which was a case–control trial. One used recombinant tPA, three streptokinase, three urokinase, and one used either tPA or urokinase. Three studies employed intermittent thrombolytic infusion [20, 22, 24] and five studies employed continuous infusion [1, 21, 23, 25, 26]. Pooled rates of outcome were 43.3% (95% CI 24.6–64.2) for conversion to VPS, 12.5% (95% CI 6.3–23.5) for infection, 11.6% (95% CI 9.9–17.1) for mortality, 7.8% (95% CI 3.6–16.0) for secondary hemorrhage, and 50.0% (95% CI 38.0–96.2) for cognitive disability. There was significant heterogeneity in conversion to VPS (Q = 21.77, I2 67.8%, p = 0.003, Tau2 = 0.95) and cognitive disability (Q = 4.11, I2 75.7%, p = 0.043, Tau2 = 4.20).
Drainage-irrigation-fibrinolytics therapy (DRIFT)
Results from three articles described findings from DRIFT treatment. Two articles described the same cohort and were pooled into one instance [6, 12]. Pooled rates across the two cohorts were 34.3% (20.5–51.3) for conversion to VPS, 4.7% (95% CI 0.8–22.8) for infection, 6.0% (95% CI 2.5–16.3) for mortality, 20.0% (95% CI 4.2–58.8) for secondary hemorrhage, and 53.7% (95% CI 40.4–66.5) for cognitive disability. There was significant heterogeneity in rates of secondary bleeding (Q = 4.76, I2 = 80.0, Tau2 = 1.13).
Neuroendoscopic lavage (NEL)
Five studies reported on the use of NEL. All were retrospective cohorts and used a similar protocol consisting of insertion of the endoscope into the lateral ventricle, septostomy, and continuous irrigation with lactated ringers with passive outflow until the CSF cleared. One study did not leave a reservoir or EVD after lavage [30], three studies left a ventricular reservoir for possible later CSF aspiration [8, 27, 29], and one left an EVD for possible later drainage [28]. Pooled rates of outcome were 54.8% (95% CI 46.7–62) for conversion to VPS, 11.0% (95% CI 5.6–20.6) for infection, 4.9% (95% CI 2.4–9.7) for motility, 6.9% (95% CI 3.6–12.7) for secondary hemorrhage, and 50.9 (95% CI 38.1–63.6) for cognitive disability. None of the outcomes had significant heterogeneity.
Comparison of outcomes
Meta-regression showed a significant interaction between treatment modality and secondary hemorrhage (Q = 19.61, p < 0.001) and rate of death (Q = 16.15, p = 0.001). However, after adjusting for year of publication, treatment modality was predictive of secondary hemorrhage only (Q = 19.91, p < 0.001) but not death (Q = 4.05, p = 0.2560). Later year of publication on its own was also associated with lower death rates (Z = − 3.48, p = 0.0005), but not when treatment modality was also entered into the model (Z = − 0.63, p = 0.5264). Year was not associated with secondary hemorrhage in either model (p = 0.6984 and 0.8984). DRIFT had higher rates of secondary hemorrhage compared to NEL (z = − 3.5, p < 0.001), EVD (z = − 3.9, p = 0.001), and thrombolytics (z = − 2.9, p = 0.004), but the others did not differ among each other. Prior to correction for year, NEL had lower mortality compared to EVD (z = 3.67, p < 0.001) and thrombolytics (z = 2.48, p = 0.013). DRIFT also had lower mortality compared to EVD (z = 2.26, p = 0.024).
There was no significant overall interaction between treatment modality and rate of conversion to VPS (Q = 4.05, p = 0.256; Q = 5.16, p = 0.1605 adjusted), infection (Q = 2.11, p = 0.549; Q = 2.04, p = 0.5648 adjusted), and cognitive disability (Q = 0.69, p = 0.8753; Q = 1.38, p = 0.7113 adjusted). Year of publication was neither associated with VPS (z = − 0.27, p = 0.7908; z = − 1.10, p = 0.2716 adjusted), infection (z = − 0.82, p = 0.4140; z = − 0.83, p = 0.4076 adjusted) nor cognitive disability (z = − 0.71, p = 0.4781; z = − 1.13, p = 0.2575 adjusted), see Figs. 2, 3, 4, 5, 6.
Discussion
We studied interventions that reduce blood products involved in the pathogenesis of PHH, rather than treating sequelae of increased ICP. While temporizing measures such as lumbar puncture, fontanelle taps, Rickham or Ommaya reservoir placement, and subgaleal shunts also withdraw blood breakdown products along with CSF, we defined active intervention aimed at the blood products as the intervention of interest. We included EVD into this definition due to continuous drainage of blood and inflammatory products while it is in use, though it could fall halfway in this spectrum since there is no active break down of a blood clot. To our knowledge, this is the first study to critically investigate the literature and perform a meta-analysis to provide updated information regarding the risks and benefits of NEL compared to previously attempted means of blood product removal.
Meta-analysis demonstrated that rate of secondary hemorrhage for NEL was significantly lower than DRIFT and not different from the other modalities. This is reassuring since the use of DRIFT was discontinued and the trial terminated early due to substantial numbers of infants experiencing secondary hemorrhage [31]. None of the modalities compared in our analysis showed any significant differences in rates of conversion to VPS, infection, and cognitive disability. After adjusting for year of publication, none of the modalities showed differences in mortality as well. Thus, NEL is at least as effective as the other modalities but with an improved safety profile compared to DRIFT. NEL may consequently provide a safer controlled means for removal of blood clots than earlier attempts with fibrinolysis. NEL has benefits over DRIFT and thrombolytics because it allows direct visual access to the location and burden of clots while aspirating rather than the passive infusion of thrombolytics, which act indiscriminately throughout the entire ventricular system. For EVD without thrombolytics, the duration to allow for normal physiology to clear the hematoma is much longer, exposing the brain to inflammatory substances for a longer period of time.
Although the DRIFT trial was terminated early, subsequent follow-up of children treated with DRIFT demonstrated improved 2- and 10-year neurodevelopmental outcomes relative to traditional CSF diversion (namely, reservoir taps) [7]. Thus, although no direct comparison with CSF diversion methods were made; comparable cognitive outcomes to DRIFT may translate to better outcomes between NEL and CSF diversion methods. Since none of the blood product removal techniques included in our meta-analysis differed in cognitive outcomes compared to DRIFT, it may be that the active removal of blood products may decrease the duration of inflammatory processes and reactions the brain is exposed to, leading to improved long-term outcomes. However, this remains to be speculative. A randomized controlled study to directly compare NEL against CSF diversion will be required to make more concrete conclusions.
Based on our results, NEL appears to be a viable treatment strategy to pursue in further careful study for infants with IVH of prematurity. However, we underscore the importance of considering all aspects of a patient’s medical condition (including severity and burden of disease, comorbidities, clinical condition) and tailoring treatment to the individual before selecting the best treatment protocol.
Blood product removal and shunt rates compared to CSF diversion
While our meta-analysis was limited to comparison of interventions that had a primary focus on blood product removal, other temporizing approaches—ventriculosubgaleal shunt (VSGS) implants, ventricular access devices (VAD)–that rely on CSF diversion are currently more commonly utilized. A previous meta-analysis published in 2015 reported that only 13.9% and 17.5% of patients treated with VSGS and VAD survive without conversion to VPS [32]. A subsequently published retrospective study comparing VSGS and VAD performed at a single institution (n = 46 and 44) found a VP shunt rate of 76.1% for VSGS and 77.3% for VAD [33]. Then in 2017, the Hydrocephalus Clinical Research Network reported on a multi-centered prospective cohort with a permanent shunt rate of 86% and 69% for VSGS and VAD [34]. While VSGS and ventricular reservoirs are commonly heralded as the mainstay treatment for IVH of prematurity, these high rates compared to our pooled estimate of 54% for NEL and the 34% for DRIFT–though not significantly different than the control group in the trial–suggest that removal of blood products compared to CSF diversion may have greater efficacy in treating the underlying pathophysiology of IVH to prevent development of permanent PHH. DRIFT did have a non-significantly lower rate of shunting in our meta-analysis; however, the risks associated with DRIFT preclude further use or study of this intervention. Again, studies that directly compare between NEL and CSF diversion will need to be conducted.
Limitations
This review only included published studies that had full-text manuscripts, which renders the results to publication bias and an overestimation of the number of positive and significant study results. Furthermore, only studies written in or translated to English were included, potentially excluding successful interventions and studies from other areas of the world. Additionally, the overall quality of evidence reviewed was moderate since most studies were retrospective cohorts. Statistical limitation with the meta-analysis included the heterogeneity among the studies. Since there is no standardized management guideline, each institution adapts their own protocol in terms of when to intervene, how to intervene, and when to shunt patients with PHH. Another limitation is that many of the studies were conducted nearly two decades ago. Medical therapy and biomedical technology have improved drastically since then, so it is also feasible that the mentioned interventions might prove to be more efficacious when implemented with the current standard of care. We found significantly higher rates of mortality in publications published earlier and higher rates of mortality for EVD and thrombolytics before controlling for year of publication, methods that were more commonly employed earlier (average 1995 and 2000 versus 2018 for NEL) and now generally not used as first line treatment. Indeed, the effect of treatment modality on mortality was no longer significant after controlling for year of publication.
Conclusion
We present a systematic review of the treatment options targeting intervention in the disease pathogenesis for IVH of prematurity, comparing the risks and benefits of a recent strategy of blood product removal (NEL) to previous modalities including EVD, thrombolytics, and DRIFT. We found that NEL was associated with statistically significant lower rates of secondary hemorrhage compared to DRIFT, and similar rates of death, infection, conversion to permanent VPS, and cognitive outcome. While shunting rates were numerically the lowest for DRIFT, this form of intervention carried a significantly high risk of secondary hemorrhage. The impact on cognitive outcomes, neurological implications, and functional status remain unclear. Further study regarding the optimal treatment modalities in the acute phase of IVH of prematurity is needed.
References
Akisu M, Yalaz M, Arslanoglu S, Kultursay N (2003) Intraventricular administration of recombinant tissue plasminogen activator for intraventricular hemorrhage in the newborn. Neurosurg Rev 26:266–268. https://doi.org/10.1007/s10143-003-0282-9
Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni AV (2015) Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. J Neurosurg Pediatr 16:545–555. https://doi.org/10.3171/2015.3.PEDS14630
Garton T, Hua Y, Xiang J, Xi G, Keep RF (2017) Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets 21:1111–1122. https://doi.org/10.1080/14728222.2017.1397628
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67:1–8. https://doi.org/10.1203/PDR.0b013e3181c1b176
Behrens P, Tietze A, Walch E, Bittigau P, Buhrer C, Schulz M, Aigner A, Thomale UW (2020) Neurodevelopmental outcome at 2 years after neuroendoscopic lavage in neonates with posthemorrhagic hydrocephalus. J Neurosurg Pediatr 1–9. https://doi.org/10.3171/2020.5.PEDS20211
Berger A, Weninger M, Reinprecht A, Haschke N, Kohlhauser C, Pollak A (2000) Long-term experience with subcutaneously tunneled external ventricular drainage in preterm infants. Childs Nerv Syst 16:103–109. discussion 110. https://doi.org/10.1007/s003810050022
Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P (1997) Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Childs Nerv Syst 13:369–374. https://doi.org/10.1007/s003810050102
d’Arcangues C, Schulz M, Buhrer C, Thome U, Krause M, Thomale UW (2018) Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg 116:e217–e224. https://doi.org/10.1016/j.wneu.2018.04.169
Eisenhut M, Choudhury S (2017) In premature newborns intraventricular hemorrhage causes cerebral vasospasm and associated neurodisability via heme-induced inflammasome-mediated interleukin-1 production and nitric oxide depletion. Front Neurol 8:423. https://doi.org/10.3389/fneur.2017.00423
Etus V, Kahilogullari G, Karabagli H, Unlu A (2018) Early endoscopic ventricular irrigation for the treatment of neonatal posthemorrhagic hydrocephalus: a feasible treatment option or not? A multicenter study. Turk Neurosurg 28:137–141. https://doi.org/10.5137/1019-5149.JTN.18677-16.0
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Hansen AR, Volpe JJ, Goumnerova LC, Madsen JR (1997) Intraventricular urokinase for the treatment of posthemorrhagic hydrocephalus. Pediatr Neurol 17:213–217. https://doi.org/10.1016/s0887-8994(97)00130-6
Harbaugh RE, Saunders RL, Edwards WH (1981) External ventricular drainage for control of posthemorrhagic hydrocephalus in premature infants. J Neurosurg 55:766–770. https://doi.org/10.3171/jns.1981.55.5.0766
Hudgins RJ, Boydston WR, Hudgins PA, Morris R, Adler SM, Gilreath CL (1997) Intrathecal urokinase as a treatment for intraventricular hemorrhage in the preterm infant. Pediatr Neurosurg 26:281–287
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Luciano R, Velardi F, Romagnoli C, Papacci P, De Stefano V, Tortorolo G (1997) Failure of fibrinolytic endoventricular treatment to prevent neonatal post-haemorrhagic hydrocephalus. A case-control trial. Childs Nerv Syst 13:73–76. https://doi.org/10.1007/s003810050045
Luyt K, Jary S, Lea C, Young GJ, Odd D, Miller H, Kmita G, Williams C, Blair PS, Fernandez AM, Hollingworth W, Morgan M, Smith-Collins A, Thai NJ, Walker-Cox S, Aquilina K, Pople I, Whitelaw A (2019) Ten-year follow-up of a randomised trial of drainage, irrigation and fibrinolytic therapy (DRIFT) in infants with post-haemorrhagic ventricular dilatation. Health Technol Assess 23:1–116. https://doi.org/10.3310/hta23040
Manaenko A, Lekic T, Barnhart M, Hartman R, Zhang JH (2014) Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke 45:828–834. https://doi.org/10.1161/STROKEAHA.113.003754
Marro PJ, Dransfield DA, Mott SH, Allan WC (1991) Posthemorrhagic hydrocephalus. Use of an intravenous-type catheter for cerebrospinal fluid drainage. Am J Dis Child 145:1141–1146. https://doi.org/10.1001/archpedi.1991.02160100073026
Park YS, Kotani Y, Kim TK, Yokota H, Sugimoto T, Nakagawa I, Motoyama Y, Nakase H (2020) Efficacy and safety of intraventricular fibrinolytic therapy for post-intraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. https://doi.org/10.1007/s00381-020-04766-5
Rhodes TT, Edwards WH, Saunders RL, Harbaugh RE, Little CL, Morgan LJ, Sargent SK (1987) External ventricular drainage for initial treatment of neonatal posthemorrhagic hydrocephalus: surgical and neurodevelopmental outcome. Pediatr Neurosci 13:255–262. https://doi.org/10.1159/000120339
Richard E, Cinalli G, Assis D, Pierre-Kahn A, Lacaze-Masmonteil T (2001) Treatment of post-haemorrhage ventricular dilatation with an Ommaya’s reservoir: management and outcome of 64 preterm infants. Childs Nerv Syst 17:334–340. https://doi.org/10.1007/s003810000418
Schulz M, Buhrer C, Pohl-Schickinger A, Haberl H, Thomale UW (2014) Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr 13:626–635. https://doi.org/10.3171/2014.2.PEDS13397
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Tirado-Caballero J, Rivero-Garvia M, Arteaga-Romero F, Herreria-Franco J, Lozano-Gonzalez A, Marquez-Rivas J (2020) Neuroendoscopic lavage for the management of posthemorrhagic hydrocephalus in preterm infants: safety, effectivity, and lessons learned. J Neurosurg Pediatr 1–10. https://doi.org/10.3171/2020.2.PEDS2037
Wang JY, Amin AG, Jallo GI, Ahn ES (2014) Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr 14:447–454. https://doi.org/10.3171/2014.7.PEDS13552
Wellons JC, 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD, Jr., Whitehead W, Browd S, Rozzelle C, Simon TD, Tamber MS, Oakes WJ, Drake J, Luerssen TG, Kestle J, Hydrocephalus Clinical Research N (2017) Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr 20:19–29. https://doi.org/10.3171/2017.1.PEDS16496
Weninger M, Salzer HR, Pollak A, Rosenkranz M, Vorkapic P, Korn A, Lesigang C (1992) External ventricular drainage for treatment of rapidly progressive posthemorrhagic hydrocephalus. Neurosurgery 31:52–57. discussion 57–58. https://doi.org/10.1227/00006123-199207000-00008
Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, Swietlinski J, Simpson J, Hajivassiliou C, Hunt LP, Pople I (2007) Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics 119:e1071-1078. https://doi.org/10.1542/peds.2006-2841
Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, Hunt L, Carter M, Pople I (2010) Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125:e852-858. https://doi.org/10.1542/peds.2009-1960
Whitelaw A, Pople I, Cherian S, Evans D, Thoresen M (2003) Phase 1 trial of prevention of hydrocephalus after intraventricular hemorrhage in newborn infants by drainage, irrigation, and fibrinolytic therapy. Pediatrics 111:759–765. https://doi.org/10.1542/peds.111.4.759
Whitelaw A, Rivers RP, Creighton L, Gaffney P (1992) Low dose intraventricular fibrinolytic treatment to prevent posthaemorrhagic hydrocephalus. Arch Dis Child 67:12–14. https://doi.org/10.1136/adc.67.1_spec_no.12
Whitelaw A, Saliba E, Fellman V, Mowinckel MC, Acolet D, Marlow N (1996) Phase I study of intraventricular recombinant tissue plasminogen activator for treatment of posthaemorrhagic hydrocephalus. Arch Dis Child Fetal Neonatal Ed 75:F20-26
Yapicioglu H, Narli N, Satar M, Soyupak S, Altunbasak S (2003) Intraventricular streptokinase for the treatment of posthaemorrhagic hydrocephalus of preterm. J Clin Neurosci 10:297–299
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication
No part of this work has been previously published.
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kandula, V., Mohammad, L.M., Thirunavu, V. et al. The role of blood product removal in intraventricular hemorrhage of prematurity: a meta-analysis of the clinical evidence. Childs Nerv Syst 38, 239–252 (2022). https://doi.org/10.1007/s00381-021-05400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05400-8