Abstract
Background
An important feature of hydrocephalus is the alteration of the cerebral spinal fluid (CSF) homeostasis. New insights in the understanding of production, secretion, and absorption of CSF, along with the discovery of the glymphatic system (GS), can be useful for a better understanding and treatment of hydrocephalus in disorders with CSF overproduction.
Case description
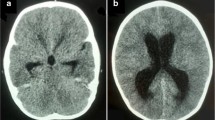
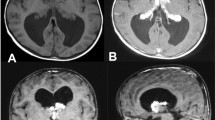
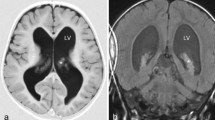
A 1-year-old patient was diagnosed with communicating hydrocephalus; ventricle peritoneal shunt (VPS) is installed and ascites developed. VPS is exposed, yielding volumes of 1000-1200ml/day CSF per day. MRI is performed showing generalized choroidal plexus hyperplasia. Bilateral endoscopic coagulation of thechoroid plexus was performed in 2 stages (CPC) however the high rate of CSF production persisted, needing a bilateral plexectomy through septostomy, which finally decreased the CSF outflow.
Discussion
New knowledge about the CSF physiology will help to propose better treatment depending on the cause of the hydrocephalus. The GS is becoming an additional reason to better study and develop new therapies focused of the modulation of alternative CSF reabsorption.
Conclusion
Despite the current knowledge about hydrocephalus, we remain without a complete understanding of the pathophysiology of this condition. GS could be more important than conventional concept of reabsorption of CSF in the arachnoid villi, therefore GS could be a new key point, which will guide future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is a diverse integration of conditions characterized by a disorder on cerebral spinal fluid (CSF) physiology that usually drives to an abnormal enlargement of the cerebral ventricles and is regularly linked with raised intracranial pressure [1, 2]. If untreated, hydrocephalus could produce brain herniation and subsequent decease [1]. In addition, it is a frequent cause of pediatric disease and death, representing a foremost monetary burden on health care budget [3]. In the pediatric population, hydrocephalus acquires complexity in its anatomy and mechanisms [4, 5]. Clinical manifestations are linked to the age of onset; children in early infancy complain more commonly for headache, usually associated with progressive macrocephaly and visual disturbances, and older kids present impairment and decreased levels of consciousness due to raised intracranial pressure [2, 5]. Hydrocephalus might disturb cerebral development and prompt motor, sensitive, and cognitive deficits [6]. The management of hydrocephalus aims to relieve the symptoms that regularly implicates the placement of ventricle-peritoneal shunts (VPSs) which require neurosurgical intervention [3]. Current paradigm of impairment in the reabsorption of CSF in arachnoid villi could be renewed because of increasing knowledge of CSF dynamics. Alternative pathways of CSF drainage are under extensive research, and their relevance in the management of hydrocephalus could be higher than we think. Besides, management alternatives for hydrocephalus remain unchanged during the last years [7]. Nevertheless, evidence shows that in order to describe its pathogenesis, genetic factors get a relevant role in several types of hydrocephalus [8]. Therefore, we report a bilateral hyperplasia of choroid plexus (CP) with severe CSF production in a 1-year-old boy, and we review the underlying physiology of the CSF in children and new insights about the relation between hydrocephalus and the glymphatic system (GS).
Case description
A 1-year-old patient, product of preterm gestation because of premature rupture of membranes, and previously healthy, presents with vomiting and irritability. Communicating hydrocephalus is evident in computed tomography (CT); VPS is placed, and 45 days after surgery, the patient developed ascites. CT abdomen shows free fluid in the cavity, without a solid or hollow viscera lesion. VPS is externalized, reporting drainage volumes of 1000–1200 ml/day of CSF, without signs of infection. Given such high output, magnetic resonance imaging (MRI) is performed showing bilateral CP hyperplasia (Figs. 1 and 2). The bilateral endoscopic procedure is performed in 2 stages: first, only CP cauterization (CPC). Subsequently, CSF flow decreases by 800 ml/day, and bilateral plexectomy was performed using a right frontal approach and performing a posterior septostomy, decreasing CSF outflow to 120 ml/day. It is decided to install VPS. A biopsy confirmed the diagnosis of CP hyperplasia.
Historic model of CSF physiology
In the last century, the “bulk-flow” model of CSF homeostasis was the standard in which pathogenesis of hydrocephaly had the best understanding [5, 9]. In this paradigm, the CSF is excreted in the CP in the brain ventricles, then leaves into the subarachnoid space where it flows and is absorbed by the arachnoid granulations in the deep vein draining. This model states hydrocephalus is due to the obstruction of CSF circulation anywhere along the aforementioned pathway.
Relatively new, another “hydrodynamic model” where the role of the atypical intracranial pulsations might cause the pathological condition [10]. Better accounts for annotations cannot be explained with the bulk flow model and are founded in the following premises:
-
1.
Functional arachnoid granulations cannot be found in some pediatric populations (infants < 2 years) [11].
-
2.
The ependyma and other structures different from CP might supply a significant quantity of CSF [12].
-
3.
Increasing the intra-ventricular CSF osmolarity is sufficient to cause experimental hydrocephalus [13].
-
4.
Despite unobstructed flow and normal mean CFS pressures, increasing intra-ventricular fluid pulsation amplitudes by itself are enough to produce hydrocephalus [14].
Some types of hydrocephalus appear (mostly) in the pediatric population in which pathogenesis has been neglected toward CSF production; rather, it is attributed, lastly, to an anomalous accumulation of CSF. Nevertheless, pharmacological (e.g., acetazolamide) and non-conservative (e.g., CPC) alternatives that reduce CSF excretion have demonstrated effectiveness for particular hydrocephalus types.
CSF secretion and production
The CP is a vastly vascularized capillary bed of fenestrated vessels fenced by polarized cube-shaped epithelial cells faced through tight junctions [15]. In contrast to the blood–brain barrier (BBB), which is constituted by tight junction, the blood-CSF barrier is constituted by the tight junctions of CP epithelia. The fenestrated capillaries of the CP have the feature of not being completely impermeable and, unlike the brain endothelial cells, willingly allow the diffusion of ions and other smalls particles [16]. Epithelial cells in the CP have diverse ion channels and transporters that are responsible for most of CSF secretion: [17]
-
1.
Na/K-ATPase is disposed toward the lumen (apical membrane), is central to CSF production, and prompts the hydro-electrolytic gradient for Na+ that is imported utilizing: (a) Na+/H+ exchanger, (b) NHE, (c) Na + /HCO3− cotransporter, (d) NCBE basolaterally
-
2.
Co-import of HCO3− via NCBE and hydration of CO2 by carbon anhydrase (CA) increases the concentrations of HCO3− intracellularly, which prompts a hydro-electrolytic gradient which modulates the efflux of HCO3− basolaterally situated Cl/HCO3− exchanger, AE2, and apically expressed HCO3− channels
-
3.
The role of AE2 prompts an increment in Cl− concentrations intracellularly, modulating the apically Cl− exporter employing the NKCC1 and Cl− channels
The ending outcome of the aforementioned procedures at the CP epithelial cells is a net flux of Na+, HCO3−, and Cl−, from the vascular compartment through the epithelial cells of the cerebral ventricles, which prompts the hydro-electrolytic gradient that induces water diffuse across AQP1, thus generating the CSF (Fig. 3).
CSF production. Amiloride (K-save diuretic) reduces CSF production by 50%. Ouabaina inhibits K/Na ATPase and can reduce CSF production near to 50–60%. NKCCl can send sodium, clorum, and potassium inward cell for the remaining intracellular equilibrium: Na/Cl/HCO3:18/15/3. These NKCCls are associated to a special protein named SPAK which is sensitive to changes in intracellular clorum level, osmotic stress, and inflammation
The transcellular pathway is the main transporter for CSF [18], and ending solute concentrations of the CSF are carefully modulated and persist quite unchanged [19].
The terminal membrane of CP epithelial cells has vast water leak [20], and the passive movement of water through the transcellular way from the vascular compartment to the ventricles is performed mostly through AQP1 [21]. That is demonstrated thanks to animal studies with AQP1 knockout mice where the permeability of CPE is reduced by 80% [21]. However, an increase in AQP1 expression does not always lead to a rise in the excretory capacity of the CPE by itself, given that water movements require a driving force (osmotic force made by Na/K ATPase and others) [1].
CSF absorption
The CP epithelium (CPE) produces about 80% of CSF, while the remaining 20% is generated from brain interstitial fluid (BIF) [22]. The CPE is between the main competent excretory epithelium in the human organism. It generates a rate of 0.4 ml/min/g of tissue and an excretion rate that is just matched by the proximal tubule of the nephrons and the canals of the exocrine pancreas [19]. The entire amount of CSF is around 150 ml; nevertheless, it is calculated that 500–600 ml is excreted daily. Then, the CSF is reabsorbed by arachnoid granulations. Nonetheless, several of the non-human models which attain to study hydrocephalus [23] and early infancy [24] do not appear to express functional arachnoid granulations. So, there must be other factors, such as BIF, which generate approximately 20% of CSF volume, as aforementioned [25]. The flow of the BIF is estimated between 0.1 and 0.29 μg/g of tissue/min [25]. Besides, BIF is dynamic; it pursues a mostly periventricular pathway and crosses the intricate microanatomy of Virchow-Robin spaces (VRS) [22]. It has been demonstrated that the circulation of BIF is not in a single direction and might influence equally the net CSF excretion and absorption. Therefore, there is persistent intercommunication among BIF and CSF [17]; the makeup of this dynamic system is termed glymphatic system (GS), which is a paravascular path which eases the flow of subarachnoid CSF into BIF and, thus, out through the deep vein draining [17] (Fig. 4).
Neurovascular unit. The “neurovascular unit” is constituted by astrocytes, pericytes, microglia, and even neurons. Contrary to early assumptions, the endothelial barrier carries no AQP4 transporters. Instead, water may cross the endothelium by diffusion, vascular transport, and even against osmotic gradients by means of co-transport with ions and glucose. CSF, cerebrospinal fluid; AQP, aquaporin
These paravascular networks are attached by astrocyte feet expressing Aquaporin 4 (AQP4) [26], which, once it is dysfunctional, can influence or worsen the progression of hydrocephalus [26]. The CP owns the maximum rate of water and ion diffusion of any epithelia in humans [19].
Microscopic anatomy of the Virchow–Robin space
The pia covers the artery unlike on the vein, which is uncovered by pia in the VRS (Fig. 5). The pia sheathes the arteries, but not venous vessels extend into the VRS. In studies of rodents, the VRS space is filled by fluid, electron-microscopic–dense material [27] macrophages, and other blood-borne inflammatory cells [28]. The pia in humans is a barrier constituted by a seemingly continuous stratum of cells, which are united by desmosomes and gap junctions but have no apparent tight junctions [29]. Notably, the injection of tracers into the brain shows no drainage throughout the perivenous canals except if there is a distraction of circulation in cerebral amyloid angiopathy when entering some tracer through the perivenous spaces [30].
The glymphatic system
The dense distribution of lymph vessels is proportional to the rate of tissue metabolic function in each tissue [31]. While the brain and spine are differentiated by a dissimilarly great metabolic rate [32] and the synaptic transmission is finely susceptible to variations in their situations, these lack of traditional lymphatic vessels. CSF is drained into the conventional lymphatic system (lymphatic nodes) by efflux via the olfactory bulb and throughout peripheral nervous fibers [33]. Lately, the relevance of arachnoid granulations in CSF reabsorption has been interrogated [34]. Therefore, efflux throughout peripheral nerve fibers and the olfactory path can signify the most important efflux ways for CSF [33].
The discovery of the glymphatic system
A lymphatic drainage percentage of 50% was calculated based on injections of radio-iodinated albumin (RISA) in the brain of rabbits. Remarkably, considerable RISA presented a draining through the brain perivenous spaces along with that by the route from the subarachnoid space of olfactory lobes into the submucosal spaces of the nose (therefore to the lymphoid vessels) [35].
The dynamics of the glymphatic system
CSF flows into the tissue, then it diffuses by convection through BIF within the tissue on the way to the perivascular space and flows out of the brain into the cervical lymphoid structures [33].
In 2012, employing two-photon microscopic was characterized for the first time in vivo in a mouse model [34]. Moreover, using injected fluorescent tracers in the CSF within the cisterna magna, a study demonstrated CSF quickly arrives at the brain via pial blood vessels situated in the cortex. This penetration was followed by influx into the VRS throughout penetrating arterioles. It was obvious that CSF tracers, instead of being widely and randomly spread in the tissue, arrived at the tissue via periarterial route neighboring the muscle cells in vessels united by perivenous astrocytic end-feet, and ex vivo suggestion demonstrated that tracers quickly left the brain mostly throughout the central deep vessels and the anterolateral caudal rhinal veins [34]. The paravascular glymphatic route guided by AQP4 bulk flow-dependent represents a foremost elimination route of interstitial fluid substance from the nervous tissue [36].
AQPs and other models of water transport
It is well-known that diffusion transportation lacks specificity and is a very low way to move; in contrast, water canals such as the AQPs confer a quick way to diffuse and own a great competence and a high selectivity to transport molecules [37]. There are five different types of AQPs [5]. Trials assessing the structure and function of AQPs showed data suggesting that whether the AQP channels are permeable or not might be modulated and can also present compromise in pathologic states of the brain [38]. Remarkably, AQP1 is found in cells of endothelium along with the organism but cannot be found in the BBB, except in the structures adjacent to ventricles. AQP1 is expressed in the cells disposed into ventricles of CP epithelia, signifying an important role in this structure for CSF production.
Controversially, literature stated that extrachoroidal CSF secretion was notably higher than CSF generated in CP, rather be the most important producer of CSF [39]. The posterior concept is reinforced by the comment that after its intravenous administration, the infiltration and stable concentration of H217O are markedly decreased in ventricular CSF in AQP4 but not in AQP1 knockout mouse models. The authors comment that in conclusion, AQP4 is a higher CSF producer than AQP1 [40]. AQP4 is vastly found in astrocyte foot processes located in the BBB, glia limits with brain surface and VRS, ventricular ependymal cells and subependymal astrocytes [41], and astrocytic end-feet at the presynaptic space of nerve cells and is expressed in the olfactory epithelial cells [42]. Nowadays, it is well known that water penetrates the endothelial cells by simple diffusion and vesicular transport and through the astrocyte foot processes mainly via AQP4 channels [43].
Implications in hydrocephalus
Clinical presentation
The rhythms of CSF secretion and reabsorption have to be balanced. The excess of production can be seen in:
-
(a)
CP hyperplasia [44] (our case)
-
It is also named diffuse villous hyperplasia or villous hypertrophy. It is a rare congenital disorder that yields enlarged and hyper-secreting CSF. There is an increase in the number of CPE cells [1], which denotes an increase in blood flow to choroid plexus, a wider surface of filtration, and, therefore, a higher rate of production of CSF. As we detailed in this case, once ascites were reported, EVD quantified an output rate as high as 1000–1200 ml per day, which doubles the normal production and saturates the drainage systems of CSF we aforementioned. To note, rates of arachnoid villi drainage are pressure-dependent essentially following a kind of first-order kinetic model of reabsorption. When intracranial pressure is 0, 10, 20, or 30 mmH2O, then reabsorption rate is up to 0, 1.52, 6.44, and 18.04 ml/min, respectively. This rate of reabsorption was even more sensitive to changes in pressure in the glymphatic drainage system, which results in a more pronounced reabsorption activity in hydrocephalus [45]. This data reinforces the idea that possibly, the glymphatic pathway becomes even more relevant than arachnoid villi in reabsorption of CSF when there is intracranial hypertension, and therefore, in the onset of communicating hydrocephalus, the modulation of the glymphatic system could be a potential therapeutic target in the management of this disease.
-
(b)
CP papilloma (CPP) [46]
-
It represents 1–4% of all cerebral neoplasm in children. It is a different bulk separated from the CPE and is regularly seen within 2 years of birth [1].
The diagnosis of hydrocephalus with CPP or CP hyperplasia origin is decisive given that the standardized management for them is not a VP shunt; in contrast, tumor or excessive CPE should be resected [44]. The diagnosis is challenging and usually can be confirmed when a performed shunt fails or there is development of ascites, and if the shunt is externalized, the excessive amount of CSF makes the diagnosis [1]. The normal secretion of CSF is 500 mL/day. If CPP or CP hyperplasia appears, then the rate could be as high as 5000 mL/day, and a higher rate is associated with worse hydrocephalus [44]. After surgery (CP cauterization or tumor resection), the rates of CSF production decrease [44], and in some cases, there was no further need for a shunt [46].
Management
Conservative treatment of hydrocephalus by targeting CSF production
Diuretics are the medications more frequently used [1]; however, these drugs are regularly non-effective, develop adverse effects, and have off-target properties in the kidney [1].
-
(a)
Sulfonamide-type acetazolamide generates about 30–60% reduction in CSF rate and 24-h excretion [47]. The fractional result of this inhibitor is described by the expression of a group of CAIII receptors which are not sensible to acetazolamide. This subtype of receptors has been isolated in normal persons and different species models [48].
-
(b)
Loop diuretics: There is evidence showing that furosemide as a KCC inhibitor and bumetanide as an NKCC1 inhibitor, single or combined with the aforementioned, reduce the CSF rate of output in dog and cat models [49].
Animal information also exposes the result of furosemide in interrupting ion transport along the blood-CSF barrier, which decreases the rate of CSF excretion [50]. Given that the outcomes of these drugs were also described in animal-based models in which nephrectomy was performed, there was reported likely secondary diuretic or hemodynamic alterations prompted by renal hydro-electrolytic dysregulation as well as the apparition of acid-base disorders, which uncertainly explicate the reduction in CSF secretion [51].
Even with the theoretical success and hopeful outcomes from animal models, furosemide and acetazolamide have been administered in patients where the posterior period of hemorrhagic hydrocephalus has been reached (n = 177). The outcome unexpectedly showed a representative crossover to the shunt surgery and an augmented rate of neurological manifestations in this group [52]. The literature stated that a representative fraction of the pediatric population progressed into nephron-calcinosis as a consequence of the administration of this drug [53].
Then, according to the Cochrane review, the combination of acetazolamide + furosemide is not effective and neither safe in managing post-hemorrhagic hydrocephalus.
Modulation of CSF production by the surgical intervention of the CP
Surgical procedures that compromise targeting CSF are widely defined by several authors in the last century. Dandy [54] illustrated the first surgery for managing hydrocephalus by ablating the CP.
Current techniques are:
-
(a)
Plexectomy: Some authors informed 37% of successful cases, as dodging of CSF deviation interventions [55]. The first animal study was performed by Milhorat et al. [56], and it was done on monkeys and demonstrated a reduction of CSF production near to 37–40%.
-
(b)
Cauterization (CPC): The study of Pople et al. demonstrated that 36% of the cohort did not crossover to shunt surgery in the mean follow-up period of 10.5 years; the best outcome was in those cases that developed communicating hydrocephalus and in cases with decelerated evolution of ventriculomegaly [57].
Warf et al. [58] in Uganda have used ETV (endoscopic third ventriculostomy) and CPC surgery using an elastic endoscope and monopolar cautery to coagulate the whole CP through both lateral ventricles; they emphasize that ETV might serve as a pulsation absorber.
In comparison with single-ETV, ETV-CPC generated greater outcomes in infants < 1 year of age [58] and in all mentioned etiology subtypes [59,60,61]. The efficacy of ETV-CPC is related to the quantity of CP cauterized [62] and does not harmfully affect cognition in comparison to shunt placement or single-ETV [63]. Physiological adjustment to a modification in the regular secretion of CSF could suggest compensation by the residual CP tissue not cauterized in typical procedure or by upregulation of secondary mechanism of production.
Conclusions
There are several types of hydrocephalus, and its differential diagnosis is a major concern, such as what happens in CP hyperplasia and CPP diagnosis. This kind of hydrocephalus commonly leads to high rate of CSF production. The best mechanism of diagnoses is the MRI; the ideal treatment according to our experience corresponds to total plexectomy followed by total CPC. Despite the current knowledge about hydrocephalus, we remain without a complete understanding of the pathophysiology of this condition. GS could be more important than conventional concept of reabsorption of CSF in the arachnoid villi; therefore, GS could be a new key point, which will guide future investigations. The new concepts of AQPs 1 and 4 are involved in the physiology of the CSF production and open the possibilities of new pharmacological approaches. It is even possible that disorders in AQP1 on endothelial cells in specialized circumventricular organs like subcommisural structure might be associated with congenital hydrocephalus. There are few cases like ours written in the literature; we believe this kind of example switches on the alarms and should be taken into account always in the mind of the neurosurgeons. Further studies are required to corroborate these premises and elucidate the pathophysiological mechanisms underlying CSF circulation diseases.
References
Karimy JK, Duran D, Hu JKJK et al (2016) Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus 41:E10. https://doi.org/10.3171/2016.8.FOCUS16278
Kahle KT, Kulkarni AV, Limbrick DD, Warf BC (2016) Hydrocephalus in children. Lancet 372:788–799. https://doi.org/10.1016/S0140-6736(15)60694-8
Boivin MJ, Kakooza AM, Warf BC et al (2015) Reducing neurodevelopmental disorders and disability through research and interventions. Nature 527:S155–S160
Tully HM, Ishak GE, Rue TC et al (2016) Two hundred thirty-six children with developmental hydrocephalus: causes and clinical consequences. J Child Neurol 31:309–320. https://doi.org/10.1177/0883073815592222
Di Rocco C, Frassanito P (2019) Hydrocephalus: generalities and clinical presentations. In: Di Rocco C, Pang D, Rutka JT (eds) Textbook of Pediatric Neurosurgery. Springer International Publishing, Cham, pp 1–46
Radic JAE, Vincer M, McNeely PD (2015) Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr 15:580–588
McAllister JP, Williams MA, Walker ML et al (2015) An update on research priorities in hydrocephalus: Overview of the third National Institutes of Health-sponsored symposium “opportunities for Hydrocephalus Research: Pathways to Better Outcomes.” J Neurosurg 123:1427–1438. https://doi.org/10.3171/2014.12.JNS132352
Kousi M, Katsanis N (2016) The genetic basis of hydrocephalus. Annu Rev Neurosci 39:409–435. https://doi.org/10.1146/annurev-neuro-070815-014023
Dandy WE (1919) Experimental hydrocephalus. Ann Surg 70:129. https://doi.org/10.1097/00000658-191908000-00001
Bering EA (1962) Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J Neurosurg 19:405–413. https://doi.org/10.3171/jns.1962.19.5.0405
Bateman GA, Brown KM (2012) The measurement of CSF flow through the aqueduct in normal and hydrocephalic children: from where does it come, to where does it go? Child’s Nerv Syst 28:55–63. https://doi.org/10.1007/s00381-011-1617-4
Milhorat TH (1969) Choroid plexus and cerebrospinal fluid production. Science 166:1514–1516. https://doi.org/10.1126/science.166.3912.1514
Krishnamurthy S, Li J, Schultz L, McAllister JP (2009) Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus. Cerebrospinal Fluid Res 6:1–9. https://doi.org/10.1186/1743-8454-6-16
Di Rocco C, Pettorossi VE, Caldarelli M et al (1978) Communicating hydrocephalus induced by mechanically increased amplitude of the intraventricular cerebrospinal fluid pressure: experimental studies. Exp Neurol 59:40–52. https://doi.org/10.1016/0014-4886(78)90199-1
Redzic ZB, Segal MB (2004) The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev 56:1695–1716
Segal MB (1993) Extracellular and cerebrospinal fluids. J Inherit Metab Dis 16:617–638. https://doi.org/10.1007/BF00711896
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40:2583–2599. https://doi.org/10.1007/s11064-015-1581-6
Spector R, Keep RF, Robert Snodgrass S et al (2015) A balanced view of choroid plexus structure and function: focus on adult humans. Exp Neurol 267:78–86
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:1847–1892
Oshio K, Watanabe H, Song Y et al (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J 19:76–78. https://doi.org/10.1096/fj.04-1711fje
Nielsen S, Smith BL, Christensen EI, Agre P (1993) Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA 90:7275–7279. https://doi.org/10.1073/pnas.90.15.7275
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:1–16
Strahle J, Garton HJL, Maher CO et al (2012) Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhagE. Transl Stroke Res 3:25–38
Bateman GA, Alber M, Schuhmann MU (2014) An association between external hydrocephalus in infants and reversible collapse of the venous sinuses. Neuropediatrics 45:183–187. https://doi.org/10.1055/s-0033-1363092
Cserr HF (1988) Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci 529:9–20. https://doi.org/10.1111/j.1749-6632.1988.tb51415.x
Iliff JJ, Chen MJ, Plog BA et al (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34:16180–16193. https://doi.org/10.1523/JNEUROSCI.3020-14.2014
Krisch B, Leonhardt H, Oksche A (1984) Compartments and perivascular arrangement of the meninges covering the cerebral cortex of the rat. Cell Tissue Res 238:459–474. https://doi.org/10.1007/BF00219861
Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58:1–10
Alcolado R, Weller RO, Parrish EP, Garrod D (1988) The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol 14:1–17. https://doi.org/10.1111/j.1365-2990.1988.tb00862.x
Hawkes CA, Härtig W, Kacza J et al (2011) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121:431–443. https://doi.org/10.1007/s00401-011-0801-7
Liao S, Padera TP (2013) Lymphatic function and immune regulation in health and disease. Lymphat Res Biol 11:136–143. https://doi.org/10.1089/lrb.2013.0012
Wang Z, Ying Z, Bosy-Westphal A et al (2011) Evaluation of specific metabolic rates of major organs and tissues: comparison between men and women. Am J Hum Biol 23:333–338. https://doi.org/10.1002/ajhb.21137
Johnston M, Zakharov A, Papaiconomou C et al (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1:1–13. https://doi.org/10.1186/1743-8454-1-2
Biceroglu H, Albayram S, Ogullar S et al (2012) Direct venous spinal reabsorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits - laboratory investigation. J Neurosurg Spine 16:394–401. https://doi.org/10.3171/2011.12.SPINE11108
Bradbury MWB, Cserr HF, Westrop RJ (1981) Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol - Ren Fluid Electrolyte Physiol 9:329–336. https://doi.org/10.1152/ajprenal.1981.240.4.f329
Iliff JJ, Nedergaard M (2013) Is there a cerebral lymphatic system? In: Stroke. Stroke
Agre P (2006) The aquaporin water channels. Proc Am Thorac Soc 3:5–13
Zelenina M (2010) Regulation of brain aquaporins. Neurochem Int 57:468–488
Manley GT, Oshio K, Binder DK et al (2003) Aquaporin-1 expression in human glial tumors suggests a potential novel therapeutic target for tumor-associated edema. Acta Neurochir Suppl 499–502. https://doi.org/10.1007/978-3-7091-0651-8_102
Igarashi H, Tsujita M, Suzuki Y et al (2013) Inhibition of aquaporin-4 significantly increases regional cerebral blood flow. NeuroReport 24:324–328. https://doi.org/10.1097/WNR.0b013e32835fc827
Orešković D, Klarica M (2014) A new look at cerebrospinal fluid movement. Fluids Barriers CNS 11:1–3
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277
Tait MJ, Saadoun S, Bell BA, Papadopoulos MC (2008) Water movements in the brain: role of aquaporins. Trends Neurosci 31:37–43
Aziz AA, Coleman L, Morokoff A, Maixner W (2005) Diffuse choroid plexus hyperplasia: an under-diagnosed cause of hydrocephalus in children? Pediatr Radiol 35:815–818. https://doi.org/10.1007/s00247-005-1456-0
Boulton M, Armstrong D, Flessner M et al (1998) Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol 275:R889–R896. https://doi.org/10.1152/ajpregu.1998.275.3.R889
D’Ambrosio AL, O’Toole JE, Connolly ES, Feldstein NA (2003) Villous hypertrophy versus choroid plexus papilloma: a case report demonstrating a diagnostic role for the proliferation index. Pediatr Neurosurg 39:91–96. https://doi.org/10.1159/000071320
Carrion E, Hertzog JH, Medlock MD et al (2001) Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child 84:68–71. https://doi.org/10.1136/adc.84.1.68
Nógrádi A, Kelly C, Carter ND (1993) Localization of acetazolamide-resistant carbonic anhydrase III in human and rat choroid plexus by immunocytochemistry and in situ hybridisation. Neurosci Lett 151:162–165. https://doi.org/10.1016/0304-3940(93)90011-9
Javaheri S, Wagner KR (1993) Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for NaCl cotransport in the central nervous system. J Clin Invest 92:2257–2261. https://doi.org/10.1172/JCI116829
Johanson CE, Murphy VA, Dyas M (1992) Ethacrynic acid and furosemide alter Cl, K, and Na distribution between blood, choroid plexus, CSF, and brain. Neurochem Res 17:1079–1085. https://doi.org/10.1007/BF00967284
McCarthy KD, Reed DJ (1974) The effect of acetazolamide and furosemide on cerebrospinal fluid production and choroid plexus carbonic anhydrase activity. J Pharmacol Exp Ther 189:194–201
Kennedy CR, Campbell M, Elbourne D et al (1998) International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. Lancet 352:433–440. https://doi.org/10.1016/S0140-6736(97)12390-X
Libenson MH, Kaye EM, Rosman NP, Gilmore HE (1999) Acetazolamide and furosemide for posthemorrhagic hydrocephalus of the newborn. Pediatr Neurol 20:185–191. https://doi.org/10.1016/S0887-8994(98)00127-1
Dandy WE (1918) Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 68:569–579. https://doi.org/10.1097/00000658-191812000-00001
Lapras C, Mertens P, Guilburd JN et al (1988) Choroid plexectomy for the treatment of chronic infected hydrocephalus. Child’s Nerv Syst 4:139–142. https://doi.org/10.1007/BF00270903
Milhorat TH, Hammock MK, Fenstermacher JD et al (1971) Cerebrospinal fluid production by the choroid plexus and brain. Science (80- ) 173:330–332. https://doi.org/10.1126/science.173.3994.330
Pople IK, Ettles D (1995) The role of endoscopic choroid plexus coagulation in the management of hydrocephalus. Neurosurgery 36:698–702. https://doi.org/10.1227/00006123-199504000-00009
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg Pediatr 103:475–481. https://doi.org/10.3171/ped.2005.103.6.0475
Warf BC, Dewan M, Mugamba J (2011) Management of Dandy-Walker complex-associated infant hydrocephalus by combined endoscopic third ventriculostomy and choroid plexus cauterization: Clinical article. J Neurosurg Pediatr 8:377–383. https://doi.org/10.3171/2011.7.PEDS1198
Warf BC (2013) The impact of combined endoscopic third ventriculostomy and choroid plexus cauterization on the management of pediatric hydrocephalus in developing countries. World Neurosurg 79:S23.e13–S23.e15. https://doi.org/10.1016/j.wneu.2011.02.012
Warf BC, Tracy S, Mugamba J (2012) Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants: Clinical article. J Neurosurg Pediatr 10:108–111. https://doi.org/10.3171/2012.4.PEDS1253
Warf BC, Mugamba J, Kulkarni AV (2010) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success: Clinical article. J Neurosurg Pediatr 5:143–148. https://doi.org/10.3171/2009.9.PEDS09196
Warf B, Ondoma S, Kulkarni A et al (2009) Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda: Clinical article. J Neurosurg Pediatr 4:564–570. https://doi.org/10.3171/2009.7.PEDS09136
Acknowledgements
We would like to make special mention to Dr. Jorge Torres for the support provided throughout the accomplishment of this project. Also special thanks to Dr. Claudio Rivas for providing valuable information for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paez-Nova, M., Andaur, K., Campos, G. et al. Bilateral hyperplasia of choroid plexus with severe CSF production: a case report and review of the glymphatic system. Childs Nerv Syst 37, 3521–3529 (2021). https://doi.org/10.1007/s00381-021-05325-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05325-2