Abstract
Fetal ventriculomegaly (VM) refers to the enlargement of the cerebral ventricles in utero. It is associated with the postnatal diagnosis of hydrocephalus. VM is clinically diagnosed on ultrasound and is defined as an atrial diameter greater than 10 mm. Because of the anatomic detailed seen with advanced imaging, VM is often further characterized by fetal magnetic resonance imaging (MRI). Fetal VM is a heterogeneous condition with various etiologies and a wide range of neurodevelopmental outcomes. These outcomes are heavily dependent on the presence or absence of associated anomalies and the direct cause of the ventriculomegaly rather than on the absolute degree of VM. In this review article, we discuss diagnosis, work-up, counseling, and management strategies as they relate to fetal VM. We then describe imaging-based research efforts aimed at using prenatal data to predict postnatal outcome. Finally, we review the early experience with fetal therapy such as in utero shunting, as well as the advances in prenatal diagnosis and fetal surgery that may begin to address the limitations of previous therapeutic efforts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fetal ventriculomegaly (VM) refers to the enlargement of the cerebral ventricles diagnosed in utero and occurs in up to 2 per 1000 births [1, 2]. This condition is one of the most commonly detected fetal anomalies at the mid-trimester ultrasound (US). VM is heterogeneous, with multiple etiologies and a wide range of neurodevelopmental outcomes. In this article, we review the diagnosis, work-up, and management of fetal VM. We then discuss prognosis of VM as it relates to parental counseling and report recent studies in which prenatal data is used to make predictions on postnatal outcome. Finally, we examine limitations of early studies of in utero CSF diversion and advances in the current era of prenatal imaging and fetal surgery as they relate to fetal VM.
Definitions
Fetal VM is defined based on measurements at the atrium of the lateral ventricle, which is the convergence of the body, occipital horn, and temporal horn of the ventricle. Whereas normal values of atrial diameter (AD) range from 6.2 ± 1.2 to 7.6 ± 0.6 mm [3, 4], an AD greater than 10 mm, which is three to four standard deviations above the mean, defines fetal VM at any stage of pregnancy [5, 6]. VM may be further classified into subgroups based on AD, which include mild (10 to 12 mm), moderate (13 to 15 mm), and severe (>15 mm) [7]. Other groups refer to mild fetal VM as AD 10–15 mm and severe as >15 mm, although the former classification will be used in this article. Figure 1 provides illustrations of the ventricular system in a fetus with and without VM.
Isolated fetal VM occurs when in utero enlargement of the cerebral ventricles is observed in the absence of other apparent anomalies, such as Chiari malformations, neural tube defects, Dandy Walker malformations, agenesis of the corpus callosum, or genetic syndromes [8]. In a subset of patients with severe VM, it is not uncommon for anomalies to be revealed postnatally. Whereas VM denotes enlargement of the cerebral ventricles, the term hydrocephalus refers to pathologic dilatation of the ventricles due to increased pressure [9]. The presence of fetal VM has been associated with postnatal hydrocephalus. However, these two conditions are not synonymous. Hydrocephalus is a clinical diagnosis that results in increased pressure as the result of altered cerebrospinal fluid (CSF) dynamics. Fetal VM is the dilation of the cerebral ventricles, and it does not always result from altered CSF dynamics. It does not always result in increased intracranial pressure. Fetal VM can result from hydrocephalus, but it can also result from the loss of brain tissue or generalized brain atrophy, which are conditions not associated with increased intracranial pressure [6]. While it is difficult to determine intracranial pressure in the prenatal setting, as compared to the postnatal setting, [10], VM and hydrocephalus should not be used interchangeably when applied to the fetus.
Diagnosis
Imaging studies of the fetus that assess AD are used to diagnose fetal VM. AD has several favorable attributes that support its diagnostic use. In fetal VM, the occipital horn of the lateral ventricle is the first area to dilate [6], and the atrium dilates to a greater extent than do other ventricle regions, such as the frontal horns [4]. The atrium lacks constriction from the striatum, compared to the more anterior portion of the ventricle. In addition, in accordance with Laplace’s law, it takes less pressure to dilate an already larger atrium compared with other parts of the ventricle system [11]. Furthermore, AD remains stable between 20 and 40 weeks of gestation [3, 4, 12]. Finally, the walls of the ventricular atrium are perpendicular to the US beam in the transaxial plane, making them, along with the choroid plexus, readily identifiable on US [6]. By convention, AD is measured in an axial plane at the level of the atria of the lateral ventricles, and measurements are made from the inner walls of the atria at the level of the glomus of the choroid plexus [13]. Measurements are perpendicular to the long axis of the lateral ventricle. In cases of fetal VM, the choroid plexus appears to “dangle” or fall toward the dependent ventricular wall and occupies less of the ventricle than in cases without VM. In non-VM cases, choroid plexus usually fills at least half or more of the ventricle [4]. Measurement of AD can be quickly performed, and it has low intra- and interobserver variation, with a low false-positive rate for AD >10 mm. Patients with unilateral VM have similar courses as those with bilateral VM [14,15,16].
Etiology
The etiology of fetal VM differs across patients and ranges from idiopathic to structural or chromosomal. Causes of fetal VM can be broadly divided into loss of cerebral tissue, obstruction of the ventricular system, or excessive CSF production [6]. Cerebral atrophy leads to loss of brain tissue such that the ventricles appear enlarged. It may occur from metabolic disorders, infections, or cerebral hypoxia or infarction, and it usually results in asymmetry between both lateral ventricles. Obstruction commonly occurs due to aqueductal stenosis (AS), or narrowing of the cerebral aqueduct, which may occur with X-linked hydrocephalus, infection with subsequent fibrosis of the aqueduct, or intraventricular hemorrhage [8]. It occurs in 22.5% of fetal VM patients [6].The most common cause of AS is intrauterine infection or intracerebral hemorrhage. Infections, such as cytomegalovirus or toxoplasmosis may also result in isolated VM as a result of cerebral atrophy or inflammation of arachnoid granulations. Hemorrhage can also interfere with reabsorption of CSF. Other causes of fetal VM include cortical malformations, brain masses, or migrational abnormalities. Fetal VM itself may be viewed as a marker in certain cases for serious central nervous system (CNS) disease, chromosomal abnormalities, infection, or brain malformations [17, 18].
Work-up
Following a diagnosis of fetal VM, a search for an etiology and associated anomalies is undertaken, the results of which can aid in patient management and counseling. A comprehensive US examination of the CNS is performed targeting each ventricle, as well as the thalamus, corpus callosum, germinal matrix region, and cerebellum [8]. Intracerebral or periventricular calcification may indicate an infectious etiology, which can be supported by maternal serologies or polymerase chain reaction analysis of amniotic fluid. A detailed anatomic survey should also be performed, as findings of hepatosplenomegaly, ascites, or intra-abdominal calcifications may further support infection. Also, VM may be a component of several genetic syndromes that have specific manifestations outside of the CNS, and associated anomalies of the CNS or other regions have been reported to occur in 10 to 76% of cases [17, 19,20,21].
History and genetic testing
The radiographic findings should be interpreted in the context of the maternal medical history, including maternal illness or drug exposure [11]. Family history is reviewed to determine the presence of L1 cell adhesion molecule mutations, which alters neuronal migration and differentiation and can cause hydrocephalus [8]. DNA testing for L1 spectrum mutations is recommended in male patients with isolated severe VM or in those with a positive family history. For all patients, fetal karyotype and chromosomal microarray should be offered. In patients with isolated mild VM, 4.7% had an abnormal karyotype [22].
Fetal MRI
Fetal magnetic resonance imaging (MRI), as shown in Fig. 2, is increasingly used to improve the diagnosis of structural abnormalities suspected on US. Fetal MRI is especially useful for detecting small foci of brain hemorrhage, depicting corpus callosum anomalies, and assessing cortical development [23]. Furthermore, certain abnormalities, such as cortical malformations, porencephaly, migrational abnormalities, and white matter pathologies may be better detected on fetal MRI than US. Unlike US, MRI allows for complete visualization of the ventricles and brain parenchyma without artifacts from surrounding skull ossification or fetal position [24] (Fig. 3). The major limitations of fetal MRI are poor image quality due to fetal motion, cost, and availability [23]. Fetal brain MRI is usually performed at 20 to 24 weeks of gestation, as developmental milestones are more evident at these times and discontinuation of pregnancy may still be an option [8].
There remains debate on the additive benefit of fetal brain MRI when US shows no abnormalities, especially in cases of mild or moderate VM, and rates of additional findings by fetal MRI vary [23, 25]. In a prospective study of 59 fetuses, additional findings by fetal MRI were found in 17% of cases [18]. Although categorical assessments of ventricular size by fetal MRI were concordant with US in 90% of cases, other abnormalities were revealed in 25 of 147 fetuses (17%) [7]. When looking specifically at the degree of VM, previously unidentified abnormalities were found in 5 of 90 patients (6%) with mild VM, 4 of 29 patients (14%) with moderate VM, and 16 of 28 patients (57%) with severe VM [7]. Of note, the likelihood of finding an abnormality on MRI and not on US depends, in part, on the quality of the US examination and varies overall from 5 to 50% in other studies [26,27,28,29]. Agenesis of the corpus callosum was the most common anomaly to be detected by MRI but missed on US [7]. However, in moderate or severe VM, the enlarged ventricles may obscure the corpus callosum, whereas color Doppler may be used on US to look for the pericallosal artery to confirm partial or complete corpus callosum absence. Some groups recommend fetal MRI in all cases of isolated ventriculomegaly [8]. Also, it is important to note that the absolute value of AD may differ on US and MRI.
Follow-up studies
Repeat US examinations are obtained during gestation to assess the in utero course of fetal VM and to further assess for associated anomalies [11]. The natural history of fetal VM, as shown in Fig. 4, is resolution of VM in 29% of cases, stability in 57% of cases, and progression in 14% of cases [6, 30, 31]. Among 63 fetuses with mild isolated VM, 26 fetuses (41%) showed normalization of the lateral ventricles, 10 fetuses (16%) showed progression, and 27 fetuses (43%) remained stable [32]. Finally, among 106 live-born infants with VM, 50 cases (47%) resolved [20]. Also, a follow-up US may detect abnormalities not seen on the initial scan in as many as 13% of cases [13]. Thus, at least one additional imaging study should be performed between 28 and 34 weeks of gestation, and monthly evaluations to monitor progression are commonly recommended [8].
Natural in utero history of ventriculomegaly. Serial imaging studies in utero show that ventricular size remains stable in slightly more than half of cases, whereas it regresses in 30% of cases and progresses in 15% of cases [6]
Prognosis
The overall outcome of a patient diagnosed in utero with VM is primarily dependent on the severity of ventriculomegaly and the presence or absence of associated anomalies. For isolated VM, the outcome is highly correlated with ventricular enlargement, with isolated mild ventriculomegaly having the most favorable outcome [22, 33]. For example, in a study of 176 VM patients, survival rates were 98% for mild cases, 80% for moderate cases, and 33% for severe cases [20]. In another series, Beeghly et al. found that the survival rate for patients with isolated mild VM was 93%, whereas the survival rate for patients with moderate to severe VM was 60% [34]. Again, the worse outcome with increased ventricular size is related to the underlying reason for the VM and the associated abnormalities in the more severe group and not just to the absolute AD size. Severe VM is more commonly associated with chromosomal anomalies and, in turn, with less favorable outcomes.
In addition to survival, prognosis may be studied in terms of neurological outcome after birth. At mean evaluation of 45.9 months, approximately one third of mild VM patients had a mild degree of developmental delay in terms of motor function [35]. Several systematic reviews of mild to moderate ventriculomegaly demonstrate an overall risk of handicap ranging from 10.4 to 36%, with a lower rate in the absence of chromosomal abnormality or infection [6, 30, 36, 37]. In a meta-analysis by Pagnani et al. that included over 700 patients with isolated mild or moderate VM, development delay occurred in approximately 8% at a mean age of 30 months [22]. Conversely, in reviews of severe VM patients, normal neurodevelopment was present in only 5–8% of patients [20, 38, 39]. Also, poor neurological outcome is more commonly associated with progression of VM in utero. Among mild to moderate VM patients, the risk of neurological abnormality was lower in stable versus progressive VM with an odds ratio of 0.29 (confidence interval 0.15–0.58) [36]. In another study, postnatal abnormalities were noted in 66.7% of patients with in utero progression, 15.6% of patients with stable VM, and none of patients with resolution of VM [40].
Counseling
Prenatal counseling is a complex and challenging discussion between the parents of a fetus with VM and the healthcare provider, and counseling regarding prognosis is very difficult for several reasons. First, fetal VM is associated with a wide range of potential outcomes for each pregnancy. Second, the etiology of fetal VM is not always identified, even with the use of fetal MRI. Among patients presenting for neurosurgical consultation, over half of patients had additional anomalies that were not detected prenatally [41], and it is known that certain etiologies have improved outcomes compared to others. Third, the natural history of fetal VM is not completely known because pregnancy terminations may bias reported outcomes to the less severe cases that were carried to delivery [6]. Fourth, definitions of neurodevelopmental outcome and follow-up periods vary between studies. In order to provide effective counseling, the physician should focus on four areas: (1) determining the degree of VM; (2) identifying any additional structural CNS anomalies or other fetal abnormalities that may affect outcome; (3) performing a full chromosomal, genetic, and infectious analysis; and (4) determining the change or progression of VM over time.
All options, including expectant management, pregnancy termination, or preterm delivery should be discussed, and cases should be reviewed at a multidisciplinary meeting [6]. The option for early delivery in cases of progressive and severe VM must be weighed against the higher reported incidence of shunt-related complications at younger ages [42]. Although expectant delivery may theoretically carry the risk of ongoing neurological injury from hydrocephalus, there is little to no evidence that this occurs to any significant degree, and prematurity has established clinical and developmental risks for patients. Additionally, treatment options are limited in premature infants, making preterm delivery counter-productive. [10]. The advantages and disadvantages of management approaches should be thoroughly discussed between the parents and healthcare provider. Certain associated anomalies, such as chromosomal anomalies, make imaging and the management options less relevant, as these patients are known to have poor neurological outcomes regardless of ventricle size [8, 11]. Although there is still some controversy, in most instances, waiting as long as possible for delivery of patients with VM is preferred as long as the head is able to be safely delivered. Most fetal VM patients have normal head circumference for age, rather than macrocephaly; therefore, cesarean delivery is not required, except for the standard obstetric indications or a macrocephalic head circumference greater than 40 cm [8].
Identification of an etiology can be helpful in assessing the risk of recurrence in a future pregnancy. The recurrence risk in cases of isolated VM without identified etiology is less than 4% [43], although recurrence rates are higher for patients at risk for x-linked hydrocephalus, and these parents should be offered genetic counseling [6]. Several authors recommend a detailed US examination at 18 to 20 weeks of gestation in a future pregnancy to assess for fetal VM and an amniocentesis for a known prior genetic abnormality [8].
Postnatal management
A physical exam after birth should focus on signs of hydrocephalus and the presence of associated anomalies. Head circumference should be measured and followed over time. US through the anterior fontanelle should be performed followed by a MRI in the first week of life [6], as it may detect neuronal migration disorders or subtle white matter abnormalities not seen on US. There has been little change to the management of fetal VM after birth [10]. In the postnatal period, VM patients are monitored closely, usually in a neonatal intensive care unit, for the development of signs of increased intracranial pressure, which include decreased activity, vomiting, feeding intolerance, sun-setting eyes, splayed sutures, bulging fontanelle, or apnea. The degree and rate of progression of VM is assessed when considering surgical intervention, as progression of VM may be accelerated after birth. For patients with definite abnormalities of CSF dynamics, ventricular dilatation, or increasing intracranial pressure, CSF diversion via ventricular shunt, endoscopic third ventriculostomy, or external ventricular drainage should be offered [10]. Currently, there are no definitive methods to determine in the prenatal period which patients will require surgical intervention after birth, although research is underway to improve such predictive abilities.
Outcome prediction
To better identify etiology and guide prognosis, several groups have examined prenatal imaging, usually with the aid of computer-assisted image analysis, in efforts to better predict postnatal outcome in fetal VM patients. Haratz et al. examined the relationship between morphology of the lateral ventricles and etiology and found that cases with agenesis of the corpus callosum presented with thin ventricles with dilation of the posterior horn, whereas cases of trisomy 18 and cytomegalovirus presented with ventricle wall irregularities suggestive of parenchymal damage [44]. By performing a volumetric analysis of fetal MRI data, a higher ratio of ventricular volume to parenchymal volume was more likely to portend a poor prognosis in isolated VM patients [45]. In a similar study, Pier et al. found that larger ventricular volume and higher AD were associated with poor birth outcomes; however, none of the imaging features studied was predictive of postnatal neurodevelopmental outcome [24]. Although imaging features are useful in estimating postnatal survival, functional outcome is harder to predict. In addition to quantification of ventricular volume, fetal MRI offers the ability to study cortical maturation, which was assessed as another predictor of postnatal outcome. For fetuses with isolated VM in which pregnancies were continued to birth, the visualization of the parieto-occipital or cingulate sulcus on fetal MRI was associated with an odds ratio of normal postnatal development ranging from 5.6 to 7.8 compared to cases in which these sulci were not visualized on fetal MRI [46]. The authors suggest that fetuses with hydrocephalus may have effacement of sulci due to increased ventricular pressure. In addition, because sulci development begins early in gestation, any disturbance in cortical maturation may reflect global problems with CNS development and therefore be linked to a poor neurodevelopmental outcome.
Other groups have gone further and attempted to determine which VM patients will require CSF diversion after birth. In a study of 38 patients presenting for neurosurgical consultation following a diagnosis of fetal VM, Hankinson et al. found that AD positively correlated with shunt placement, with all patients with an AD greater than or equal to 20 mm requiring ventricular shunt placement, regardless of gestational age. Two patients with AD over 20 mm did not require a shunt, and no difference in shunt insertion rates was found between isolated and non-isolated fetal VM [47]. Finally, our group has used machine learning and image analysis techniques to identify AD, area of the smaller occipital horn of the lateral ventricle, and ventricular volume as predictive of postnatal need for shunt with approximately 80% accuracy [Pisapia et al., unpublished results]. In addition to improving prognosis, such predictive models are useful for patient selection in terms of identifying fetuses most likely to benefit from potential in utero surgical treatment.
Fetal surgery
Despite unfavorable outcomes during the early experience with in utero CSF diversion, improvements in prenatal diagnosis and fetal surgery have led to a renewed interest in fetal ventriculo-amniotic fluid (VA) shunting.
Preclinical studies
Large animal models of fetal VM were developed in order to assess the feasibility of in utero CSF diversion and improve understanding of the pathophysiology of fetal VM. In a rhesus monkey model, investigators used serial intramuscular injections of the corticosteroid triamcinolone acetonide to induce fetal hydrocephalus [48]. The authors then successfully treated the hydrocephalus with a small indwelling valve inserted into the skull to divert CSF into surrounding amniotic fluid. In a lamb model, Nakayama et al. utilized cisternal injection of kaolin, a local irritant that produces an inflammation-related obstruction at the exit foramina of the fourth ventricle, to induce hydrocephalus [49]. The investigators then inserted a ventriculo-amniotic fluid shunt via hysterotomy and treated the hydrocephalus. Although each model allowed for assessment of the underlying mechanisms of fetal hydrocephalus, each model had limitations. For instance, corticosteroid administration was associated with the development of other major congenital anomalies besides VM, and kaolin injection was suspected to potentially cause periventricular white matter changes due to local irritant effects [50]. Nonetheless, animals that underwent CSF diversion showed clinical improvement compared to control animals. For example, fetal rhesus monkeys with induced hydrocephalus that underwent in utero valve placement had increased rates of normal growth and survival, as compared to untreated fetuses, which rarely survived more than 2 weeks after birth [48]. In addition, the majority of shunted lambs showed decreased head circumference and normalization of ventricle size [51]. Such favorable preliminary data generated by animal models led the way to trials of in utero CSF diversion in humans. The rationale for VA shunting was that CSF diversion at an early, in utero stage might prevent irreversible brain damage due to prolonged increased intracranial hypertension by relieving intracranial pressure, decreasing ventricle size, and normalizing cerebral blood flow [52].
Clinical studies
Initial attempts at in utero CSF diversion in humans involved cephalocentesis, in which ventricular puncture was performed under US guidance [53]. However, the need for repeat taps made this approach impractical, and it carried a cumulative risk of infection and injury to surrounding structures. Instead, a more continuous form of CSF diversion was made possible through VA shunting. Clewell et al. reported the placement of a Silastic shunt with a one-way valve in a patient with fetal hydrocephalus using a percutaneous US-guided needle technique [54]. Early attempts involved placement of a needle trocar into the uterus and through the parietal bone of the fetus into the lateral ventricle. The shunt tubing, known as the Denver shunt, was then passed through the trocar into the desired position and the trocar was withdrawn, leaving behind 2–3 cm of distal tubing draining into the amniotic fluid [9]. As interest in fetal surgery increased, the International Society of Fetal Medicine and Surgery was created in June 1982, and it established a voluntary international registry of fetal surgery [55]. Between 1982 and 1985, 39 cases of in utero shunting were reported to the registry [56]. Among these cases, the overall fetal survival rate was 83% and the procedure-related death rate was 17%. In terms of disability, over half of the patients had serious neurological handicaps, while 12% had mild to moderate handicaps and 35% developed normally [11]. Based on accumulating data, a moratorium was issued on further in utero shunting procedures. Between 1999 and 2003, with Institutional Review Board approval, four fetuses with hydrocephalus were treated with VA shunting via hysterotomy [9]. Although all shunts were intact at the time of delivery, demonstrating technical feasibility of shunt placement and maintenance, infection was a major complication that affected three of the four patients, and one death occurred in the neonatal period. In a separate study of patients treated between 2005 and 2007, five pregnancies were treated at gestational ages of 27 to 31 weeks with placement of Al-Anazi VA shunts [57]. These shunts where performed through a 1-cm hysterotomy. One infant was delivered 18 h after surgery due to an abruption. Despite the shunt performing well and reducing VM, the outcome in the four surviving patients was poor. Two developed shunt infections, one developed sepsis, and all had developmental delay.
Limitations
Several issues have been cited to explain the suboptimal outcomes associated with the early experience of in utero VA shunting. Poor patient selection arose from limited knowledge of fetal VM outcomes and disease course and from lower-quality imaging modalities than are available today [58]. Patients undergoing VA shunting commonly had other associated conditions, such as Dandy-Walker malformation, Chiari malformation, and holoprosencephaly [56, 59]. Also, patients with x-linked hydrocephalus were not excluded from the analysis [52]. As fetal surgery was at its beginning, less refined surgical techniques were used, and technical difficulties such as shunt obstruction, malposition, or migration were commonly reported [54, 60]. Finally, early studies lacked concurrent, untreated control groups.
Improvements
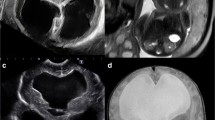
Several advances in prenatal diagnosis, fetal surgery, and shunt technology have addressed limitations of earlier studies of in utero shunting for fetal hydrocephalus. Developments such as 3D US and fetal MRI now facilitate the detection of associated anomalies, which are linked to poor outcome regardless of early intervention in many cases. Advanced imaging is also better able to detect ventricle size and configuration such that pathologies more likely to be amenable to in utero shunting, such as AS, may be more accurately diagnosed. An example of the fetal MRI appearance of AS is shown in Fig. 5. Emory et al. correctly identified all cases of isolated AS in 110 fetuses and differentiated it from other subtle CNS anomalies, such as the presence of cerebellar hypoplasia or a tectal mass [61]. Accurate patient selection increases the likelihood of favorable outcomes in treated patients. For instance, further analysis of data from the early VA shunting experience showed that patients deemed neurologically normal all had the diagnosis of AS. Among AS patients, 43% were without handicap, 7% had mild to moderate handicap, and 50% had severe handicaps [52]. Further improvements in prenatal diagnosis relate to genetic testing, including L1-CAM screening, microarray, and whole-exome sequencing. In addition, shunt technologies and techniques of in utero VA shunt placement continue to improve. Current VA shunts may be inserted percutaneously and have an anchoring mechanism to prevent migration inside or outside of the skull [52, 60]. Newer tubing is designed to be kink resistant and have a length and lumen size optimized based on computational flow dynamic studies to minimize occlusion [62]. All shunts are equipped with one-way valves to prevent the reflux of amniotic fluid, which can potentially lead to ventriculitis [54]. Surgical techniques and instruments developed in the successful treatment of in utero myelomeningocele repair have ushered in safe and effective practices of hysterotomy and fetal surgery [58]. Better anesthetics and tocolytics, important considerations in fetal surgery, have addressed issues of preterm labor and premature rupture of membranes [63]. Standardized techniques now exist for fetal MMC closure [64], and the increased volume of fetal surgeries since its inception has led to better outcomes. In addition, VA shunts placed with the distal end tunneled to exit at the interscapular position is thought to decrease the risk of the fetus pulling out the catheter [65]. It also leaves the anterior chest untouched for future conversion to a standard ventriculoperitoneal shunt and does not require repositioning of the fetus at the time of hysterotomy [9].
Fetal MRI of aqueductal stenosis. Axial (a), sagittal (b), and coronal (c) images were obtained by half-Fourier acquisition single-shot turbo spin-echo fetal MRI at 35 weeks and show severe ventriculomegaly (atrial diameter > 15 mm). Preferential enlargement of the occipital horns and the third ventricle with a normal-sized fourth ventricle indicate aqueductal stenosis
Future directions
Patient selection will be an important factor for the successful implementation of in utero VA shunting. Because of the risk of complications associated with fetal shunting, only those patients who are severely affected with the greatest chance of improvement and viability should be considered. Further guidelines on patient selection include singleton pregnancy, normal fetal karyotype, amniotic fluid without evidence of infection, absence of associated anomalies, progressive ventricular dilation, and gestational age less than 32 weeks and fetal lung maturity [11, 65, 66]. Fetuses with progressive ventricular enlargement and cortical thinning before 28 weeks gestation may be better candidates due to the possibility of irreversible brain damage after 32 weeks [58]. Centers considering fetal VA shunting should have access to a multidisciplinary team with members from maternal-fetal medicine, neonatology, anesthesia, pediatric surgery, pediatric neurosurgery, and bioethics. Along with identification of proper patients, further animal studies of hydrocephalus, particularly ones that can recapitulate isolated hydrocephalus, will help to further understand in utero pathophysiology. Finally, the development of a standard surgical method will be necessary in the future as well for reproducibility of outcomes.
Work is currently underway to prospectively assess the performance of prenatal US and MRI in the diagnosis of patients most likely to benefit from in utero VA shunting, namely fetuses with isolated AS. The effort is made possible through the North American Fetal Therapy Network (NAFTNet), which consists of 30 medical institutions in the USA and Canada that perform fetal surgery in a multidisciplinary center [52]. In addition to further defining the natural history of VM in a standardized fashion, the cohort will be followed prospectively into the postnatal period, and it will serve as a comparison to future studies of prenatal management. Also, endoscopic third ventriculostomy has been raised as another treatment alternative in the fetus; however, success rates in infancy are low (approximately 25%) [67], current endoscopy instruments may not have reached the required size for work in the fetal brain, and there is high risk of injury to surrounding structures.
Conclusion
Although fetal VM is clearly defined as in utero enlargement of the cerebral ventricles, it is a heterogeneous condition with multiple etiologies and varied neurological outcomes. VM may occur in isolation or in the setting of associated anomalies that, when present, usually adversely impact the overall outcome. Several studies have demonstrated improvements in diagnostic ability when fetal MRI is performed in addition to US. More accurate diagnosis can lead to improved patient counseling, and research efforts focusing on image analysis are ongoing to use prenatal data to better predict postnatal outcomes. Although in utero VA shunting remains investigational at this time, advances in fetal diagnosis and treatment have renewed interest in fetal surgery, and further animal investigations and prospective fetal studies of VM are underway. Patient selection will be integral in determining whether fetal hydrocephalus will join the list of current indications for fetal surgery.
Abbreviations
- AD:
-
Atrial diameter
- AS:
-
Aqueductal stenosis
- CSF:
-
Cerebrospinal fluid
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasound
- VA:
-
Ventriculo-amniotic fluid
- VM:
-
Ventriculomegaly
References
Edwards JH (1958) Congenital malformations of the central nervous system in Scotland. Br J Prev Soc Med 12:115–130
Laurence KM, Carter CO, David PA (1968) Major central nervous system malformations in South Wales. I. Incidence, local variations and geographical factors. Br J Prev Soc Med 22:146–160
Almog B, Gamzu R, Achiron R et al (2003) Fetal lateral ventricular width: what should be its upper limit? A prospective cohort study and reanalysis of the current and previous data. J Ultrasound Med 22:39–43
Cardoza JD, Goldstein RB, Filly RA (1988) Exclusion of fetal ventriculomegaly with a single measurement: the width of the lateral ventricular atrium. Radiology 169:711–714. doi:10.1148/radiology.169.3.3055034
Goynumer G, Yayla M, Arisoy R, Turkmen O (2014) The criterion value of fetal cerebral lateral ventricular atrium width for diagnosis of ventriculomegaly. Clin Exp Obstet Gynecol 41:67–71
McKechnie L, Vasudevan C, Levene M (2012) Neonatal outcome of congenital ventriculomegaly. Semin Fetal Neonatal Med 17:301–307. doi:10.1016/j.siny.2012.06.001
Griffiths PD, Reeves MJ, Morris JE et al (2010) A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. AJNR Am J Neuroradiol 31:106–111. doi:10.3174/ajnr.A1767
Norton M (2016) Fetal cerebral ventriculomegaly. In: UpToDate
Bruner JP, Davis G, Tulipan N (2006) Intrauterine shunt for obstructive hydrocephalus—still not ready. Fetal Diagn Ther 21:532–539. doi:10.1159/000095668
Wang K-C, Lee JY, Kim S-K et al (2011) Fetal ventriculomegaly: postnatal management. Childs Nerv Syst 27:1571–1573. doi:10.1007/s00381-011-1556-0
Von Koch CS, Gupta N, Sutton LN, Sun PP (2003) In utero surgery for hydrocephalus. Childs Nerv Syst 19:574–586. doi:10.1007/s00381-003-0775-4
Pilu G, Reece EA, Goldstein I et al (1989) Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles: II. The atria. Obstet Gynecol 73:250–256
Melchiorre K, Bhide A, Gika AD et al (2009) Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol 34:212–224. doi:10.1002/uog.7307
Sadan S, Malinger G, Schweiger A et al (2007) Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG 114:596–602. doi:10.1111/j.1471-0528.2007.01301.x
Atad-Rapoport M, Schweiger A, Lev D et al (2015) Neuropsychological follow-up at school age of children with asymmetric ventricles or unilateral ventriculomegaly identified in utero. BJOG 122:932–938. doi:10.1111/1471-0528.12976
Durfee SM, Kim FM, Benson CB (2001) Postnatal outcome of fetuses with the prenatal diagnosis of asymmetric hydrocephalus. J Ultrasound Med 20:263–268
Gaglioti P, Oberto M, Todros T (2009) The significance of fetal ventriculomegaly: etiology, short- and long-term outcomes. Prenat Diagn 29:381–388. doi:10.1002/pd.2195
Kandula T, Fahey M, Chalmers R et al (2015) Isolated ventriculomegaly on prenatal ultrasound: what does fetal MRI add? J Med Imaging Radiat Oncol 59:154–162. doi:10.1111/1754-9485.12287
Hannon T, Tennant PWG, Rankin J, Robson SC (2012) Epidemiology, natural history, progression, and postnatal outcome of severe fetal ventriculomegaly. Obstet Gynecol 120:1345–1353. doi:10.1097/AOG.0b013e3182732b53
Gaglioti P, Danelon D, Bontempo S et al (2005) Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 25:372–377. doi:10.1002/uog.1857
Sethna F, Tennant PWG, Rankin J, Robson SC (2011) Prevalence, natural history, and clinical outcome of mild to moderate ventriculomegaly. Obstet Gynecol 117:867–876. doi:10.1097/AOG.0b013e3182117471
Pagani G, Thilaganathan B, Prefumo F (2014) Neurodevelopmental outcome in isolated mild fetal ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 44:254–260. doi:10.1002/uog.13364
Cardoen L, De Catte L, Demaerel P et al (2011) The role of magnetic resonance imaging in the diagnostic work-up of fetal ventriculomegaly. Facts Views Vis ObGyn 3:159–163
Pier DB, Levine D, Kataoka ML et al (2011) Magnetic resonance volumetric assessments of brains in fetuses with ventriculomegaly correlated to outcomes. J Ultrasound Med 30:595–603
Parazzini C, Righini A, Doneda C et al (2012) Is fetal magnetic resonance imaging indicated when ultrasound isolated mild ventriculomegaly is present in pregnancies with no risk factors? Prenat Diagn 32:752–757. doi:10.1002/pd.3896
Benacerraf BR, Shipp TD, Bromley B, Levine D (2007) What does magnetic resonance imaging add to the prenatal sonographic diagnosis of ventriculomegaly? J Ultrasound Med 26:1513–1522
Salomon LJ, Ouahba J, Delezoide A-L et al (2006) Third-trimester fetal MRI in isolated 10- to 12-mm ventriculomegaly: is it worth it? BJOG 113:942–947. doi:10.1111/j.1471-0528.2006.01003.x
Manganaro L, Savelli S, Francioso A et al (2009) Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med 114:1013–1023. doi:10.1007/s11547-009-0434-2
Morris JE, Rickard S, Paley MNJ et al (2007) The value of in-utero magnetic resonance imaging in ultrasound diagnosed foetal isolated cerebral ventriculomegaly. Clin Radiol 62:140–144. doi:10.1016/j.crad.2006.06.016
Kelly EN, Allen VM, Seaward G et al (2001) Mild ventriculomegaly in the fetus, natural history, associated findings and outcome of isolated mild ventriculomegaly: a literature review. Prenat Diagn 21:697–700
Vergani P, Locatelli A, Strobelt N et al (1998) Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol 178:218–222
Parilla BV, Endres LK, Dinsmoor MJ, Curran L (2006) In utero progression of mild fetal ventriculomegaly. Int J Gynaecol Obstet 93:106–109. doi:10.1016/j.ijgo.2006.01.026
Melchiorre K, Liberati M, Celentano C et al (2009) Neurological outcome following isolated 10-12 mm fetal ventriculomegaly. Arch Dis Child Fetal Neonatal Ed 94:F311–F312. doi:10.1136/adc.2007.134312
Beeghly M, Ware J, Soul J et al (2010) Neurodevelopmental outcome of fetuses referred for ventriculomegaly. Ultrasound Obstet Gynecol 35:405–416. doi:10.1002/uog.7554
Kutuk MS, Ozgun MT, Uludag S et al (2013) Postnatal outcome of isolated, nonprogressive, mild borderline fetal ventriculomegaly. Childs Nerv Syst 29:803–808. doi:10.1007/s00381-013-2020-0
Devaseelan P, Cardwell C, Bell B, Ong S (2010) Prognosis of isolated mild to moderate fetal cerebral ventriculomegaly: a systematic review. J Perinat Med 38:401–409. doi:10.1515/JPM.2010.048
Laskin MD, Kingdom J, Toi A et al (2005) Perinatal and neurodevelopmental outcome with isolated fetal ventriculomegaly: a systematic review. J Matern Fetal Neonatal Med 18:289–298. doi:10.1080/14767050500329775
Breeze ACG, Alexander PMA, Murdoch EM et al (2007) Obstetric and neonatal outcomes in severe fetal ventriculomegaly. Prenat Diagn 27:124–129. doi:10.1002/pd.1624
Graham E, Duhl A, Ural S et al (2001) The degree of antenatal ventriculomegaly is related to pediatric neurological morbidity. J Matern Fetal Med 10:258–263
Baffero GM, Crovetto F, Fabietti I et al (2015) Prenatal ultrasound predictors of postnatal major cerebral abnormalities in fetuses with apparently isolated mild ventriculomegaly. Prenat Diagn 35:783–788. doi:10.1002/pd.4607
Lee CS, Hong SH, Wang K-C et al (2006) Fetal ventriculomegaly: prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg 105:265–270. doi:10.3171/ped.2006.105.4.265
McGirt MJ, Leveque J-C, Wellons JC 3rd et al (2002) Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg 36:248–255
Varadi V, Toth Z, Torok O, Papp Z (1988) Heterogeneity and recurrence risk for congenital hydrocephalus (ventriculomegaly): a prospective study. Am J Med Genet 29:305–310. doi:10.1002/ajmg.1320290209
Haratz KK, Nardozza LMM, de Oliveira PS et al (2011) Morphological evaluation of lateral ventricles of fetuses with ventriculomegaly by three-dimensional ultrasonography and magnetic resonance imaging: correlation with etiology. Arch Gynecol Obstet 284:331–336. doi:10.1007/s00404-010-1666-z
Gezer NS, Guleryuz H, Gezer C et al (2015) The prognostic role of prenatal MRI volumetric assessment in fetuses with isolated ventriculomegaly. Turk J Pediatr 57:266–271
Li Y, Estroff JA, Mehta TS et al (2011) Ultrasound and MRI of fetuses with ventriculomegaly: can cortical development be used to predict postnatal outcome? AJR Am J Roentgenol 196:1457–1467. doi:10.2214/AJR.10.5422
Hankinson TC, Vanaman M, Kan P et al (2009) Correlation between ventriculomegaly on prenatal magnetic resonance imaging and the need for postnatal ventricular shunt placement. J Neurosurg Pediatr 3:365–370. doi:10.3171/2009.1.PEDS08328
Michejda M, Hodgen GD (1981) In utero diagnosis and treatment of non-human primate fetal skeletal anomalies. I. Hydrocephalus. JAMA 246:1093–1097
Nakayama DK, Harrison MR, Berger MS et al (1983) Correction of congenital hydrocephalus in utero I. The model: intracisternal kaolin produces hydrocephalus in fetal lambs and rhesus monkeys. J Pediatr Surg 18:331–338
Glick PL, Harrison MR, Halks-Miller M et al (1984) Correction of congenital hydrocephalus in utero II: efficacy of in utero shunting. J Pediatr Surg 19:870–881
Edwards MS, Harrison MR, Halks-Miller M et al (1984) Kaolin-induced congenital hydrocephalus in utero in fetal lambs and rhesus monkeys. J Neurosurg 60:115–122. doi:10.3171/jns.1984.60.1.0115
Emery SP, Greene S, Hogge WA (2015) Fetal therapy for isolated aqueductal stenosis. Fetal Diagn Ther 38:81–85. doi:10.1159/000382015
Birnholz JC, Frigoletto FD (1981) Antenatal treatment of hydrocephalus. N Engl J Med 304:1021–1023. doi:10.1056/NEJM198104233041706
Clewell WH, Johnson ML, Meier PR et al (1982) A surgical approach to the treatment of fetal hydrocephalus. N Engl J Med 306:1320–1325. doi:10.1056/NEJM198206033062202
Harrison MR, Filly RA, Golbus MS et al (1982) Fetal treatment 1982. N Engl J Med 307:1651–1652. doi:10.1056/NEJM198212233072623
Manning FA, Harrison MR, Rodeck C (1986) Catheter shunts for fetal hydronephrosis and hydrocephalus. Report of the international fetal surgery registry. N Engl J Med 315:336–340. doi:10.1056/NEJM198607313150532
Al-Anazi AR (2010) In utero ventriculo-uterine shunt treatment for fetal hydrocephalus: preliminary study of Al-Anazi ventriculo-uterine shunt. Neurosurg Q 20(1):1–4
Sutton LN, Sun P, Adzick NS (2001) Fetal neurosurgery. Neurosurgery 48:124
Cavalheiro S, Moron AF, Zymberg ST, Dastoli P (2003) Fetal hydrocephalus—prenatal treatment. Childs Nerv Syst 19:561–573. doi:10.1007/s00381-003-0772-7
Al-Anazi A, Al-Mejhim F, Al-Qahtani N (2008) In uteroventriculo-amniotic shunt for hydrocephalus. Childs Nerv Syst 24:193–195. doi:10.1007/s00381-007-0451-1
Emery SP, Hogge WA, Hill LM (2015) Accuracy of prenatal diagnosis of isolated aqueductal stenosis. Prenat Diagn 35:319–324. doi:10.1002/pd.4520
Chen Y, Emery SP, Maxey AP et al (2016) A novel low-profile ventriculoamniotic shunt for foetal aqueductal stenosis. J Med Eng Technol 40:186–198. doi:10.3109/03091902.2016.1154617
Saadai P, Runyon T, Farmer DL (2011) Fetal neurosurgery: current state of the art. Future Neurol 6:165–171. doi:10.2217/fnl.11.3
Heuer GG, Adzick NS, Sutton LN (2015) Fetal myelomeningocele closure: technical considerations. Fetal Diagn Ther 37:166–171. doi:10.1159/000363182
Davis GH (2003) Fetal hydrocephalus. Clin Perinatol 30:531–539
Frigoletto FDJ, Birnholz JC, Greene MF (1982) Antenatal treatment of hydrocephalus by ventriculoamniotic shunting. JAMA 248:2496–2497
Kulkarni AV, Drake JM, Mallucci CL et al (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155:254–9.e1. doi:10.1016/j.jpeds.2009.02.048
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding source
Pediatric Hydrocephalus Foundation Research Grant.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Pisapia, J.M., Sinha, S., Zarnow, D.M. et al. Fetal ventriculomegaly: Diagnosis, treatment, and future directions. Childs Nerv Syst 33, 1113–1123 (2017). https://doi.org/10.1007/s00381-017-3441-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3441-y