Abstract
Purpose
Chyloperitoneum is an extremely rare finding following myelomeningocele (MMC) repair in neonates. We aimed to describe the characteristics of such a case and explore its clinical significance.
Case report
A male baby born at term with open MMC and hydrocephalus underwent MMC repair surgery with rotational flaps on the first postnatal day. The procedure was uneventful. Three days later, he underwent a right ventriculoperitoneal shunt (VPS) insertion. On opening the peritoneum, a remarkable amount of yellowish opaque fluid was observed. Chyloperitoneum was suspected, but the VPS procedure was completed as planned. Biochemical analysis was consistent with that of chyle.
Discussion
Neonatal chylous ascites is a rare condition; hence, available data on pathophysiology and therapy in the literature are scarce. It is postulated that the MMC repair in neonates causes abdominal tautness, which leads to rupture of small lymphatics and raised intraportal pressure. The combination of these two processes results in extravasation of chyle from the gastrointestinal tract. Presence of chyloperitoneum is not a contraindication for VPS insertion.
Conclusion
Chyloperitoneum is an extremely rare sequela of MMC repair in neonates. Pediatric neurosurgeons should be aware of it, especially when a VPS procedure is to follow a repair, in order to know how to deal with it and avoid unnecessary abandonment of the shunt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chyloperitoneum or (in its severe form) chylous ascites, is the accumulation of chyle (lymph) in the peritoneal cavity and was first described by Morton in 1694 [1]. It is an unusual finding with reported incidence between 0.5 and 8.6/100,000 [1,2,3]. It usually occurs due to trauma and rupture of abdominal lymphatic vessels or increased abdominal lymphatic pressure secondary to obstruction [4]. Among the various causes for chylous ascites, malignancy and cirrhosis account for the majority of cases in developed countries, whereas infections, such as tuberculosis and filariasis, are the leading cause in developing countries [4].
Chyloperitoneum is a rare entity in pediatric population, and only 3.7% of neonatal ascites is attributed to accumulation of chyle [5]. Chyloperitoneum is an extremely rare finding following myelomeningocele (MMC) repair in neonates [6]. The primary aim of this article is to describe a case treated in our unit and explore the clinical significance of this finding, in order to make suggestions on its management.
Case report
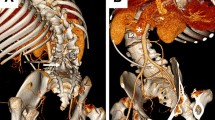
A male baby was born at term with a thoracolumbar open MMC (Fig. 1) and hydrocephalus (Fig. 2), which were diagnosed antenatally. The spinal defect measured approximately 6 × 4 cm (cranial-caudal and transverse dimensions). He underwent MMC repair surgery with bilateral rotational skin flap closure by the plastic surgical team (Fig. 3) on the first postnatal day. The procedure was uneventful, and the baby tolerated the procedure well. Post-operatively, he was nursed in prone position. Three days later, he underwent a right ventriculoperitoneal shunt (VPS) insertion to treat his hydrocephalus. Prior to the shunt surgery, the neonate exhibited no signs or symptoms of abdominal pathology. On opening the peritoneum, remarkable amount of yellowish opaque fluid was found within the peritoneal cavity. Samples (Fig. 4) were sent to the clinical chemistry and microbiology laboratories. Chyloperitoneum was suspected based on previous experience of the senior surgeon (DR), and the VPS procedure was completed as planned.
The collected fluid within minutes formed a coagulum. The biochemical analysis of the fluid revealed triglycerides 6.01 mmol/L (532 mg/dL), total protein 22 g/L, albumin 12 g/L, sodium 140 mmol/L, and potassium 4 mmol/L. The paired plasma total protein value was 44 g/L and albumin level was 23 g/L. These findings were consistent with the diagnosis of chyloperitoneum. The microbiological analysis of the fluid revealed no neutrophils, no organisms, and no growth on culture.
The patient recovered from the VPS surgery well with no concerns regarding the shunt’s function, no signs or symptoms suggesting infection or inflammation, and no abnormal abdominal clinical findings. He was started on oral feeding a few hours after surgery and had no VPS-related issues at three-month follow-up.
Discussion
Neonatal chylous ascites is an uncommon condition; hence, available data on pathophysiology and therapy of chylous ascites in worldwide literature are scarce [1,2,3]. The most common cause, up to 60%, of neonatal chylous ascites is malformation of lymphatic vessels, including stenosis or atresia of the major lacteals, lymphangiomatosis, and mesenteric cysts. In half of neonatal cases, it is idiopathic due to a condition termed “leaky lymphatics” [2], due to immature lacteals. Another frequent cause, up to 25%, is external compression leading to obstruction of the lymphatics, as in the cases of intestinal malrotation, incarcerated hernia, intussusception, inflammatory enlargement of lymph nodes, and malignancy. In 15–20% of cases, chylous ascites is the result of trauma related to surgery, accidents, or child abuse [2]. Furthermore, chyloperitoneum can be caused by portal hypertension. Promoted production of hepatic lymph and rise in lymphatic pressure, as a result of portal hypertension, can cause endothelial compromise or rupture of serosal dilated lymphatic channels and ascites formation [4].
Clinical presentation of chylous ascites is similar to ascites of other causes with abdominal distension, “shifting dullness” on precussion, etc. Analysis of ascitic fluid is crucial for differentiating ascites of various etiologies. Macroscopically, chyle has milky, turbid appearance [4, 7] if the patient is orally fed [2]. Otherwise, it is straw-colored [2], as in our case. Occasionally, the fluid does not become milky despite enteral feeding [2]. It is odorless [2, 8] and separates into a top creamy layer on standing [8]. Microscopically, a cell count of > 500/μL is usually found, predominantly (70–90%) lymphocytes [2, 7]. Cytology may be positive in malignancy and culture in tuberculosis [7].
Biochemically, chyle is alkaline [2, 8], with bacteriostatic properties, and specific gravity > 1.012 [2, 8]. Its concentration of triglycerides is higher than 110–200 mg/dL, usually > 200 mg/dL [2,3,4,5, 7] (532 mg/dL in our case), with predominance of chylomicrons. Its fat globules can be stained with Sudan Red [2, 8]. The gold standard investigation is the identification of chylomicrons with lipoprotein electrophoresis [7], where available. Chyle is the only fluid with greater fat content than that of plasma [1], and triglyceride level is therefore vital for diagnosing chylous effusions. Cholesterol in chylous ascites is low (ascites/serum ratio < 1) [7]. Its protein content is approximately half of that in plasma [1] (50% in our case), with a concentration of 2.5–7 g/dL [2, 7] and serum-ascites albumin gradient < 1.1 [7] (0.52 in our case). The latter may be higher in cirrhosis. Glucose is usually < 100 mg/dL, and lactate dehydrogenase ranges between 110 and 200 IU/L. Additionally, amylase may be elevated in cases of pancreatitis and adenosine deaminase in tuberculosis [7].
The diagnostic challenge is to determine the underlying cause of chylous ascites [2]. Radiological diagnostic investigations include ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) of the abdomen, MRI of the retroperitoneum, as well as upper and lower gastrointestinal tract studies [2]. Lymphoscintigraphy is the imaging modality of choice [1,2,3]. Regarding management, chyloperitoneum cases can be treated conservatively with dietary measures. Implementation of high-protein and low-fat diet with medium-chain triglycerides reduces the production and flow of chyle [7]. If this fails, medical therapy (orlistat and somatostatin), peritoneo-venous shunt, or surgical ligation of the lymphatic leaks is among the available treatment options [1, 6].
Chyloperitoneum can be asymptomatic and only detected as an incidental finding during abdominal surgery, e.g., in VPS procedures. Tubbs et al. [6] reported three cases of chyloperitoneum identified during VPS following MMC repair in neonates. To the best of our knowledge, this article reports the 4th case of neonatal chyloperitoneum following MMC repair worldwide so far. As potential pathophysiological mechanism, Tubbs et al. [6] suggested that the repair of the MMC causes tautness of the abdomen leading to rupture of small lymphatic vessels or alternatively causes raised intraportal pressure resulting in extravasation of chyle from the gastrointestinal tract. We agree with their hypothesis, and this case adds to the body of evidence which is scarce. In our patient, the pathophysiology was probably a combination of (1) increased intraportal pressure and (2) rupture of small and immature lymphatics, secondary to abdominal wall tautness from the MMC closure. In our case, the patient’s skin defect required closure by plastic surgery. This created further tension around the flanks, which was transmitted to the anterior and lateral abdominal walls. This association of a large defect, and therefore large repair, strengthens the hypothesis that abdominal wall tautness is a contributing factor.
As demonstrated in our case, the possibility of finding chyloperitoneum following a MMC repair should be borne in mind when inserting a VPS. As with any peritoneal fluid, samples should be sent for detailed biochemical and microbiological investigations, including triglycerides. The current case suggests that a finding of chyloperitoneum is not a contraindication for VPS insertion as it settles without any specific medical or surgical treatment.
Conclusion
Chyloperitoneum is an extremely rare finding following MMC repair in neonates. Pediatric neurosurgeons should be aware of it, especially when a VPS procedure is going to follow. Such awareness enables them to (1) suspect the diagnosis, (2) arrange the appropriate laboratory tests to confirm the diagnosis, and (3) make an appropriate intraoperative decision to proceed and avoid unnecessary abandonment of the insertion of the VPS.
Abbreviations
- CT:
-
computed tomography
- MMC:
-
myelomeningocele
- MRI:
-
magnetic resonance imaging
- VPS:
-
ventriculoperitoneal shunt
References
Aalami OO, Allen DB, Organ CH Jr (2000) Chylous ascites: a collective review. Surgery 128(5):761–778
Mouravas V, Dede O, Hatziioannidis H, Spyridakis I, Filippopoulos A (2012) Diagnosis and management of congenital neonatal chylous ascites. Hippokratia 16(2):175–180
Press OW, Press NO, Kaufman SD (1982) Evaluation and management of chylous ascites. Ann Intern Med 96(3):358–364
Bhardwaj R, Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY (2018) Chylous ascites: a review of pathogenesis, diagnosis and treatment. J Clin Transl Hepatol 6(1):105–113
Griscom NT, Colodny AH, Rosenberg HK, Fliegel CP, Hardy BE (1977) Diagnostic aspect of neonatal ascites: report of 27 cases. AJR Am J Roentgenol 128(6):961–969
Tubbs RS, Tyler-Kabara EC, Wellons JC 3rd, Blount JP, Oakes WJ (2005) Unusual findings during abdominal placement of a ventriculoperitoneal shunt: report of three cases. J Neurosurg 102(4suppl):423–425
Cárdenas A, Chopra S (2002) Chylous ascites. Am J Gastroenterol 97(8):1896–1900
Hibbeln JF, Wehmueller MD, Wilbur AC (1995) Chylous ascites: CT and ultrasound appearance. Abdom Imaging 20(2):138–140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mavridis, I.N., Basnet, A., Wimalachandra, W.S.B. et al. Chyloperitoneum following open myelomeningocele repair: dealing with an extremely rare finding. Childs Nerv Syst 37, 995–998 (2021). https://doi.org/10.1007/s00381-020-04793-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04793-2