Abstract
Purpose
Medulloblastoma is one of the most common malignant brain tumors in the pediatric population. Recent studies identified four distinct medulloblastoma subgroups with different molecular alterations and pathways, and natural courses and outcomes. To evaluate the results of surgical and medical treatments of patients with medulloblastoma and compare them among the medulloblastoma subgroups.
Methods
The clinical and radiological features, medical and surgical management and treatment outcomes and their correlation with molecular subgroups of 58 patients treated for medulloblastoma in the last 20 years were evaluated.
Results
Fifty-eight patients, of whom 35 were male and 23 were female, were evaluated. The median age was 6 years (range, 1–19 years). The most common symptoms were nausea and vomiting (60%). Forty-three percent of the patients had headache and 40% had ataxia. Previous pathology reports revealed that 43 (74%), eight (14%), five (8%), and two (3%) had classic, desmoplastic, desmoplastic/nodular, and anaplastic morphologies, respectively. After the subgroup analyses, five patients (12%) were attributed to the wingless subgroup (WNT) group; 14 (32.5%), to the sonic hedgehog subgroup (SHH) group; and 24 (56%), to the non-WNT non-SHH group. On the basis of immunohistochemical analysis results, 15 patients could not be attributed to any subgroups. The clinical risk groups (average vs high-risk) and age at diagnosis (≥ 3 years vs < 3 years of age) were significant for 5-year event free survival (86% vs 43%, p:0.011 and 59% vs 36%, p:0.039). There was no significant difference in survival or event free survival according to molecular subtypes in this cohort.
Conclusion
In corporation of molecular features to the clinicopathologic classification leads to risk-adapted treatment. Although the molecular subgroups did not affect outcome significantly in this study, more studies with larger numbers of patients are needed to understand the tumor pathophysiology of medulloblastoma and design the future medical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma is one of the most common childhood malignant brain tumors, with an incidence of 0.74 per 100,000 population [27]. Most cases are found in the posterior fossa; 30% can occur with spinal and leptomeningeal disseminations [8]. Ten to 25% of the patients are adults [40]. The standard treatment modalities are surgery, chemotherapy, and radiotherapy. Until recently, treatment was assigned according to risk stratification which included the residual tumor postoperatively (> 1.5 cm2), cerebrospinal fluid cytology and spinal axis metastasis.
The 2007 World Health Organization classification system categorized medulloblastomas into four groups as follows: classic, desmoplastic, large cell anaplastic, and nodular. In 2016, this classification system was upgraded, and the molecular properties of tumors were added in the diagnostic criteria. Studies have shown four medulloblastoma subgroups, namely the wingless (WNT) subgroup, sonic hedgehog (SHH) subgroup, group 3, and group 4, with different histological and molecular properties, and natural courses and outcomes [43].
In 2014, the International Medulloblastoma Work Group added genetic studies in the diagnostic algorithm of medulloblastomas. However, as laboratory studies are time-consuming and expensive and the results of genetic studies using formalin-fixed paraffin-embedded tissues are not reliable, these studies are not integrated into routine practice. Recently, immunohistochemistry and fluorescence in situ hybridization (FISH) studies have been used to identify these subgroups [16]. Further investigations suggest that these subgroups can be divided into more detailed subtypes according to genomic analyses [5, 38]. However, due to financial constraints, limited time and manpower, these studies are not routinely done in many centers, especially in low and middle income countries (LMIC), including our center.
Here, we present a series of 58 pediatric patients with medulloblastomas, their demographic characteristics, pathological features, clinical and molecular risk groups and outcome. We aimed to correlate the clinical group and molecular groups.
Materials and methods
A retrospective analysis of patients with medulloblastoma treated in the Department of Neurosurgery, Istanbul Medical Faculty, Istanbul University and Oncology Institute, Divisions of Pediatric Hematology- Oncology and Division of Radiation Oncology, Istanbul University between 1997 and 2017 was performed. The patient’s clinical features, radiological imaging studies, and postoperative medical treatments were evaluated. Follow-up data were collected from inpatient and outpatient clinic files, and the patients and their relatives were contacted if available. All the patients were indicated to undergo maximal tumor resection and postoperative magnetic resonance imaging (MRI) within 24 h from surgery for investigating the extent of resection. Gross total resection (GTR) was defined as no residual tumor postoperatively. Patients with residual tumor of > 1.5 cm2 in size on postoperative imagings were considered high risk. Routine histopathological analysis included examination for local leptomeningeal invasion (LLMI) [3].
Postoperative cranial MRI scan with and without contrast was performed preferably within 24 h post-surgery. All patients had a spinal axis MRI with contrast and cerebrospinal fluid (CSF) cytology for risk stratification. Spinal MRI with and without contrast was done either preoperatively or within 28 days of surgery if done postoperatively. Lumbar CSF cytology was obtained > 14 days postoperatively to rule out surgically induced false positives.
All patients were scheduled to receive postoperative craniospinal radiotherapy (CSRT) followed by chemotherapy. Patients with CNS metastasis (intracranial, spinal metastasis or CSF positivity) or residual tumor of > 1.5 cm2 in size on postoperative imaging were considered high risk and received CSRT to doses of 36 Gy with a boost to the posterior fossa for a cumulative dose of 54–55.8 Gy. Patients with local leptomeningeal invasion in our institution were also treated as metastatic (M1 +) tumors [3].
Other patients were considered average risk and received 23.4 Gy CSRT with a boost to the posterior fossa or tumor bed for cumulative boost of 54 Gy. Vincristine 1.5 mg/m2 was given once per week concomitantly during CSRT.
Following radiation and a 6-week rest period, patients received adjuvant (maintenance) chemotherapy. Adjuvant chemotherapy regimens used over the years were: eight in one chemotherapy during 1990–1996; vincristine (VCR), cyclophosphamide, CCNU during 1997–2008; from 2008 to date cisplatin (CDDP), vincristine and cyclophosphamide (modified from COG 99,701, Regimen B) for high risk patients in 28-day cycles for a total of 6 cycles; CCNU, CDDP, VCR (modified from CCG A9961) for low risk patients in 42- day cycles for 8 cycles. [2].
Radiotherapy was postponed to after 3 years of age. In patients younger than 3 years of age; the patients received conventional chemotherapy modified according to age and weight. None of the patients younger than 3 years of age in this study received high dose chemotherapy followed by autologous stem cell transplantation (HDC + ASCT). However, HDC + ASCT is being used recently as the facility became available more widely.
The patients’ paraffin blocks were deparaffinized and rehydrated using xylene. On the next day, citrate buffer was used for pretreatment. Tissue sections were blocked for 10 min with a hydrogen peroxidase solution and then incubated with primary antibody solutions by following the manufacturer-recommended steps for each stain. Light microscopy was used to evaluate the staining patterns of the sections. The patients were subgrouped on the basis of previous consensus reports detailing the staining features of each group. The staining patterns are summarized in Table 1.
p53 protein overexpression was assessed immunohistochemically by using p53 monoclonal antibody (DO-7, Dako, Denmark). The percentage of p53 positive tumor cells was estimated in the SHH subgroup by counting the number of immunoreactive cells. Cases with more than 5% immunopositive tumor cells were scored as positive.
N-myc was assessed with the FISH technique. In each case, hematoxylin–eosin slides were reevaluated, the tumor areas were selected and 4 µm thick sections were obtained from the paraffin embedded tumor blocks. Two-color interphase FISH was performed using a target probe for MYCN (Vysis LSI N-MYC (2p24) Spectrum Green/CEP 2 Spectrum Orange Probe). Signals were scored in at least 100 non-overlapping, intact nuclei.
The immunohistochemistry for β-catenin, YAP-1 and GAB-1and FISH for N-myc were provided by a grant (Scientific Research Projects Coordination Unit of Istanbul University. Project number: 34871).
The survival analyses were performed by the Statistical Package for Social Sciences (SPSS) version 13.0. The Kaplan–Meier analysis was used for evaluation of survival rates, the log-rank test was used for comparing survival among the groups. Overall survival (OS) was estimated as the time interval from the date of diagnosis to the date of death from any cause or time of latest follow-up. Event-free survival (EFS) was defined as the relapsed time between the date of diagnosis and the date of first relapse, progression or death from any cause.
Results
Of the 58 patients, 35 (60%) were male and 23 (40%) were female. The median age was 6 years (range, 8–19 years), and 11 patients (19%) were aged < 3 years. The most common symptom during admission was nausea and vomiting (35/58, 60%). Most of the patients had headache (25/58, 43%) and ataxia (22/58, 40%). The patients’ characteristics are summarized in Table 2.
The neurological examination results of 15 patients were normal. The examination results of nine patients improved postoperatively. Among the patients, two had papilledema, one had upward gaze palsy, three had various degrees of cognitive decline and three had cerebellar signs, all of which resolved postoperatively. Eight patients were neurologically worse after surgery. Two patients had a previously nonexistent dysmetria, two had CN VII palsy, and one had CN VI palsy. Two patients developed cerebellar mutism, which resolved months after surgery. One patient had a cognitive decline. One patient with recurrent and metastatic lesions only 1 week after surgery died thereafter. The remaining patients were neurologically stable postoperatively (40/58, 69%).
Thirty-one patients had hydrocephalus at the initial admission (31/58, 53%). Eighteen patients were shunt-dependent after tumor resection (18/31, 58%). Among the hydrocephalic patients, four were attributed to the WNT group, nine to the SHH group, and ten to the non-WNT non-SHH group. Eight of the 31 patients could not be classified. The hydrocephalus rates showed no statistically significant differences between the subgroups (p = 0.332). Among these 18 patients 14 had metastatic disease of any kind, which may be the reason why temporary solutions such as external ventricular drainages didn’t work and further shunting operations had to be done in these patients. The presence of hydrocephalus did not have an effect on mortality (p = 0.144).
Forty-two patients (72%) had metastatic disease according to spinal MRI, histopathology and cytology. Among these patients 21 (50%) showed atypical cells in cytological studies, 22 (52%) had metastatic lesions in spinal MRI and 28 patients (67%) were found to have LLMI in histopathological examination. Of the 28 with LMMI, 17 had also metastatic disease either in the CSF cytology or spinal axis MRI. Detailed data can be found in Table 3.
GTR was achieved in 44 patients (76%). Thirteen patients (22%) underwent a subtotal resection (STR). Only one patient underwent surgery only for tissue sampling; the patient who had a diffuse lesion all over the cerebellum and was admitted to the emergency department in a pre-exitus state due to tonsillar herniation. After an urgent external ventricular drain insertion, an emergent posterior fossa decompression was performed, and a biopsy sample was taken from the cerebellar tissue, which was later found to be a medulloblastoma. Out of 13 patients who underwent STR, two patients were reoperated aiming GTR in early postoperative period. However, due to patients’ morbidities or surgically challenging localization of residual tumor, 11 patients didn’t receive further resection.
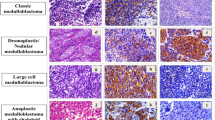
After immunohistochemical staining, 43 patients could be assigned to various subgroups as follows: five in the WNT group (5/43, 12%), 14 in the SHH group (32.5%), and 24 in the non-WNT non-SHH group (56%). We could not determine the subgroup in 15 patients owing to technical issues. The demographic characteristics of each group are summarized in Table 4. The immunostaining results for all the patients are shown in Fig. 1.
Positive staining of β-catenin, both nuclear and cytoplasmic a; positive YAP-1 staining b; and negative GAB-1 staining c are typical in the WNT subgroup. In the SHH subgroup, β-catenin staining is positive only in the cytoplasm d, whereas both YAP-1 and GAB-1 staining are positive e and f. The non-WNT non-SHH subgroup is characterized by cytoplasmic β-catenin staining g with neither YAP-1 nor GAB-1 staining i
Radiogenomics of the patients was also assessed in order to find a relationship between tumor localization and subgroup. Four patients (%80) out of five WNT subgroup patients had tumors that are were localized in 4th ventricle with either uni- or bilateral lateral recess extension. SHH subgroup patients had ten midline tumors and only four patients had hemispheric tumor. Mean age of these patients are 6 (4–14). Non- WNT non-SHH subgroup mostly had midline tumors (21, 87.5%). Radiogenomic characteristics of the patients are summarized in Table 5.
The median follow-up period of all patients was 6.7 years (1 month to 13 years).
Survival analyses were performed to investigate the impact of clinical and molecular characteristics on prognosis. The 5-year overall survival (OS) and event-free survival (EFS) of the 58 patients were 72% and 54.8%respectively. Of the 58 patients 25 recurred at a median time of 26 months (1–158 months).
Survival data of 58 patients were evaluated according to clinical risk classification. Sixteen patients (28%) received average risk regimens, whereas 42 patients (72%) were treated as high-risk patients. The 5-year OS and EFS of average risk patients were both 86.5%. The 5-year OS and EFS of high-risk patients were 66.1% and 43.3% respectively. The OS (p-value:0.092) and EFS (p-value:0.011) were lower in the high-risk group in comparison to the average risk group.
In the WNT group, one patient recurred, similarly there was one mortality (the 5-year OS 80%; EFS: 80%). The 5-year OS and EFS in the SHH subgroup were 68.2% and 57.1% respectively. The survival data of the SHH group were grouped according to their p53 mutation status. Among three of nine patients with p53 mutation, three had a recurrence (33%) and three died (33%) (5-year OS: 64.8%; EFS: 66.7%). Among the patients who were p53 wild type, three had a recurrence (60%) whereas one died (20%) (5-year OS: 75%, EFS: 40%) (Table 6). In non-WNT non-SHH subgroup, seven patients died, whereas eleven patients had a recurrence (OS: 81.6%; EFS: 51.8%). There was no statistically significant difference among OS and EFS of subgroups (p-values: 0.810, 0.429).
We investigated whether differences existed among the different age groups. Seven of 11 patients aged < 3 years and 18 of 47 patients aged > 3 years had a recurrence (36.4% vs 59.2%, p-value = 0.039). The 5-year OS for patients < 3 years of age was lower compared to patients ≥ 3 years of age (54.4% vs 76.2%, p-value:0.177). All patients under 3-years of age were attributed to the high-risk group. Among 47 patients ≥ 3 years of age, 31 patients were attributed to high-risk, whereas 16 patients were managed as average risk patients. 5- year OS of these high vs average risk patients were 63.9% and 86.5% respectively (p-value:0.08). Similarly, 5-year EFS of these patients were 45.7% and 86.7% (p-value:0.22).
We also investigated the incidence of metastatic disease. Patients with LMMI in our center were treated as CSF cytology positive tumors. 5-year EFS of LMMI positive patients was 55%, whereas EFS was 58% in negative group (p:0.809). Similarly, no statistically significant difference in mortality rates were found (LMMI ( +) OS 66%; LMMI (-) OS 62.5%; p-value: 0.980).
Discussion
Medulloblastoma is a malignant brain tumor that is predominantly common in the pediatric population [8]. Recent studies demonstrated different subgroups of medulloblastoma with distinct clinical or molecular features. We aimed to classify surgically treated pediatric medulloblastoma cases in our institution into clinical and molecular subgroups and investigate whether their clinical courses correlate with those of the cases reported in the literature.
CNS tumors constitute 20% of all pediatric malignancies treated in the Istanbul University, Oncology Institute (IUOI) [17]. This study included all children with medulloblastoma that were operated in the Istanbul University, Department of Neurosurgery and further treated in the IUOI. The median age at disease onset was reported to be 8 years, and the incidence was slightly dominant among males [11]. Similar to that reported in the literature, the median age in our series was 6 years. The male-to-female ratio was 1.5:1 as in previous series (35 males and 23 females) [40]. In a previous series, the most common symptoms were cognitive decline, nausea and vomiting, headache, and ataxia. In our series, the most common symptom was nausea and vomiting (60%), followed by headache (43%) and ataxia (40%). However, in our series, cognitive decline was not as common as previously reported (13%) [28]. As in the literature, classic morphology was the most common (43, 74%) and anaplastic morphology was the least common in our patients (2, 3%).
In developed countries, the survival for patients with average-risk medulloblastoma treated with adjuvant radiation and chemotherapy is over 80% at 5 years [15]. Results of average risk patients in our study are in parallel with the literature as the OS and EFS of the average risk patients were 86.5%. In the high-risk patients using CSRT with chemotherapy, 45–50% of patients are disease-free at 5 years. When chemotherapy dose intensity is increased with more aggressive regimens, higher survival rates have been reported. Amar et al., have reported 5- year EFS of 58.7% in high risk patients older than 3 years old age [4, 15]. The OS and EFS of the high-risk patients ≥ 3 years of age (%71 and %59) in our study are comparable with these results. In the recent COG-high risk study, the 5-year EFS was 62.9% and OS was 73.4% (CI 66.7%-80.1%) [20].
In our study, the EFS of high-risk patients is significantly lower in comparison to average risk patients and the EFS in patients < 3 years of age is lower than those ≥ 3 years old. Although, the OS in both respective groups is numerically lower than the compared group, it is not statistically significant. This may be due to the extended survival time by the use of salvage treatments (reoperations, second and further line chemotherapies, reirradiation).
The identification of molecular features in the tumor specimen and the incorporation of these features to the clinicopathologic classification has been studied aiming to be used in risk adapted treatment. In the International phase III trial (SJMB03), a comprehensive clinicomolecular risk factor analysis identified three low-risk groups (WNT, low-risk SHH, and low-risk combined groups 3 and 4) with excellent (5-year EFS > 90%) and two very high-risk groups (high-risk SHH and high-risk combined groups 3 and 4) with poor survival (5-year EFS < 60%) [15]. In the recent, COF high risk trial, 5-year OS differed by molecular subgroups (p-value:0.006): WNT pathway activated; SHH pathway activated, 53.6%; group 3, 73.7%; and group 4, 76.9%; the addition of carboplatinum concomitant with radiotherapy increased the EFS significantly only in the group 3 subgroup [20].
In our center, routine molecular studies were not performed in medulloblastoma patients in the pathology department until very recently. Thus, in this retrospective study, we aimed to investigate the molecular features and the correlation with demographic, clinical characteristics and outcome in our cohort of medulloblastoma patients.
Though the rarest (10%), the WNT subgroup has the best prognosis and is characterized by somatic mutations of the CTNNB1 gene [7, 19, 49]. Mortality is usually due to therapy complications or secondary tumors [14]. In our series, there were only five WNT patients, thus making comparison with results in the literature is difficult. In our WNT subgroup one had a recurrence and one died in the 5-year postoperative period. In addition, three patients had CNS metastatic disease (cytologic, spinal axis or LMMI) at the time of diagnosis, which is contrary to the previously reported rates of 5–10% [43]. However, in contrast to previous studies with equal sex distribution, our study consisted mostly of female patients (4/5). The mean age was 6 years. With classic morphology being predominant, histomorphological studies were in accordance with the literature [21, 25]. One patient had hemispheric tumor, while four (80%) had tumors with lateral recess extension, which were comparable with previous reports, since WNT subgroup tumors are reported to locate in the midline with lateral extension in accordance with their origin [22, 29, 30]. None of our patients had intratumoral hemorrhage preoperatively, which is uncommon in WNT subgroup tumors. Similarly, Keil et al. reported absence of hemorrhage in their WNT subgroup patients however, their series are comprised of adult patients [18]. Three of the five patients (60%) had p53 mutations. Similar to the report of Ramaswamy et al., p53 mutation in the WNT subgroup did not have any effect on mortality and recurrence [31].
The SHH subgroup has mutations in the SHH signaling pathway and mainly has a desmoplastic/nodular and large cell morphology. This subgroup is the most common [42] and shows immunostaining for GAB-1 [13]. The SHH subgroup, which accounts for 30% of medulloblastomas, consisted of 14 patients in our series. Though ranking as second in our patient group, because we combined groups 3 and 4 into the non-WNT non-SHH group, the incidence of this subgroup correlated with that reported in the literature. Contrary to previous reports, our study did not have an equal sex distribution (11 males and three females). The SHH subgroup is known to have a desmoplastic morphology predominantly. As majority of the patients in the SHH subgroup had a desmoplastic histology. Vast majority of patients had midline/vermian tumor (10/14) while four had hemispheric tumors. Our results are incompatible with literature since SHH subgroup tumors are known to be prone to locate in cerebellar hemispheres as they originate from external granular layer of cerebellum [9]. Rate of lateral localization in SHH subgroup tumors increases with age as was the case within our series [10]. All four patients who had laterally located tumors in this subgroup were older than 3 years of age (median:6, 4–14).
p53 mutation in the SHH subgroup leads to more aggressive tumor, worse prognosis [48], and drug resistance. Several studies have been conducted to block the SHH signaling pathway [23, 37]. p53 mutation not only leads to higher mortality but also is suggested to cause less radiosensitive tumors [47]. Rausch et al. reported that p53-mutated SHH subgroup tumors are more likely to have n-myc amplification [36]. Among our patients, nine had the p53 mutant (64%), although in the literature, the p53-mutated SHH subgroup constituted 20% of all cases [46]. Only three patients were metastasis free, which is compatible with the aggressive nature of p53 mutated SHH subgroup. One patient in the group showed n-myc amplification, consistent with the literature. Three patients recurred in each subgroup, p53 positivity was not found to be significant for OS and EFS. Overall OS and EFS in SHH subgroup are 68.2% and 57% respectively.
The pathophysiology of group 3 is through n-myc amplification, with the worst prognosis [24]. Patients in group 3 mostly have the classic morphology, and most cases with large cell morphology, which usually metastasizes, belong to this subgroup [35]. Though n-myc amplification can be seen in Group 3, the major concern as a poor prognostic marker is MYC/MYC-C amplification. However, we were not able to search for MYC-C amplification in our cohort. This group has high rates of leptomeningeal spread (45–50%) [33]. Group 4 is a prototype of medulloblastomas and has the classic morphology with moderate prognosis [1, 6, 34]. Groups 3 and 4 are generally regarded as non-WNT non-SHH medulloblastomas, as distinguishing these subgroups are not generally possible by immunohistochemistry [26]. Our non-WNT non-SHH subgroup consisted of 24 patients. The mean age was found to be 6 years (1–16), and male dominance was observed as expected (16 males and eight females) [26]. Lesions of this subgroup usually locate in the midline, similarly in our patient group (22/25, 88%) [45]. Dasgupta et al. reported that these tumors can be seen as ill-defined infiltrative lesions [10]. One of our patients, who undergone emergent posterior fossa decompression and cerebellar tissue sampling without significant tumor appearance, was also attributed to non-WNT non-SHH subgroup. The most common histomorphological pattern was classic medulloblastoma, consistent with the literature. Overall, morphological studies revealed the anaplastic variant in two patients, both belonging to the non-WNT non-SHH group, as in the literature. Eberthart et al. concluded that n-myc amplification is a poor prognostic marker mostly found in anaplastic variants [12]. Similarly, one of the two patients with the anaplastic variant in our series had n-myc amplification. Although group 3 is known to have a large cell morphology, this was not found in any of our patients. 18 patients (75%) showed various kinds of metastatic disease, as this subgroup is known to be metastatic. Eleven patients recurred in this subgroup while seven patients died (OS: 82%; EFS: 52%). In the recent COG-high risk trial, the OS of the groups 3 (73.7%); and group 4 (76.9%) were higher than the SHH pathway activated subgroup (53.6%) [20], which was similar in our study (SHH subgroup, OS: 68.2% EFS: 57.1%; non-WNT non-SHH subgroup, OS: 81.6%; EFS: 51.8%).
The prognosis of patients with medulloblastoma is determined on the basis of tumor histology, morphology, tumor dissemination, and postoperative residual tumor volume. These criteria allow us to divide patients into standard and high-risk groups. The standard-risk group consists of patients aged > 3 years with no metastatic disease and residual tumor < 1.5 cm2 [44]. Depending on the risk group, different oncological treatment modalities are applied and survival rates vary [19, 41]. However, in addition to the risk group, molecular features of the tumor also effect the clinical outcome. Ramaswamy et al. divided pediatric patients with medulloblastoma into four risk groups based on their clinical and molecular features [32, 34] (Table 7). This classification enables physicians to prescribe more individualized multimodal therapies, but our knowledge is insufficient to design case-specific treatments to reduce mortality rates. Recent studies have shown that rapid determination of the tumor subgroup may lead physicians to create novel personalized treatments, but this is still not used routinely in everyday practice [39].
However, in our patients we did not observe a difference in OS or EFS according molecular subgroups. This may be due to the fact that we had a high number of high-risk patients in each molecular subgroup and they were treated as high-risk.
In this study patients were treated per clinical staging. All radiological diagnostics, all treatment, routine pathological evaluation is reimbursed by the government for all patients. The addition of the IHC for beta catenin, YAP-1, GAB-1 and N-myc by FISH (provided by a grant for this study) resulted in a financial load of 130 Euros for each patient in addition to the extra work for the limited number of technicians in the lab. These studies could be done in private pathology labs, however, most of the families could not afford it. The recent advances in oncology by the addition of molecular/genetic studies is remarkable. The relevant question is which of these molecular studies are cost effective and indispensable for LMICs and would lead to relevant changes in treatment such as less toxic treatments in low risk groups and aggressive treatments in high risk groups. According to the results of this study, we cannot conclude whether, the addition of molecular features would have changed our treatment strategy in our patients. However, there are limitations in this study, it is a retrospective evaluation, it is a single center study, the number of patients is limited.
Conclusion
Medulloblastoma is the most common malignant brain tumor in childhood. Risk stratification of patients is useful for physicians to plan risk-adapted treatments. Recent studies have classified the tumor into subgroups with distinct molecular features and clinical outcomes. However, current practice does not involve routine immunohistochemistry profiling of all tumors in LMIC. This study reports the demographic, clinical characteristics and outcome of a large cohort of consecutive pediatric medulloblastoma patients treated multidisciplinarily in a university hospital in a middle income country which is a major referral center for neurooncology [17]. The study aims to evaluate the molecular subgrouping and its correlation to clinical subgrouping. A clinicomolecular risk stratification may provide less toxic treatments for low risk and more aggressive treatments for high-risk patients. Further studies with larger groups are needed to reveal the cost effectiveness of the addition of molecular grouping to clinical grouping especially for LMIC.
References
Archer TC, Mahoney EL, Pomeroy SL (2017) Medulloblastoma: Molecular Classification-Based Personal Therapeutics. Neurotherapeutics. https://doi.org/10.1007/s13311-017-0526-y
Ayan I, Darendeliler E, Kebudi R, Barlas O, Ayan N, Bayindir Ç, Bahar S, Bilge N (1995) Evaluation of response to postradiation eight in one chemotherapy in childhood brain tumors. J Neurooncol. https://doi.org/10.1007/BF01054770
Ayan I, Kebudi R, Bayindir Ç, Darendeliler E (1997) Microscopic local leptomeningeal invasion at diagnosis of medulloblastoma. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/s0360-3016(97)00083-7
Bouffet E (2021) Management of high-risk medulloblastoma. Neurochirurgie. https://doi.org/10.1016/j.neuchi.2019.05.007
Cavalli FMG, Remke M, Rampasek L et al (2017) Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell. https://doi.org/10.1016/j.ccell.2017.05.005
Cho YJ, Tsherniak A, Tamayo P et al (2011) Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. https://doi.org/10.1200/JCO.2010.28.5148
Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW (2006) Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell cycle (Georgetown, Tex). https://doi.org/10.4161/cc.5.22.3446
Coluccia D, Figuereido C, Isik S, Smith C, Rutka JT (2016) Medulloblastoma: Tumor biology and relevance to treatment and prognosis paradigm. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-016-0644-7
Dasgupta A, Gupta T (2018) Radiogenomics of medulloblastoma: imaging surrogates of molecular biology. Journal of Translational Genetics and Genomics. https://doi.org/10.20517/jtgg.2018.21
Dasgupta A, Gupta T, Pungavkar S et al (2019) Nomograms based on preoperative multiparametric magnetic resonance imaging for prediction of molecular subgrouping in medulloblastoma: Results from a radiogenomics study of 111 patients. Neuro Oncol. https://doi.org/10.1093/neuonc/noy093
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. https://doi.org/10.1093/neuonc/nos218
Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, Dang CV, Burger PC (2004) Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and Anaplasia. J Neuropathol Exp Neurol. https://doi.org/10.1093/jnen/63.5.441
Ellison DW, Dalton J, Kocak M et al (2011) Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. https://doi.org/10.1007/s00401-011-0800-8
Ellison DW, Kocak M, Dalton J et al (2011) Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. https://doi.org/10.1200/JCO.2010.30.2810
Gajjar A, Robinson GW, Smith KS et al (2021) Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. https://doi.org/10.1200/JCO.20.01372
Kaur K, Kakkar A, Kumar A, Mallick S, Julka PK, Gupta D, Suri A, Suri V, Sharma MC, Sarkar C (2016) Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. https://doi.org/10.1111/bpa.12293
Kebudi R, Alkaya DU (2021) Epidemiology and survival of childhood cancer in Turkey. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.28754
Keil VC, Warmuth-Metz M, Reh C et al (2017) Imaging biomarkers for adult medulloblastomas: Genetic entities may be identified by their MR imaging radiophenotype. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5313
Kool M, Korshunov A, Remke M et al (2012) Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. https://doi.org/10.1007/s00401-012-0958-8
Leary SES, Packer RJ, Li Y et al (2021) Efficacy of Carboplatin and Isotretinoin in Children With High-risk Medulloblastoma. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2021.2224
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1545-1
Mata-Mbemba D, Zapotocky M, Laughlin S, Taylor MD, Ramaswamy V, Raybaud C (2018) MRI characteristics of primary tumors and metastatic lesions in molecular subgroups of pediatric medulloblastoma: A single-center study. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5578
Neumann JE, Swartling FJ, Schüller U (2017) Medulloblastoma: experimental models and reality. Acta Neuropathol. https://doi.org/10.1007/s00401-017-1753-3
Northcott PA, Korshunov A, Witt H et al (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. https://doi.org/10.1200/JCO.2009.27.4324
Northcott PA, Shih DJH, Peacock J et al (2012) Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. https://doi.org/10.1038/nature11327
Orr BA (2020) Pathology, diagnostics, and classification of medulloblastoma. Brain Pathol. https://doi.org/10.1111/bpa.12837
Özişik PA, Akalan N, Palaoǧlu S, Topçu M (2002) Medulloblastoma in a child with the metabolic disease L-2-hydroxyglutaric aciduria. Pediatr Neurosurg. https://doi.org/10.1159/000065097
Park TS, Hoffman HJ, Hendrick EB, Humphreys RP, Becker LE (1983) Medulloblastoma: Clinical presentation and management. Experience at the Hospital for Sick Children, Toronto, 1950–1980. J Neurosurg. https://doi.org/10.3171/jns.1983.58.4.0543
Patay Z, DeSain LA, Hwang SN, Coan A, Li Y, Ellison DW (2015) MR imaging characteristics of wingless-type-subgroup pediatric medulloblastoma. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4495
Perreault S, Ramaswamy V, Achrol AS et al (2014) MRI surrogates for molecular subgroups of medulloblastoma. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A3990
Ramaswamy V, Nör C, Taylor MD (2016) p53 and meduloblastoma. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a026278
Ramaswamy V, Remke M, Adamski J et al (2016) Medulloblastoma subgroup-specific outcomes in irradiated children: Who are the true high-risk patients? Neuro Oncol. https://doi.org/10.1093/neuonc/nou357
Ramaswamy V, Remke M, Bouffet E et al (2013) Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. https://doi.org/10.1016/S1470-2045(13)70449-2
Ramaswamy V, Remke M, Bouffet E et al (2016) Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1569-6
Ramaswamy V, Taylor MD (2017) Medulloblastoma: From myth to molecular. J Clin Oncol. https://doi.org/10.1200/JCO.2017.72.7842
Rausch T, Jones DTW, Zapatka M et al (2012) Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. https://doi.org/10.1016/j.cell.2011.12.013
Rodriguez-Blanco J, Li B, Long J et al (2019) A CK1a Activator Penetrates the Brain and Shows Efficacy Against Drug-resistant Metastatic Medulloblastoma. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-1319
Schwalbe EC, Lindsey JC, Nakjang S et al (2017) Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. https://doi.org/10.1016/S1470-2045(17)30243-7
Schwalbe EC, Lindsey JC, Straughton D et al (2011) Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-10-2210
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2012.04.009
Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, Burger P, Barnes P, Kun L (2013) High-risk medulloblastoma: A pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. https://doi.org/10.1200/JCO.2012.43.9984
Taylor MD, Mainprize TG, Rutka JT (2000) Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: A review. Neurosurgery. https://doi.org/10.1097/00006123-200010000-00020
Taylor MD, Northcott PA, Korshunov A et al (2012) Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. https://doi.org/10.1007/s00401-011-0922-z
Thomas A, Noël G (2019) Medulloblastoma: Optimizing care with a multidisciplinary approach. J Multidiscip Healthc. https://doi.org/10.2147/JMDH.S167808
Wefers AK, Warmuth-Metz M, Pöschl J et al (2014) Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol. https://doi.org/10.1007/s00401-014-1271-5
Wei Y, Maximov V, Morrissy SA, Taylor MD, Pallas DC, Kenney AM (2019) P53 function is compromised by inhibitor 2 of phosphatase 2A in sonic hedgehog medulloblastoma. Mol Cancer Res. https://doi.org/10.1158/1541-7786.MCR-18-0485
Williams JR, Zhang Y, Zhou H, Gridley DS, Koch CJ, Russell J, Slater JS, Little JB (2008) A quantitative overview of radiosensitivity of human tumor cells across histological type and TP53 status. Int J Radiat Biol. https://doi.org/10.1080/09553000801953342
Zhukova N, Ramaswamy V, Remke M et al (2013) Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. https://doi.org/10.1200/JCO.2012.48.5052
Zurawel RH, Chiappa SA, Allen C, Raffel C (1998) Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res
Acknowledgements
We are thankful to Prof. Dr. Bilge Bilgiç, senior neuropathologist of our university, for her helps with reevaluating pathology slides and immunohistochemistry stains. Furthermore, we are grateful to our senior radiation oncologists Prof. Dr. Mehmet Emin Darendeliler, with our deepest condolences upon his unexpected loss, and Prof Dr. Fulya Ağaoğlu.
Funding
This study was funded by the Scientific Research Projects Coordination Unit of Istanbul University (project No. 34871). Institutional ethics committee approved this study (file No. 2018/280). Informed consent was signed by patients’ relatives before surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rejin Kebudi is a senior author.
Rights and permissions
About this article
Cite this article
Aras, Y., Dölen, D., İribas Çelik, A. et al. Effects of different molecular subtypes and tumor biology on the prognosis of medulloblastoma. Childs Nerv Syst 37, 3733–3742 (2021). https://doi.org/10.1007/s00381-021-05350-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05350-1