Abstract
Introduction
Transient cerebellar mutism has been well recognized in literature as a complication of posterior fossa tumor resection. It is marked by profound impairment of fluency, articulation, and modulation of speech, irritability and autistic features and typically resolves within days to months. Underlying pathophysiology is debated, but currently unknown.
Methods
We present a case of a child with similar clinical findings after cerebellitis, demonstration of diffuse cerebellar signal changes, swelling, and protruding tonsils at the level of foramen magnum.
Discussion
To support the hypothesis that this clinical syndrome may occur in a non-surgical context, we present a review of literature of non-surgical transient cerebellar mutism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term “mutism” refers to the inability of an awake and conscious patient to produce verbal output [1]. It can reflect aphasia, anarthria, or aphonia, and has been attributed to damage to Broca’s area, the supplementary motor cortex, the reticular activating system and can be seen in bilateral hemispheric lesions [1, 2]. Cerebellar mutism is a form of dysarthria characterized by a complete or transient disruption of speech following surgery for posterior fossa tumors [3]. It most frequently occurs in children, but occasionally in adults, and is often attributed to damage to the cerebellar vermis or hemispheres [2, 4]. It typically manifests as a profound impairment of fluency, articulation, and modulation of speech, irritability and autistic features and typically resolves within days to months [2].
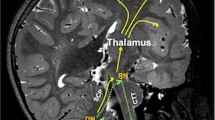
The anatomical substrate of cerebellar mutism is not fully understood, but conventional hypotheses implicate the dentato-thalamo-cortical tract, the cerebellar cortex, and the dentate nuclei [1, 5]. The data on cerebellar representation of language control is still debated and uncertain [5]. While postoperative cerebellar mutism from posterior fossa surgery is well-documented in the literature, we define non-surgical cerebellar mutism as a similar syndrome that would occur following trauma, inflammatory diseases, and infections, in the absence of recent posterior fossa surgery. However, there is sparse literature regarding these causes [6]. It is suspected that the underlying pathologic mechanism for non-surgical cerebellar mutism is similar to that after posterior fossa tumor surgery.
We present the case of a young girl who developed anarthria and behavior mimicking a postoperative cerebellar mutism due to severe acute cerebellitis and cerebellar edema. We additionally review the literature with respect to development of non-surgical cerebellar mutism following cerebellitis.
Methods
Literature review
A systematic literature search was conducted using PubMed and Ovid. Search terms included “Cerebellar Mutism,” “Cerebellar Mutism AND Cerebellitis,” “Cerebellar Mutism AND Nonsurgical,” “Cerebellar Mutism AND Inflammation,” “Cerebellar Mutism AND Trauma,” and “Cerebellar Mutism AND Infection” as the search criteria. Reported patients with a diagnosis of non-surgical cerebellar mutism fulfilled our inclusion criteria. Poster presentations, conference abstracts, and non-English articles were excluded.
Data extracted from eligible articles included the number of patients with non-surgical cerebellar mutism, age of presentation, sex, development of hydrocephalus, diagnosis, radiological imaging findings, extent of cerebellar involvement, duration of cerebellar mutism, surgical intervention (if any), and length of follow-up. Not all studies reported on all the above variables. Descriptive statistics were used to quantify the data extracted from studies that fulfilled our inclusion criteria.
Results
Case report
History
A nine-year-old girl presented with a two-week history of headache, intermittent vomiting, and verbal regression. Her symptoms began a few days after having had an orthodontic plate inserted into her upper jaw in preparation for reconstructive surgery on her palate. In the week prior to presentation, she stopped talking altogether. As no other cause for her symptoms was identified, the possibility of them being related to the orthodontic surgery was raised and the rod removed. Her symptoms however, did not improve. Her medical history was significant for a cleft palate repair in early childhood. She had no known allergies and took no regular medication. She was educationally and developmentally normal.
Examination
Neurological examination revealed anarthria, though she was able to comprehend commands and respond with “yes” and “no” non-verbal gestures. Otherwise, she was awake and alert, had a normal cranial nerve examination with no evidence of papilledema. She had full strength in her upper and lower extremities, and no obvious dysmetria. Systemic examination was normal. She was afebrile with no clinical suspicion of ongoing infection.
Laboratory findings
Serology for Lyme disease was non-reactive, and mycoplasma pneumoniae testing was negative. Virology was negative for active CMV, EBV, HSV, VZV, and HTLV I and II. Cerebrospinal fluid and blood cultures were negative as was the test for tuberculosis. We had performed cytology testing which showed no tumor cells in CSF on histopathological testing.
Radiology
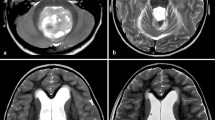
Admission non-contrast CT imaging demonstrated posterior fossa edema with early hydrocephalus and no clear brain stem compression. An MRI scan demonstrated diffuse bilateral cerebellar hemispheric parenchymal T2 and FLAIR increased signal intensity in keeping with edema (Fig. 1). There was no evidence of contrast enhancement or diffusion restriction. This was consistent with cerebellitis. Additionally, there was compression of the fourth ventricle, tonsillar crowding of the foramen magnum, and obstructive hydrocephalus.
Clinical course
Forty-eight hours after admission, the patient’s GCS suddenly dropped to 3, she developed fixed and dilated (7 mm) pupils and suffered a respiratory arrest. Following resuscitation, a right frontal external ventricular drain (EVD) was emergently placed. The patient was admitted to the pediatric intensive care and following a rapid improvement in level of consciousness, was extubated without event. The EVD was weaned over the course of a week and then removed. She ultimately regained limited verbal function with a vocabulary of 10–20 words, and is in the early recovery stages at a rehabilitation facility. Brainstem and lower cranial nerve function was normal.
Literature review
Since being first described by Rekate et al. in 1985, there have been 15 published reports in the English literature documenting 28 patients with cerebellar mutism which did not develop as a result of posterior fossa surgery [4]. Twenty three were secondary to cerebellitis, three followed a traumatic injury with a cerebellar contusion, one was due to a midbrain infarct from a vertebral artery dissection, and one was due to vermian intratumoral hemorrhage [7,8,9,10,11]. These results are summarized in Table 1. Patients had a median age at presentation of 4 years, with a male-to-female ratio of roughly 1:2. The most frequently encountered involvement was in cerebellar cortex (62%) and vermis (46%) regions on MRI. Dentate nuclei involvement was less frequent (35%). Three patients with diffuse cerebellar involvement have been reported, involving the cerebellar hemispheres, the vermis, as well as deep cerebellar nuclei [12, 13].
Of 23 patients with cerebellitis, in 15, the pathogen responsible was rotavirus, identified by PCR in cerebrospinal fluid [13,14,15]. In two patients, the agent was thought to be the cause of an upper respiratory tract infection; in one of the patients, the agent was varicella zoster virus (VZV); another one, it was influenza B [16, 17]. In six of the patients, no offending pathogen was identified [6, 12, 18,19,20].
The imaging modality most often used to characterize cerebellar injury in the event of mutism was computed tomography (CT), followed by magnetic resonance imaging (MRI) of the brain, with many (27/28) of the patients undergoing both imaging investigations. Given the low specificity of CT screening for posterior fossa lesions, the initial screening test may be negative, hence the need for MRI to better characterize the lesion. In nine patients, restriction was noted on diffusion-weighted imaging (DWI) at the site of cerebellitis.
The natural history has a similar course to postoperative mutism. Fluency returned within 2–6 months following the initial insult and in most cases, the patients returned to baseline at 6 months with persisting dysarthria. Interestingly, only three patients had no residual dysarthria at the time of the last follow-up. Only one of the patients presented with additional hydrocephalus due to cerebellar edema and tonsillar descent, and required an insertion of a ventriculoperitoneal shunt for hydrocephalus [6]. One patient with a traumatic posterior fossa injury required evacuation of their acute subdural hematoma.
Discussion
Prevailing theory identifies the dentato-thalamo-cortical tract, the cerebellar cortex, and the dentate nuclei as the structures involved in the pathophysiology of cerebellar mutism, albeit the mechanism are still debated [1, 5]. Damage to vermis, superior cerebellar peduncle, edema of the dentate nucleus, and surgical injury to deep cerebellar structures have been implicated in the postoperative development cerebellar mutism [21,22,23]. Stereotactic ablation of the superior cerebellar peduncle and dentate nucleus has been shown to cause reversible mutism, suggesting bilateral dentato-thalamo-cortical tract involvement in its pathophysiology [6, 15, 24]. Furthermore, additional pathophysiology such as hydrocephalus, invasion of brainstem, histology of the lesion, and tumoral involvement of neural pathways pre-operatively have been associated [1, 25]. Advances in surgical technique such as the telovelar approach (spares the vermis), approaches through the cerebello-medullary fissure or a combined transventricular and supracerebellar approach may reduce the risk augmented by the use of intraoperative adjuncts such as ultrasonography [26,27,28,29].
In our case, the mutism dated back to a week prior to presentation, and thus is likely related to disruption of the dentate-thalamo-cortical tract visualized by the diffuse cerebellar edema seen on imaging. The pathophysiology of cerebellar mutism in cerebellitis is largely unclear and it may be multifactorial. While in a surgical resection, it may be clear which structures were manipulated, diffuse edema and cytotoxicity would indirectly disrupt communication for speech production in non-surgical cerebellar mutism.
In the cases where traumatic injury was the cause of cerebellar insult, the pathophysiology of cerebellar mutism seems akin to that of cases following posterior fossa surgery. The damage to the cerebellar cortex ultimately led to the disruption of speech pathways. The time until recovery certainly reflects a “stunned” state after the initial event, with all three patients in literature regaining their speech within 2 months.
In 19 of 23 patients with mutism and cerebellitis, MRI revealed transient lesions in several parts of the cerebellar anatomy. These lesions are associated with reduced white matter and nuclei diffusion between days 5 and 7 following the initial infection. These lesions disappeared afterward after 3–6 months, being followed by T2/FLAIR hyperintensity in the same regions, finally leading to cerebellar atrophy. Edema of the cerebellar cortex is also present. As expected, given limitation of CT imaging of the posterior fossa, these are often unremarkable. Cases of cerebellitis have been associated with rotavirus infection, with DWI changes in cerebellum [14]. These were thought to be associated with being a valuable predictor of cerebellar mutism. Additionally, some cases have reported the lesions disappearing by the time mutism is observed (between days 5 and 7), and this is unclear why [15]. Perhaps it is a delay in the biochemical response in nature, or perhaps the disturbance of consciousness in the acute stage lasts longer than the imaging abnormalities.
In the majority of cases, the mutism was observed following documented cerebellitis following rotavirus infection, with the largest portion of patients derived from Takanashi et al. [15]. This is often explained as direct CNS invasion by the rotavirus, despite lack of rotavirus antigen or positive detection by PCR of the CSF in some of the patients [15, 30]. It is suspected that this damage is responsible for the development of cerebellitis, which would then lead to cerebellar mutism. Ultimately, the resolution of the infection, and then subsequent development of cerebellar atrophy would explain to incomplete resolution of anarthria with residual difficulties in 17 of the patients (Table 1).
Conclusion
Non-surgical cerebellar mutism is an uncommon condition that should be included as part of clinical presentation in cerebellitis and following posterior fossa trauma. The clinical course characterized by irritability, autistic features, and anarthria is like that of those cases that develop following posterior fossa surgery. It is likely that the pathophysiology is similar between these two entities.
References
Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldarelli M, Di Rocco C (2015) Cerebellar mutism. Childs Nerv Syst 31:1841–1851. https://doi.org/10.1007/s00381-015-2803-6
Dietze DD Jr, Mickle JP (1990) Cerebellar mutism after posterior fossa surgery. Pediatr Neurosurg 16:25–31 discussion 31
Koh S, Turkel SB, Baram TZ (1997) Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol 16:218–219
Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA (1985) Muteness of cerebellar origin. Arch Neurol 42:697–698
Mariën P, Ackermann H, Adamaszek M, Barwood CH, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M, Leggio M, Marvel C, Molinari M, Murdoch BE, Nicolson RI, Schmahmann JD, Stoodley CJ, Thürling M, Timmann D, Wouters E, Ziegler W (2014) Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum Lond Engl 13:386–410. https://doi.org/10.1007/s12311-013-0540-5
Riva D (1998) The cerebellar contribution to language and sequential functions: evidence from a child with cerebellitis. Cortex 34:279–287
Erşahin Y, Mutluer S, Saydam S, Barçin E (1997) Cerebellar mutism: report of two unusual cases and review of the literature. Clin Neurol Neurosurg 99:130–134
Fujisawa H, Yonaha H, Okumoto K, Uehara H, Ie T, Nagata Y, Suehiro E, Suzuki M (2005) Mutism after evacuation of acute subdural hematoma of the posterior fossa. Childs Nerv Syst 21:234–236. https://doi.org/10.1007/s00381-004-0999-y
Kariyattil R, Rahim MIA, Muthukuttiparambil U (2015) Cerebellar mutism following closed head injury in a child. Sultan Qaboos Univ Med J 15:e133–e135
Frassanito P, Massimi L, Caldarelli M, Di Rocco C (2009) Cerebellar mutism after spontaneous intratumoral bleeding involving the upper cerebellar vermis: a contribution to the physiopathogenic interpretation. Childs Nerv Syst 25:7–11. https://doi.org/10.1007/s00381-008-0711-8
Miyakita Y, Taguchi Y, Sakakibara Y, Matsuzawa M, Kitagawa H (1999) Transient mutism resolving into cerebellar speech after brain stem infarction following a traumatic injury of the vertebral artery in a child. Acta Neurochir 141:209–213. https://doi.org/10.1007/s007010050288
Dimova PS, Bojinova VS, Milanov IG (2009) Transient mutism and pathologic laughter in the course of cerebellitis. Pediatr Neurol 41:49–52. https://doi.org/10.1016/j.pediatrneurol.2009.01.013
Shiihara T, Watanabe M, Honma A, Kato M, Morita Y, Ichiyama T, Maruyama K (2007) Rotavirus associated acute encephalitis/encephalopathy and concurrent cerebellitis: report of two cases. Brain and Development 29:670–673. https://doi.org/10.1016/j.braindev.2007.04.005
Kubota T, Suzuki T, Kitase Y, Kidokoro H, Miyajima Y, Ogawa A, Natsume J, Okumura A (2011) Chronological diffusion-weighted imaging changes and mutism in the course of rotavirus-associated acute cerebellitis/cerebellopathy concurrent with encephalitis/encephalopathy. Brain and Development 33:21–27. https://doi.org/10.1016/j.braindev.2010.04.007
Takanashi J, Miyamoto T, Ando N, Kubota T, Oka M, Kato Z, Hamano S, Hirabayashi S, Kikuchi M, Barkovich AJ (2010) Clinical and radiological features of rotavirus cerebellitis. AJNR Am J Neuroradiol 31:1591–1595. https://doi.org/10.3174/ajnr.A2131
Erol I, Ozkale Y, Saygi S, Alehan F (2014) Cerebellar mutism caused by primary varicella infection in an immunocompetent child. J Child Neurol 29:830–832. https://doi.org/10.1177/0883073813477202
Thabet FI, Khalil S, Naz F, Dyme IZ (2013) Cerebellar mutism and reversible cytotoxic edema in influenza B-associated encephalopathy. Pediatr Neurol 49:489–492. https://doi.org/10.1016/j.pediatrneurol.2013.06.014
Drost G, Verrips A, Thijssen HO, Gabreels (2000) Cerebellar involvement as a rare complication of pneumococcal meningitis. Neuropediatrics 31:97–99. https://doi.org/10.1055/s-2000-7474
Mewasingh LD, Kadhim H, Christophe C, Christiaens FJ, Dan B (2003) Nonsurgical cerebellar mutism (anarthria) in two children. Pediatr Neurol 28:59–63
Papavasiliou AS, Kotsalis C, Trakadas S (2004) Transient cerebellar mutism in the course of acute cerebellitis. Pediatr Neurol 30:71–74
Han S, Wang Z, Wang Y, Wu A (2013) Transcerebellomedullary fissure approach to lesions of the fourth ventricle: less is more? Acta Neurochir 155:1011–1016. https://doi.org/10.1007/s00701-013-1689-x
Matsushima T, Abe H, Kawashima M, Inoue T (2012) Exposure of the wide interior of the fourth ventricle without splitting the vermis: importance of cutting procedures for the tela choroidea. Neurosurg Rev 35:563–571; discussion 571-572. https://doi.org/10.1007/s10143-012-0384-3
Zaheer SN, Wood M (2010) Experiences with the telovelar approach to fourth ventricular tumors in children. Pediatr Neurosurg 46:340–343. https://doi.org/10.1159/000321539
Horowitz MB, Pang D, Hirsch W (1991) Acute cerebellitis: case report and review. Pediatr Neurosurg 17:142–145
Di Rocco C, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G (2011) Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum 10:551–562. https://doi.org/10.1007/s12311-011-0273-2
El Beltagy MA, Atteya MME (2013) The benefits of navigated intraoperative ultrasonography during resection of fourth ventricular tumors in children. Childs Nerv Syst ChNS off J Int Soc Pediatr Neurosurg 29:1079–1088. https://doi.org/10.1007/s00381-013-2103-y
Hermann EJ, Rittierodt M, Krauss JK (2008) Combined transventricular and supracerebellar infratentorial approach preserving the vermis in giant pediatric posterior fossa midline tumors. Neurosurgery 63:ONS30–35; discussion ONS35–37. doi: https://doi.org/10.1227/01.neu.0000335008.45499.22
Kellogg JX, Piatt JH (1997) Resection of fourth ventricle tumors without splitting the vermis: the cerebellomedullary fissure approach. Pediatr Neurosurg 27:28–33
von Hoff K, Kieffer V, Habrand J-L, Kalifa C, Dellatolas G, Grill J (2008) Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer 8:15. https://doi.org/10.1186/1471-2407-8-15
Nakagomi T, Nakagomi O (2005) Rotavirus antigenemia in children with encephalopathy accompanied by rotavirus gastroenteritis. Arch Virol 150:1927–1931. https://doi.org/10.1007/s00705-005-0565-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Makarenko, S., Singh, N. & McDonald, P.J. Non-surgical transient cerebellar mutism—case report and systematic review. Childs Nerv Syst 34, 535–540 (2018). https://doi.org/10.1007/s00381-017-3643-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3643-3