Abstract
Recent developments in prenatal ultrasonography and MRI neuroimaging techniques have enabled more frequent diagnoses of congenital anomalies of the central nervous system in the prenatal period. The frequency of prenatal hydrocephalus is reportedly 0.2–0.8/1000 births. However, the diagnosis of prenatal hydrocephalus always involves some uncertainty since the fetus is in utero. Differentiation from fetal ventriculomegaly, the frequency of which is 0.5–3.8/1000 births, is indispensable but not straightforward in some cases. Prenatal hydrocephalus is highly suspected if the ventriculomegaly is progressive. Such cases of progressive ventriculomegaly occur at a rate of 10–20%, while the frequency of fetal ventriculomegaly remains stable at 50–60%, with spontaneous resolution observed in about 30–50% of cases.
The prognosis of both prenatal hydrocephalus and fetal ventriculomegaly is multifactorial. The size of the ventriculomegaly and pathogenesis and the time at onset may influence outcomes. The survival rate of overall fetal ventriculomegaly is reportedly 40–50%, with some cases being terminated at the request of the parents when the diagnosis is made in the early fetal period. The peri- and postnatal mortality rates reach 10–20%. The developmental outcomes of fetal ventriculomegaly vary depending on the degree of ventriculomegaly and the presence of associated abnormalities within and without the central nervous system. Normal development is reportedly seen in 80–90% of cases of isolated mild ventriculomegaly. Even in postnatally treated prenatal hydrocephalus, normal development is expected in about 20% of cases. A poor outcome seems unavoidable in the majority of genetic, post-infectious, and syndromic hydrocephalus.
Information obtained from prenatal examinations is limited, and at present no standardized prenatal treatment exists. Neurosurgical intervention is an option postnatally, with the procedure being selected on the basis of the clinical condition of the baby.
Parents should receive prenatal counseling at every stage of the examination and diagnosis. Since the exact diagnosis and prognosis are difficult to establish prenatally, it would be appropriate to take a narrative approach to counseling the parents.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Hydrocephalus
- Prenatal hydrocephalus
- Fetal hydrocephalus
- Fetal ventriculomegaly
- Congenital hydrocephalus
- VP shunt
- Neuroendoscopy
- Cerebrospinal fluid
- Prenatal counseling
- Informed consent
- Narrative-based medicine
Introduction
With the evolution of modern diagnostic technologies, more cases of congenital central nervous system (CNS) anomalies have come to be diagnosed prenatally. Recent developments in ultrasonography, especially the introduction of high-quality transvaginal and 3D ultrasound, together with the use of fetal MRI, have enabled detailed visualization of early fetal morphology (Pooh 2009). Hydrocephaly is among those congenital CNS anomalies which have benefited greatly from these technological advances and are now often diagnosed prenatally using ultrasound.
The etiology of 55% of infantile hydrocephaly cases is reportedly prenatal, and the prevalence of pre- or perinatally diagnosed hydrocephalus was found to be 0.82 per 1000 live births or 0.49 births for infantile hydrocephaly and 0.33 births for myelomeningocele (Persson et al. 2005). The data seem compatible with those of another study, which cited the incidence of primary congenital hydrocephaly (excluding secondary hydrocephaly and association with myelomeningocele) as 0.2–0.8 per 1000 births (Chi et al. 2005).
Fetal hydrocephaly is generally rare but one of the most commonly encountered fetal CNS anomalies. It should be remembered, however, that morphological abnormalities do not necessarily correspond with functional outcomes. This is particularly the case in fetal hydrocephaly, which is characterized by ventriculomegaly. Hydrocephaly results from progressive, abnormal accumulation of cerebrospinal fluid (CSF) in the ventricles and has a number of possible etiologies. Ventriculomegaly, on the other hand, is a condition characterized simply by enlarged ventricles (Rekate 2011). Hydrocephaly is almost always accompanied by ventriculomegaly and requires surgical treatment, but ventriculomegaly does not necessarily involve the presence of hydrocephaly. Thus, prenatal counseling for parents facing the prospect of a child with fetal hydrocephaly differs markedly from that for parents expecting a child with fetal ventriculomegaly. The problem is that the differentiation is not straightforward despite recent technological improvements in diagnostic imaging (Garel et al. 2003).
Another problem surrounding fetal hydrocephaly is that even though it can be diagnosed early, fetal management options are quite limited; no practical treatment method exists, and the only recourse is often just observation. Fetal surgery for prenatally diagnosed hydrocephaly is not considered to be very effective, although controversy on this question remains (Birnholz and Frigoletto 1981; Clewell et al. 1981; Manning et al. 1986; Frigoletto et al. 1992; von Koch et al. 2003; Cavalheiro et al. 2011). Meaningful management of hydrocephaly becomes possible only postnatally. The time lag between the diagnosis and start of treatment makes prenatal counseling for parents indispensable but difficult.
This paper begins by defining and classifying fetal hydrocephaly before proceeding to a discussion of diagnosis, differences from simple ventriculomegaly, currently available postnatal treatment algorithms, and surgical options. Finally, principles for prenatal counseling for parents expecting a fetal hydrocephalic child are discussed. It should be noted that there is a wide variation in the reported incidence of any aspect of fetal hydrocephaly and fetal ventriculomegaly due to the limited number of cases and selection bias inherent in this type of hydrocephaly.
Definition and Classification of Fetal Hydrocephaly
Fetal hydrocephaly is defined as any hydrocephaly diagnosed prenatally. Most cases consist of primary hydrocephaly including genetic hydrocephaly, holoprosencephaly, Dandy-Walker syndrome, aqueduct stenosis, hydrocephaly associated with myelomeningocele, and so on. Less frequently, secondary hydrocephaly caused by fetal intraventricular hemorrhaging, CNS infection, or intrauterine trauma diagnosed during pregnancy may also be included (Morota and Yamasaki 2014; Yamasaki 2010) (Fig. 1).
Classification of perinatal hydrocephalus. All hydrocephaly cases diagnosed prenatally are classified as prenatal hydrocephaly. Congenital hydrocephaly includes all hydrocephaly cases diagnosed prenatally (prenatal hydrocephalus). Primary hydrocephaly, on the other hand, is diagnosed postnatally. Congenital hydrocephaly therefore consists of all cases of prenatal hydrocephaly and postnatally diagnosed primary hydrocephaly
Fetal and congenital hydrocephaly are often mistaken for one another. Congenital hydrocephaly encompasses diverse pathologies including any prenatally diagnosed hydrocephaly (fetal hydrocephaly) and postnatally diagnosed primary hydrocephaly, i.e., all fetal hydrocephaly as well as postnatally diagnosed primary hydrocephaly. Secondary hydrocephaly, diagnosed postnatally and sometimes referred to as acquired hydrocephaly, includes post-hemorrhagic, post-infectious, or meningitis-related hydrocephaly among others (Yamasaki 2010) (Fig. 1). Hereafter, the term “prenatal hydrocephaly” will be used instead of “fetal hydrocephaly” to differentiate fetal from congenital hydrocephaly (Fig. 1).
Diagnosis of Prenatal Hydrocephalus
The pathology of hydrocephaly is deceptively simple in that, while the pathomechanism is tied directly to ventriculomegaly, the latter condition itself is attributable to various etiologies. Some define hydrocephaly as “an increase in the fluid-containing spaces of the brain at increased pressure, resulting from an imbalance between CSF production and absorption or flow” (Abou-Hamden and Drake 2015). While this is undeniably true, it passes over crucial, etiological considerations. This picture is further complicated by the need to distinguish prenatal hydrocephaly from fetal ventriculomegaly since the postnatal management and neurodevelopmental outcomes differ widely. However, the differentiation in some case is not straightforward, as will be discussed.
In Utero Diagnosis of Prenatal Hydrocephalus
The diagnosis of prenatal hydrocephaly is based on ventriculomegaly visualized in fetal neuroimaging. Atrial width (AW), defined as the transverse diameter of the lateral ventricular atrium at the level of the glomus of choroid plexus, is used to diagnose fetal ventriculomegaly (Cardoza et al. 1988; Pillu et al. 1989). AW normally stabilizes after the second trimester when the upper limit of normal AW measures 10 mm (Cardoza et al. 1988; Rosseau et al. 1992). An AW of 10–12 mm is regarded as borderline, and a width above 12 mm is diagnosed as ventriculomegaly. Grading of ventriculomegaly has not been standardized and varies according to the source of information so that an AW of 10–12 mm is sometimes described as “mild,” 12–15 mm as “moderate,” and above 15 mm as “severe” ventriculomegaly (Gaglioti et al. 2005; Madazil et al. 2011). At present most sources agree that an AW greater than 15 mm constitutes severe and an AW between 10 and 15 mm as mild ventriculomegaly (Garel et al. 2003; D’Addario and Rossi 2012) (Fig. 2). The accuracy of the ventricular measurements obtained by fetal brain ultrasonography and MRI are reportedly the same regardless of the size of the ventricle (Perlman et al. 2014).
Definition of fetal ventriculomegaly: AW. Atrial width (AW), defined as the transverse diameter of the lateral ventricular atrium at the level of the glomus of choroid plexus, is used for the diagnosis of fetal ventriculomegaly. An AW of less than 10 mm is regarded as normal. The degree of ventriculomegaly is assessed on the basis of AW
The frequency of ventriculomegaly is reportedly 0.5–3.8 per 1000 births while that of isolated ventriculomegaly, which has no association with any other CNS anomalies, is reportedly 0.4–0.9 per 1000 births (Bannister et al. 2000; Weichert et al. 2010; Sethna et al. 2011). The degree and type of fetal ventriculomegaly differ according to the case series reported. Mild to moderate cases reportedly comprise about 40% of the total. On the other hand, severe ventriculomegaly has the same frequency range but accounts for about 60% of cases (Gaglioti et al. 2005; Joő et al. 2008; Madazil et al. 2011). Isolated fetal ventriculomegaly comprises about 40% of all cases of fetal ventriculomegaly. The remaining 60% are associated with structural abnormalities in the CNS and are often called non-isolated ventriculomegaly (Gaglioti et al. 2005; Nomura et al. 2010; Weichert et al. 2010; Yamasaki et al. 2012). Interestingly, the degree of ventriculomegaly correlates with the frequency of isolated ventriculomegaly in inverse proportion to some extent. Gaglioti et al. reported that isolated ventriculomegaly occurred in about 60% of mild cases, 24% of moderate cases, and 40% of severe cases of ventriculomegaly (Gaglioti et al. 2005). Weichert et al. reported that mild ventriculomegaly accounted for some 40% of their 47 cases of isolated ventriculomegaly and 52% of 62 cases of non-isolated ventriculomegaly (Weichert et al. 2010).
Natural Course of Fetal Ventriculomegaly
The course and prognosis of fetal ventriculomegaly are poorly understood, and ventriculomegaly itself seems to change during the pregnancy (Fig. 3). Kelly et al. analyzed the clinical course of 295 cases of isolated fetal ventriculomegaly and reported that 29% showed spontaneous resolution, while 14% progressed during pregnancy and the remaining 57% remained stable (Kelly et al. 2001). Chronological changes in fetal ventriculomegaly have also been reported in another paper with similar results in terms of a higher rate of spontaneous resolution in over 50% of isolated mild ventriculomegaly cases (Vergani et al. 1998; Breeze et al. 2005; Tugcu et al. 2014). Interestingly, Chiu et al. defined an AW of 7 to <10 mm as the “gray zone” and found that 2.8% of these cases progressed to ventriculomegaly (>10 mm) during pregnancy (Chiu et al. 2014). These data imply that fetal ventriculomegaly is rare but not unusual and that the degree of ventriculomegaly is not fixed but can vary during the course of the pregnancy. Repeated neuroimaging via ultrasound or MRI is required to clarify the prognosis of fetal ventriculomegaly. Again, it should be borne in mind that fetal ventriculomegaly does not necessarily refer to prenatal hydrocephaly.
The diagnosis of postnatal hydrocephaly is based on the clinical findings of progressive ventriculomegaly, increased head size, and tense major fontanelle (increased intracranial pressure). On the other hand, ventriculomegaly develops as a result of atrophic change or brain volume loss caused by a variety of pathologies like cortical malformation, metabolic disease, use of steroids, and the influence of CNS infectious diseases like meningitis or encephalitis. Wang et al. described the features of fetal ventriculomegaly as (1) a disturbance of CSF dynamics (true hydrocephalus), (2) brain volume loss (destruction of brain tissue by pathologic conditions during the fetal period), and (3) an abnormal dilatation of ventricles (due to neural cell migration disorders and other developmental anomalies) (Wang et al. 2011). Wang’s description implies that ventriculomegaly is a “necessary” condition, but not a “sufficient” one for the diagnosis of hydrocephaly, a critical point in the diagnosis of prenatal hydrocephaly.
The judgment of whether an infant is hydrocephalic or simply ventriculomegalic is difficult but unavoidable since the diagnosis determines the obstetric and neurosurgical management, as well as the counseling given to the parents. Comprehensive, careful evaluation including a series of fetal neuroimaging at different intervals during the pregnancy is required to establish the diagnosis of prenatal hydrocephaly.
Postnatal Management and Treatment
Hydrocephaly is first suspected when fetal neuroimaging, usually carried out by ultrasound, reveals ventriculomegaly. Distinguishing hydrocephaly from ventriculomegaly is difficult, as mentioned previously. Once ventriculomegaly is confirmed, however, the possibility of hydrocephaly needs to be considered even after birth until it can be definitely ruled out.
As yet there exists no global consensus about how to manage prenatal hydrocephaly. Part of the reason for this lack of consensus may very well be traced to social, rather than medical reasons such as religious backgrounds, insurance coverage, economic status of the family, and the availability of social support, all of which affect the management of prenatal hydrocephaly. In view of these considerations, the most that can be done by physicians is to seek the best solution compatible to the given social and medical circumstances such as the algorithm for prenatal hydrocephaly management shown in Fig. 4 (Cavalheiro et al. 2003).
Management algorithm for prenatal hydrocephaly. Prenatal hydrocephaly is first suspected when fetal ventriculomegaly is diagnosed and may be strongly suspected if the ventriculomegaly is progressive. However, the options for prenatal treatment are very limited. Fetal surgery for prenatal hydrocephaly still remains controversial
Fetal Surgery for Hydrocephalus
During the 1980s, fetal treatment of hydrocephaly was tested with the hope that early treatment would lead to better outcomes (Birnholz and Frigoletto 1981). It soon became apparent that intrauterine surgery fell short of expectations when the early surgical outcomes revealed a relatively high rate of peri- and postoperative fetal death, high rate of neurodevelopmental disability, and few cases of normal development among the survivors (Michejda et al. 1986; Manning et al. 1986). The impact of these discouraging outcomes in the early trials of fetal surgery was so serious that almost no further attempts were made since then. Currently, the focus of prenatal hydrocephaly management has shifted to postnatal treatment. Although some hospitals still attempt fetal treatment in accordance with strict surgical criteria (Cavalheiro et al. 2003, 2011), early diagnosis followed by early delivery after pulmonary maturation and early surgical intervention after a definite diagnosis of hydrocephaly are regarded as guiding principles in the management of prenatal hydrocephaly.
Timing of Delivery
The maturation of the pulmonary system and the progression of ventriculomegaly must be weighed when deciding upon the optima timing for delivery. In general, the progression of ventriculomegaly in utero is not common. If ventriculomegaly is stable, delivery at full term is preferable (Joő et al. 2008). If ventriculomegaly progresses and hydrocephaly is highly suspected, earlier delivery should be considered. Since the fetal pulmonary system matures by 36 weeks’ gestation, delivery should be planned after the 36th week (Yamasaki et al. 2012). There is no evidence that delivery by Cesarean section is safer than vaginal delivery for prenatally diagnosed hydrocephaly (Luthy et al. 1991). However, if the enlarged fetal head cannot be accommodated by the mother’s pelvis, Cesarean section should be considered based on obstetric indications.
Timing of Surgery and Surgical Procedures
Several days to months may pass before suspected prenatal hydrocephalus becomes clinically apparent postnatally. A tense major fontanelle, increased head circumference, and progression of ventriculomegaly strongly support the diagnosis of hydrocephaly.
Body weight (BW) and maturation of the pulmonary system need to be considered when selecting the initial surgical intervention for prenatal hydrocephaly (Moritake et al. 2007a). When the pulmonary function is immature, less invasive treatment is preferred before recourse is made to the VP shunt. A treatment algorithm recommended in Japan for newborn hydrocephalic infants is shown in Fig. 5 (Morota and Yamasaki 2014). When the BW is under 1000 g, a ventriculo-subgaleal shunt and/or placement of a CSF reservoir is recommended (Nagy et al. 2013; Wang et al. 2014).
Postnatal treatment algorithm for prenatal hydrocephaly. Postnatal treatment of children with prenatally diagnosed hydrocephalus is based on clinical conditions and infant body weight. A VP shunt may be placed safely if the body weight (BW) is 2500 g or greater. An experienced pediatric neurosurgeon can readily place a VP shunt in a baby with a BW between 2000 and 2500 g. A ventriculo-subgaleal shunt is a surgical option when the BW is less than 1000 g, and placement of a CSF reservoir is preferred when the BW is between 1000 and 2000 g
When the BW falls between1000 and 2000 g, placement of a CSF reservoir is recommended (Gaskill et al. 1988; Hudgins et al. 1998). After the CSF reservoir is placed, it is punctured by a 27-gauge needle under sterile conditions. The CSF is then aspirated periodically when the major fontanelle becomes tense. Alternative management of prenatal hydrocephaly in low-birth-weight infants consists of external ventricular drainage using an intravenous catheter or a peripheral inserted central venous catheter (Marro et al. 1991; Sakuma et al. 2006). The latter procedures have the advantage of enabling bedside insertion of a catheter and avoiding repeated puncturing of the CSF reservoir but may present a higher risk for infection in the long term.
The amount of CSF to be aspirated from the reservoir is determined based on the tension in the major fontanelle. Usually aspiration of 10–30 ml of CSF is sufficient for decompression. When the amount of the aspirated CSF has reached more than 40 ml or aspiration is required several times daily, recourse may be had to the next step in treatment.
If the BW exceeds 2000 g and attending neurosurgeons have sufficient skill to perform the procedure, a ventriculo-peritoneal (VP) shunt may be placed. In general, if the BW exceeds 2500 g, a VP shunt can be safely installed as an initial treatment for hydrocephaly. The type of shunt valve/system recommended for a VP shunt in prenatal hydrocephalics has been controversial for several decades. The programmable shunt valve system failed to demonstrate superiority over the traditional pressure-dependent valve system (Notarianni et al. 2009; Hatlen et al. 2012). A nationwide survey in Japan found that a low-pressure valve system was most commonly used for the initial shunting in congenital hydrocephaly treatment (Moritake et al. 2007a). The optimal length for a peritoneal catheter for insertion in the abdominal cavity also remains controversial. The author prefers using a programmable shunt valve with an antisiphon device and inserting a peritoneal catheter about 45 cm in length into the abdominal cavity. This procedure allows no shunt revision for the extension of the peritoneal catheter even after a child grows older, unless the peritoneal catheter itself is occluded. Other CSF diversion procedures can be applied when the abdominal cavity is judged unsuitable for CSF drainage.
Neuroendoscopic surgery may be indicated if any obstructive pathology in and around the ventricle is encountered. A cystic lesion causing obstructive hydrocephaly would be the best indication for endoscopic fenestration for fetal hydrocephaly. It has been commonly remarked that endoscopic third ventriculostomy (ETV) in the neonatal period poses a higher risk of failure than when performed in infants more than 6 months old (Cinalli et al. 1999; Ogiwara et al. 2010). Choroid plexus coagulation (CPC), once a major surgical procedure for hydrocephaly in the early twentieth century, has been reevaluated as a possible option for endoscopic management of hydrocephaly (Pople and Ettles 1995; Warf 2005). CPC may be indicated as a solo procedure for extreme hydrocephaly and hydranencephaly (Morota and Fujiyama 2004; Shitsama et al. 2014) or may be deployed in combination with ETV (Warf 2005; Kulkarni et al. 2014; Stone and Warf 2014; Bankole et al. 2015). Slow, progressive, and severe forms of hydrocephaly can be stabilized by solo CPC in about 30–40% of cases regardless of the pathology (Pople and Ettles 1995, Morota and Fujiyama 2004, Shitsama et al. 2014). The success rate of ETV + CPC varies from 40% to 80% based on the background pathologies. Post-infectious hydrocephaly usually showed poor outcomes (Warf 2005; Kulkarni et al. 2014; Stone and Warf 2014; Bankole et al. 2015). It should be remembered that ETV + CPC always produced a better success rate than ETV alone, but age nonetheless influences the outcome (Warf 2005; Kulkarni et al. 2014; Stone and Warf 2014; Bankole et al. 2015).
Prognosis of Prenatally Diagnosed Hydrocephalus
Fetal hydrocephaly is the natural outcome of fetal ventriculomegaly when the latter is left untreated. Despite the aforementioned confusion in terms and concepts, the two conditions should be considered distinct clinical entities. Hence the prognosis of fetal ventriculomegaly needs to be more closely examined. It should be remembered that the overall mortality rate for fetal ventriculomegaly is generally high at 37–51% according to recent reports (Gaglioti et al. 2005; Madazil et al. 2011). In addition, there is a surprising paucity of long-term follow-up data for prenatal hydrocephaly. In most reports, prenatal hydrocephaly is included in the category of congenital hydrocephaly together with postnatally diagnosed primary hydrocephaly (Moritake et al. 2007a). Strikingly, almost no studies which discussed fetal ventriculomegaly mentioned the number of cases treated neurosurgically after birth. This lack of basic data required to bridge obstetrical and neurosurgical management must be resolved in the future.
Factors influencing the prognosis of prenatally diagnosed hydrocephaly are multifactorial. A positive association between developmental delay and degree of ventriculomegaly has been widely recognized, and the presence of additional anomalies, whether CNS or non-CNS, is regarded as predictors of poor prognosis (Gaglioti et al. 2005; Madazil et al. 2011). Prenatal hydrocephaly is commonly associated with other CNS abnormalities. A nationwide survey of congenital hydrocephaly in Japan demonstrated that simple hydrocephaly constitutes less than 20% of all prenatal hydrocephaly cases (Moritake et al. 2007b).
Termination of Pregnancy
Termination of pregnancy arises as a possibility when fetal ventriculomegaly is diagnosed. Ethical, religious, cultural, legal, social, medical (gestational age at diagnosis), and various other factors influence the parents’ decision with regard to abortion, a fact reflected in the large variations in abortion reported by previous studies. When the ventriculomegaly is judged to be severe, the rate of abortion reaches 50–100% (Breeze et al. 2007; Joő et al. 2008; Chiu et al. 2014). Up to 40% of even mild cases with an AW of less than 15 mm were aborted according to one report (Joő et al. 2008). Cases of fetal ventriculomegaly with associated abnormalities were apparently terminated more often than cases with no associated abnormalities. Weichert reported an overall abortion rate of 31%, with an abortion rate of 21% (10 of 47 cases) for isolated ventriculomegaly and 39% (24 of 62 cases) for non-isolated ventriculomegaly. Others reported overall abortion rates ranging from 17% to 29% (Tugcu et al. 2014; Yamasaki et al. 2012; Chiu et al. 2014). These data generally suggest that cases of severe and/or non-isolated fetal ventriculomegaly tend to be terminated before birth.
Mortality Rate
The severity of ventriculomegaly and the associated abnormalities must also be weighed when determining prognosis even after birth. Isolated mild ventriculomegaly usually has the best prognosis with nearly no peri- or postnatal mortality (Breeze et al. 2005; Madazil et al. 2011). The mortality rate of fetuses with associated abnormalities is higher than those with isolated ventriculomegaly (Gaglioti et al. 2005; Breeze et al. 2007). Madazil et al. reported that in the absence of associated abnormalities and chromosomal aberrations, mild ventriculomegaly (the AW 10–12 mm) demonstrated a favorable outcome close to the norm for the general population (Madazil et al. 2011). Renier et al. reported a survival rate of 62% at 10 years for prenatal hydrocephaly (Renier et al. 1988). The overall death rate for fetal ventriculomegaly in the peri- and postnatal periods was reportedly 10–20% with one study reporting a rate as high as 52% (Tugcu et al. 2014; Yamasaki et al. 2012; Gaglioti et al. 2005; Madazil et al. 2011). The mortality rate for prenatal hydrocephaly surpasses the overall mortality rate for congenital hydrocephaly at 0.71 per 100 person-years (Chi et al. 2005).
Developmental Outcome
The degree of ventriculomegaly and associated abnormalities also influence the developmental outcome. Breeze et al. reported that 4 out of 21 isolated mild fetal ventriculomegaly cases showed delayed development while 7 out of 8 survivors of severe ventriculomegaly demonstrated delayed development (Breeze et al. 2005, 2007). Gaglioti et al. reported 5 cases of normal development in 8 survivors among 60 severe ventriculomegaly cases and 40 instances of normal development in 43 survivors from a total of 75 mild ventriculomegaly cases. With respect to associated abnormalities, the rate of normal development ranged from 35 to 58% for isolated ventriculomegaly and 8–9% for non-isolated ventriculomegaly (Madazil et al. 2011; Weichert et al. 2010). Overall developmental outcomes for isolated fetal ventriculomegaly were systematically reviewed by Laskin et al. with favorable outcomes seen in 85% of cases (Laskin et al. 2005). Furthermore, more than 92% of isolated mild ventriculomegaly cases showed normal development, close to the norm for the general population (Pagani et al. 2014). When ventriculomegaly improves during intrauterine follow-up, a normal outcome is reportedly more likely than when it remains unchanged (Gaglioti et al. 2005). Nonetheless, the optimistic view of developmental outcomes in mild isolated ventriculomegaly remains somewhat controversial. Kutuk et al. confirmed a mild degree of developmental delay in 9 out of 25 children (36%) for this population in the follow-up period of 24–74 months (Kutuk et al. 2013). A review of reports on isolated mild ventriculomegaly covering the last two decades showed a 10–20% rate for overall abnormal outcomes (range 0–57%) for cases of fetal ventriculomegaly (Melchiorre et al. 2009). It should be remembered that false-negative results for tests for associated abnormalities do occur. In one review, the false-negative rate was reportedly 12.8% (range, 0–50%) (Melchiorre et al. 2009).
The long-term outcomes among survivors in a series of fetal hydrocephaly cases consisted of a good outcome in 23%, and mild retardation in 26%, of cases (Yamasaki et al. 2012). Similar results were also reported from Paris with normal IQ (>80) in 28% and mild developmental delay (60 < IQ < 80) in 21% of pediatric survivors of prenatal hydrocephaly (Renier et al. 1988). On the other hand, Futagi et al. analyzed neurodevelopmental outcomes of postnatally treated fetal hydrocephaly with various concomitant pathologies and found that more than 20% of the children reached a normal IQ level (Futagi et al. 2002). Although severe ventriculomegaly is thought to have the worst prognosis, there are still a very small number of children (less than 10%) who mature without any severe deficits in the absence of associated abnormalities (Madazil et al. 2011; Gaglioti et al. 2005; Weichert et al. 2010). It should also be reminded that even isolated, nonprogressive, and mild borderline ventriculomegaly can carry the risk of delayed development (Kutuk et al. 2013; Chiu et al. 2014).
Factors Influencing the Prognosis
The prognosis of prenatal hydrocephalus is multifactorial, as mentioned above. The short- and long-term outcomes are probably influenced by the basic pathologies which gave rise to the hydrocephaly, as well as by any associated abnormalities including chromosomal and genetic aberrations. In general, the presence of chromosomal abnormalities and the severity of associated pathologies significantly influence mortality and developmental outcome.
Three major factors seem to play a critical role in the prognosis of prenatal hydrocephaly. First, the degree of ventriculomegaly and its progression can suggest the likely outcome of prenatal hydrocephalus. Spontaneous resolution or regression of ventriculomegaly may indicate a good developmental outcome. One review reported a positive outcome in 44% of progressive fetal ventriculomegaly cases and only 7% of nonprogressive cases (Melchiorre et al. 2009).
Second, the pathogenesis of hydrocephaly can provide clues to the outcome. Any prenatal hydrocephaly case resulting from infection, such as TORCH (toxoplasma, rubella, cytomegalovirus, and helps), generally has a poor developmental outcome (Melchiorre et al. 2009). Idiopathic and isolated hydrocephaly has a more favorable outcome after postnatal treatment compared with syndromic hydrocephaly associated with Dandy-Walker syndrome, encephalocele, holoprosencephaly, or hydranencephaly. A normal or near-normal developmental outcome can be expected in more than 50% of hydrocephaly cases associated with myelomeningocele (Moritake et al. 2007a, 2007b). However, even simple hydrocephaly cases without intracranial abnormalities do not necessarily have a positive outcome. It is widely known that X-linked hydrocephalus and other types of L1 syndrome whose neuroimaging findings resemble those of aqueduct stenosis often fail to show any functional improvements despite placement of a VP shunt and remains severely disabled (Yamasaki et al. 1997, 2011). The presence of chromosomal or genetic abnormalities strongly correlates with a poor outcome. In a review of isolated mild ventriculomegaly cases, the frequency of chromosomal abnormalities, mainly trisomy 21, was reported at 2.8% (ranged from 0% to 28.6%) (Melchiorre et al. 2009).
Finally, time at onset of hydrocephaly influences the functional as well as morphological development of the brain. Early onset of hydrocephaly can disturb neural cell migration, formation of vascular structures, and the progress of myelination. All of these negative episodes result in neurological deficits and delayed postnatal development (Oi 2004). Correlating these factors three-dimensionally may help produce a more precise estimate of the prognosis of prenatal hydrocephaly (Fig. 6).
Factors influencing prognosis of prenatal hydrocephaly and fetal ventriculomegaly. The prognosis of prenatal hydrocephaly and fetal ventriculomegaly is multifactorial. The degree of ventriculomegaly and its progression in utero, pathogenesis, and the timing of the onset must be all be considered as influencing the prognosis
Representative Cases
-
Case 1: Mild isolated ventriculomegaly – observation (Fig. 7).
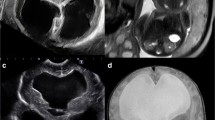
Fig. 7 A case of mild isolated ventriculomegaly. A fetal MRI taken at 32 weeks’ gestation revealed a heterogeneous intraventricular mass with moderate ventriculomegaly. The mass presented slightly high-signal intensity on T1-weighted imaging, suggesting intraventricular hemorrhage. A follow-up MRI taken at 34 weeks’ gestation showed some shrinkage of ventriculomegaly. A postnatal MRI taken 1 week after the birth showed an intraventricular hematoma attached to the choroid plexus as well as a further decrease in the size of the ventriculomegaly. An MRI taken at 2.5 months old showed a stable but mildly enlarged ventricular size. The baby continued to be observed without surgery. At the age of 3 years, the patient suffered mild delayed development, epilepsy, and bilateral cortical blindness, the combined effect of which was to greatly delay the patient’s course of development
Routine ultrasonography demonstrated severe ventriculomegaly at 31 weeks’ gestation, and the mother was referred to a maternal medical center where MRI was done at 32 weeks’ gestation. The AW was more than 15 mm, suggesting severe ventriculomegaly with a mass in the right frontal horn. The MRI finding of the mass was compatible with hemorrhaging, and intrauterine posthemorrhagic hydrocephaly was suspected. No abnormalities of any kind were found.
The parents were informed of the following:
-
1.
Possible diagnosis of hydrocephaly.
-
2.
If ventriculomegaly progressed, early delivery by cesarean section at around 36 weeks’ gestation might be required.
-
3.
After delivery, placement of a CSF reservoir or VP shunt might be performed if the diagnosis of hydrocephaly was established.
-
4.
Developmental prognosis might be difficult owing to the severity of the ventriculomegaly. On the other hand, because the case was that of isolated, simple posthemorrhagic hydrocephaly, a better prognosis could be expected given an uncomplicated course of postnatal treatment.
Follow-up MRI taken 2 weeks later revealed some shrinkage of the AW. The parents were again informed of the condition and the possible prognosis.
Since the ventriculomegaly showed signs of resolving, or at least of not progressing, the fetus was monitored carefully by weekly ultrasonography and was delivered at full term by vaginally delivery. An MRI taken a week after the birth showed further shrinkage of ventricular size. The parents were once more informed that the likelihood of hydrocephaly was very small and that neurosurgical intervention would be unnecessary. In addition, since the ventriculomegaly resolved spontaneously, and no associated abnormalities were evident in the neural structure, normal developmental prognosis could be expected. A follow-up CT scan taken 2.5 months later showed stable ventricular size. Mild developmental delay became apparent when the patient was 7 months old. At the age of 3 years, the patient suffered mild developmental delay, epilepsy, and bilateral cortical blindness, the combined effect of which resulted in severely delayed development.
-
Case 2: Severe ventriculomegaly associated with other abnormalities – VP shunt (Fig. 8).
Fig. 8 A case of severe ventriculomegaly associated with other abnormalities. Fetal ventriculomegaly was first pointed out at 22 weeks’ gestation. The first fetal MRI taken at 28 weeks’ gestation revealed marked ventriculomegaly with a bilateral AW greater than 30 mm. Hypoplasia of the cerebellar vermis, lissencephaly, and kinking of the brainstem were observed as associated abnormalities (upper low). At this point, Dandy-Walker variant was tentatively diagnosed. The baby was born at 36 weeks’ gestation by scheduled Cesarean section because ventriculomegaly showed signs of progressing even after the first MRI. Walker-Warburg syndrome was finally diagnosed by a MRI taken 2 days after the birth. Despite treatment of the hydrocephalus using a CSF reservoir followed by a VP shunt, the baby died due to cardiopulmonary arrest at the age of 9 months
Fetal ventriculomegaly was first pointed out at 22 weeks’ gestation, and the mother was referred to a maternal medical center when the progress of ventriculomegaly was confirmed at 26 weeks’ gestation. A fetal MRI first taken at 28 weeks’ gestation revealed marked ventriculomegaly with a bilateral AW greater than 30 mm. Hypoplasia of the cerebellar vermis, lissencephaly, and kinking of the brainstem were observed as associated abnormalities. At this point, Dandy-Walker variant was tentatively diagnosed. The parents were informed that the fetus was hydrocephalic, the case was probably not one of simple ventriculomegaly, and that early intervention before full term of pregnancy would be necessary. They were also informed of a high risk of delayed development based on the severity of the ventriculomegaly and associated CNS abnormalities.
The baby was delivered at 36 weeks’ gestation by scheduled Cesarean section because the ventriculomegaly showed signs of continued progression after the first MRI. Walker-Warburg syndrome was finally diagnosed by MRI done 2 days after birth. A CSF reservoir was placed on postnatal day 4 and was repeatedly aspirated to reduce tension in the major fontanelle. A VP shunt was installed at 28 days old when an increase in head circumference became clinically apparent. The baby was discharged at 3 months old but readmitted for cardiopulmonary arrest and resuscitated successfully. At age 9 months, cardiopulmonary arrest recurred, and the death was confirmed at the hospital where the patient was admitted.
Prenatal Counseling
Prognosis of prenatal hydrocephaly including fetal ventriculomegaly is usually judged on the basis of MRI and other neuroimaging (chiefly ultrasonographic) findings. Importantly, abnormal anatomical findings do not necessarily coincide with functional outcomes. Severe morphological abnormalities or ventriculomegaly suggest possible future impairment of neurodevelopment in the majority of cases. However, the actual clinical course and degree of impairment widely differ between individuals. In fact, precise clinical outcome in the long term cannot be determined without prolonged observation of patients. Information obtained pre- and postnatally is not sufficient to predict the developmental and functional outcomes with precision. We need to realize the fact that even the latest data obtained by means of cutting-edge technology is still insufficient to predict the future of children with prenatal hydrocephaly. The most likely clinical course can only be imagined. Understanding these limitations is prerequisite for counseling parents of children with prenatal hydrocephaly.
Prenatal Counseling: Providing Basic Information
Prenatal counseling starts once fetal ventriculomegaly is suspected. Precise, up-to-date information needs to be provided to the family. The parents should be informed of test results and the expected clinical course and outcome, once fetal ventriculomegaly is diagnosed. Importantly, the parents should be informed that the initial diagnosis of ventriculomegaly and the severity of the condition may change during the course of the pregnancy. Chromosomal and/or genetic testing may be recommended at this point.
Termination of pregnancy is also an option if the diagnosis of fetal ventriculomegaly is made within the legal time frame for abortion. Severe ventriculomegaly, ventriculomegaly with associated abnormalities, and ventriculomegaly with chromosomal/genetic abnormalities are aborted at a higher rate. Such cases may prove to be true instances of hydrocephaly and pose a heightened risk of delayed developmental outcomes than mild isolated ones. Nevertheless, parents should be also informed that a small number of patients in such high-risk groups can survive without developmental delay.
If the probability of hydrocephalus is high, the parents need to be informed of the following:
-
1.
Precise diagnosis of hydrocephalus.
-
2.
Possible pathogenesis, if known.
-
3.
When and how to deliver, especially if the ventriculomegaly is progressive.
-
4.
Expected postnatal clinical course.
-
5.
Timing of surgery and surgical procedures.
-
6.
Expected long-term developmental outcomes.
Providing the abovementioned information to parents, who are anxious about the fate of their in utero baby, is emotionally demanding for both parties. While it is important to give parents evidence-based information, a supportive attitude and readiness to listen to their concerns whenever needed are equally important especially in view of the uncertainty of medical predictions about the developmental course of hydrocephalic infants.
When an infant with prenatally diagnosed hydrocephaly is born, it should undergo detailed postnatal examination. The parents should be informed of the following:
-
1.
Diagnosis of hydrocephalus.
-
2.
Pathogenesis of hydrocephalus, if known.
-
3.
Any other newly discovered abnormalities.
-
4.
Expected clinical course.
-
5.
Timing of surgery and surgical procedures.
-
6.
Expected long-term outcomes.
Neurosurgeons, neonatologists, psychologists, and nurses should cooperate in providing this information. The basic purpose of such cooperation is to provide multifaceted support to, and share the latest information with, the parents. Table 1 summarizes the basic information discussed in this chapter.
EBM Versus NBM
As a pediatric neurosurgeon, I feel that most parents of hydrocephalic infants sense the gravity and risks inherent in their child’s condition even when postnatal treatments are performed without event. In such situations, it is important for all of the attending medical staff to be on hand to extend their support to the parents. This may include providing the latest evidence-based information, although in my view this is not critical at this juncture.
When parents are informed of the diagnosis and prognosis, they may express a variety of responses including denial but eventually try to accept their situation amid feelings of fear and hope. Accepting their reactions with a supportive attitude, showing them empathy, and consoling them are essential to promote a favorable relationship between the parents and medical staff, with obvious implications for any future treatment of the patient. Such an approach is regarded as central to narrative-based medicine (NBM) , a traditional approach to the caretaker-patient-family relationship and one whose role has been reevaluated even in this era of evidence-based medicine (EBM) (Greenhalgh and Hurwitz 1999; Greenhalgh 1999). The holistic approach of NBM to interactions with prospective parents of prenatally hydrocephalic infants can succeed in encouraging them to go through with the delivery and raise their child. NBM may also improve the relationship between the parents and medical staff during the postnatal clinical course, as mentioned above.
In short, counseling parents of infants with prenatal hydrocephalus by NBM is an important way of compensating for any insufficiencies of EBM. Most of the isolated mild fetal ventriculomegaly cases have a favorable outcome. Nonetheless, some may suffer a degree of developmental impairment. The role of prenatal counseling is to help prospective parents understand their situation and to lessen their apprehensions concerning the future of their baby. The aim of prenatal counseling is to support whatever decision the parents make. There is no single formula for counseling prospective parents of a prenatally hydrocephalic child, but NBM provides a framework for an individualized approach to counseling couples facing the prospect of having a prenatally hydrocephalic child.
Discussions of prenatal hydrocephalus tend to focus on the negatives. Indeed, the mortality rate is high, and delayed development in survivors seems inevitable. Nevertheless, it is important to acknowledge that more than 20% of prenatal hydrocephaly cases survive and have a normal developmental course (Renier et al. 1988; Yamasaki et al. 2012). At the time of prenatal counseling, prospective parents need to be informed of both the negatives and the positives for themselves and their child. Mentioning the positives in the situation, however few these may be, can help prospective parents cope with the difficulty of their circumstances.
Summary
This paper gave an overview of the details of prenatal hydrocephalus and fetal ventriculomegaly. The diagnosis of prenatal hydrocephaly always involves some uncertainty due to the fact that the patient is in utero. Differentiating fetal hydrocephalus from fetal ventriculomegaly is not straight forward in some cases, and prenatal hydrocephaly may be highly suspected if fetal ventriculomegaly is progressive. Information obtained by prenatal examination is limited, and no standardized prenatal treatment exists at present. Nevertheless, pediatric neurosurgeons need to plan postnatal treatment and participate in prenatal counseling as a member of a multidisciplinary team. Further, a narrative-based approach is recommended to build a strong and productive relationship with the prospective parents.
References
Abou-Hamden A, Drake JM (2015) Hydrocephalus. In: Albright AL, Pollack IF, Adelson PD (eds) Principles and practice of pediatric neurosurgery. Thieme, New York, pp 89–99
Bankole OB, Ojo OA, Nnadi M, Kanu OO, Olatosi JO (2015) Early outcome of combined endoscopic third ventriculostomy and choroid plexus cauterization in childhood hydrocephalus. J Neurosurg Pediatr 15:524–528
Bannister CM, Russel SA, Rimmer S, Arora A (2000) Pre-natal ventriculomegaly and hydrocephalus. Neurol Res 22:37–42
Birnholz JC, Frigoletto FD (1981) Antenatal treatment of hydrocephalus. N Engl J Med 304:1021–1023
Breeze AC, Alexander PM, Murdoch EM, Missfelder-lobos HH, Hackett GA, Lees CC (2007) Obstetric and neonatal outcomes in severe fetal ventriculomegaly. Prenat Diagn 27:124–129
Breeze AC, Dey PK, Lees CC, Hackett GA, Smith GC, Murdoch EM (2005) Obstetric and neonatal outcomes in apparently isolated mild fetal ventriculomegaly. J Perinat Med 33:236–240
Cardoza JD, Goldstein RB, Filly RA (1988) Exclusion of fetal ventriculomegaly with a single measurement: the width of lateral ventricular atrium. Radiology 169:711–714
Cavalheiro S, Moron AF, Zymberg ST, Dastoli P (2003) Fetal hydrocephalus-prenatal treatment. Childs Nerv Syst 19:561–573
Cavalheiro S, Moron AF, Almodin CG, Suriano IC, Hisaba V, Dastoli P, Barbosa MM (2011) Fetal hydrocephalus. Childs Nerv Syst 27:1575–1583
Chi JH, Fullerton HJ, Gupta N (2005) Time trends and demographics of death from congenital hydrocephalus in children in the United States: National Center for Health Statistics data, 1979 to 1998. J Neurosurg (Pediatr) 2(103):113–118
Chiu TH, Haliza G, Lin YH, Hung TH, Hsu JJ, Hsieh TT, Lo LM (2014) A retrospective study on the course and outcome of fetal ventriculomegaly. Taiwan J Obstet Gynecol 53:170–177
Cinalli G, Saint-Rose C, Chumas P, Zerah M, Brunelle F, Lot G, Pierre-Kahn A, Renier D (1999) Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. J Neurosurg 90:448–454
Clewell WH, Johnson ML, Meier PR, Newkirk JB, Hendee RW Jr, Bowes WA Jr, Zide SL, Hecht F, Henry G, O’Keeffe D (1981) Placement of ventriculo-amniotic shunt for hydrocephalus in a fetus. N Engl J Med 305:955
D’Addario V, Rossi AC (2012) Neuroimaging of ventriculomegaly in the fetal period. Semin Fetal Neonatal Med 17:310–318
Frigoletto FD, Bimholz JC, Greene NF (1992) Antenatal treatment of hydrocephalus by ventriculoamniotic shunting. JAMA 248:2496–2497
Futagi Y, Suzuki Y, ToribeY MK (2002) Neurodevelopmental outcome in children with fetal hydrocephalus. Pediatr Neurol 27:111–116
Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropoli S, Todros T (2005) Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 25:372–377
Garel C, Luton D, Oury JF, Gressens P (2003) Ventricular dilatations. Childs Nerv Syst 19:517–523
Gaskill SJ, Marlin AE, Rivera S (1988) The subcutaneous ventricular reservoir: an effective treatment for posthemorrhagic hydrocephalus. Childs Nerv Syst 4:291–295
Greenhalgh T (1999) Narrative based medicine in an evidence based world. BMJ 318:323–325
Greenhalgh T, Hurwitz B (1999) Why study narrative? BMJ 318:48–50
Hatlen TJ, Shurtlefe DB, Loeser JD, Ojemann JG, Avellino AM, Ellenbogen RG (2012) Nonprogrammable and programmable cerebrospinal fluid shunt valves: a 5-year study. J Neurosurg Pediatrics 9:462–467
Hudgins RJ, Boydston WR, Gilreath CL (1998) Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg 29:309–313
Joő JG, Tőth Z, Beke A, Papp C, Tőth-Pál E, Csaba A, Szigeti Z, Rab A, Papp Z (2008) Etiology, prenatal diagnostics and outcome of ventriculomegaly in 230 cases. Fetal Diagno Ther 24:254–263
Kelly EN, Allen VM, Seaward G, Windrim R, Ryan G (2001) Mild ventriculomegaly in fetus, natural history, associated findings and outcome of isolated mild ventriculomegaly: a literature review. Prenat Diagn 21:697–700
Kulkarni AV, Riva-Cambrin J, Browd SR, Drake JM, Holubkov R, Kestle JRW, Limbrick DD, Rozzelle CJ, Simon TD, Tamber M, Wellons JC III, Whitehead WE, Hydrocephalus clinical research network (2014) Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective hydrocephalus clinical research network study. J Neurosurg Pediatr 14:224–229
Kutuk MS, Ozgun MT, Uludag S, Dolanbay M, Poyrazoglu HG, Tas M (2013) Postnatal outcome of isolated, nonprogressive, mild borderline fetal ventriculomegaly. Childs Nerv Syst 29:803–808
Laskin MD, Kingdom J, Toi A, Chitayat D, Ohlsson A (2005) Perinatal and neurodevelopmental outcome with isolated fetal ventriculomegaly: a systematic review. J Matern Fetal Neonatal Med 18:289–298
Luthy DA, Wardinsky T, Shurtleff DB, Hollenbach KA, Hickok DE, Nyberg DA, Benedetti TJ (1991) Cesarean section before the onset of labor and subsequent motor function in infants with myelomeningocele diagnosed antenatally. N Engl J Med 324:662–666
Madazil R, Sal V, Erenel H, Gezer A, Ocak V (2011) Characteristics and outcome of 102 fetuses with fetal cerebral ventriculomegaly: experience of a university hospital in Turkey. J Obstet Gynecol 31:142–145
Manning FA, Harrison MR, Rodeck C, Members of the International Fetal Medicine and Surgery Society (1986) Catheter shunts for fetal hydronephrosis and hydrocephalus. Report of the International Fetal Surgery Registry. N Engl J Med 315:336–340
Marro PJ, Dransfield DA, Mott SH, Allan WC (1991) Posthemorrhagic hydrocephalus. Use of an intravenous-type catheter for cerebrospinal fluid drainage. Am J Dis Child 145:1141–1146
Melchiorre K, Bhide A, Gika AD, Pilu G, Papageorghiou AT (2009) Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol 34:212–224
Michejda M, Queenam JT, McCullough D (1986) Present status of intrauterine treatment of hydrocephalus and its future. Am J Obstet Gynecol 155:873–882
Moritake K, Nagai H, Miyazaki T, Nagasako N, Yamasaki M, Sakamoto H, Miyajima M, Tamakoshi A (2007a) Analysis of a nationwide survey on treatment and outcomes of congenital hydrocephalus in Japan. Neuro Med Chir (Tokyo) 47:453–461
Moritake K, Nagai H, Miyazaki T, Nagasako N, Yamasaki M, Tamakoshi A (2007b) Nationwide survey of the etiology and associated conditions of prenatally and postnatally diagnosed congenital hydrocephalus in Japan. Neuro Med Chir (Tokyo) 47:448–452
Morota N, Fujiyama Y (2004) Endoscopic coagulation of choroids plexus as treatment for hydrocephalus: indication and surgical outcome. Childs Nerv Syst 20:816–820
Morota N, Yamasaki M (2014) Congenital hydrocephalus. In: Neurologic syndrome (IV), 2nd edn. Nippon Rinsho, Osaka, pp 299–306, (Japanese)
Nagy A, Bognar L, Pataki I, Barta Z, Novak L (2013) Ventriculosubgaleal shunt in the treatment of posthemorrhagic and postinfectious hydrocephalus of premature infants. Childs Nerv Syst 29:413–418
Nomura ML, Barini R, De Andrade KC, Milanez H, Simoni RZ, Peralta CF, Machado IN, Zambell H, Maio KT (2010) Congenital hydrocephalus: gestational and neonatal outcomes. Arch Gynecol Obstet 282:607–611
Notarianni C, Vannemreddy P, Caldito G, Bollam P, Wylen E, Willis B, Nanda A (2009) Congenital hydrocephalus and ventriculoperitoneal shunts: influence of etiology and programmable shunts on revisions. J Neurosurg Pediatr 4:547–552
Ogiwara H, Dipatri AJ Jr, Alden TD, Bowman RM, Tomita T (2010) Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Childs Nerv Syst 26:343–347
Oi S (2004) Classification and definition of hydrocephalus: origin, controversy, and assignment of terminology. In: Cinalli G, Maixner WJ, Sainte-Rose C (eds) Pediatric hydrocephalus. Springer, Milano, pp 95–111
Pagani G, Thilaganathan B, Prefumo F (2014) Neurodevelopmental outcome in isolated mild fetal ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 44:254–260
Perlman S, Shashar D, Hoffmann C, Yosef OB, Achiron YR, Katorza E (2014) Prenatal diagnosis of fetal ventriculomegaly: agreement between fetal brain ultrasonography and MR imaging. AJNR Am J Neuroradiol 35:1214–1218
Persson EK, Hagberg G, Uvebrant P (2005) Hydrocephalus prevalence and outcome in population-based cohort of children born in 1989-1998. Acta Paediatr 94:726–732
Pillu G, Reece EA, Goldstein RB (1989) Sonographic evaluation of the normal developmental anatomy of the fetal cerebral ventricles: II. The atria. Obstet Gynecol 73:250–255
Pooh RK (2009) Neuroanatomy visualized by 2D and 3D. In: Pooh RK, Kurjak A (eds) Fetal neurology. Jaypee Brothers Medical Publishers, St. Louis, pp 15–38
Pople IK, Ettles D (1995) The role of endoscopic choroid plexus coagulation in the management of hydrocephalus. Neurosurgery 36:698–701
Rekate HL (2011) A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv Syst 27:1535–1541
Renier D, Saint-Rose C, Pierre-Kahn HJF (1988) Prenatal hydrocephalus: outcome and prognosis. Child’s Nerv Syst 4:213–222
Rosseau GL, McCullough DC, Joseph AL (1992) Current prognosis in fetal ventriculomegaly. J Neurosurg 77:551–555
Sakuma J, Horiuchi K, Munakata R, Suzuki K, Matsumoto M, Sasaki T, Kodama N, Amanuma F, Kawarada T, Ishii T, Ariga H, Ujiie N (2006) External ventricular drainage with an intravenous catheter for posthemorrhagic hydrocephalus in low-birth-wight infants. Nervous Syst Children 31:228–235, (Japanese)
Sethna F, Tennant PW, Rankin JC, Robson S (2011) Prevalence, natural history, and clinical outcome of mild to moderate ventriculomegaly. Obstet Gynecol 117:867–876
Shitsama S, Wittayanakorn N, Okechi H, Albright AL (2014) Choroid plexus coagulation in infants with extreme hydrocephalus or hydroanencephaly. J Neurosurg Pediatrics 14:55–57
Stone SS, Warf BC (2014) Endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective north American series. J Neurosurg Pediatr 14:439–446
Tugcu AU, Gulumser C, Ecevit A, Abbasoglu A, Uysal NS, Kupana ES, Yanik FF, Tardan A (2014) Prenatal evaluation and postnatal early outcomes of fetal ventriculomegaly. Eur J Paediatr Neurol 18:736–740
Vergani P, Locatelli A, Strobelt N, Cavalone M, Ceruti P, Paterlini G, Ghidini A (1998) Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol 178:218–222
von Koch CS, Gupta N, Sutton LN, Sun PP (2003) In utero surgery for hydrocephalus. Childs Nerv Syst 19:574–586
Wang KC, Lee JY, Kim SK, Phi JH, Cho BK (2011) Fetal ventriculomegaly: postnatal management. Childs Nerv Syst 27:1571–1573
Wang JY, Amin AG, Jallo G, Ahn ES (2014) Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr 14:447–454
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103(6 Suppl Pediatr):475–481
Weichert J, Hartge D, Krapp M, Germer U, Gembruch U, Axt-Fliedner R (2010) Prevalence, characteristics and perinatal outcome of fetal ventriculomegaly in 29,000 pregnancies followed at a single institution. Fetal Diagn Ther 27:142–148
Yamasaki M (2010) Explanation of technical terms. In: Editorial committee of guideline for fetal hydrocephalus (ed) Fetal hydrocephalus. Guideline for diagnosis and treatment, 2nd edn. Kimpo-Do, Kyoto, (Japanese)
Yamasaki M, Thompson P, Lemmon V (1997) CRASH syndrome: mutation in L1CAM correlated with severity of the disease. Neuropediatrics 28:175–178
Yamasaki M, Nonaka M, Suzumori N, Nakamura H, Fujita H, Namba A, Kamei Y, Yamada T, Pooh RK, Tanemura M, Sudo N, Nagasaka M, Yoshioka E, Shofuda T, Kanemura Y (2011) Prenatal molecular diagnosis of a severe type of L1CAM syndrome (X-linked hydrocephalus). J Neurosurg Pediatr 8:411–416
Yamasaki M, Nonaka M, Bamba Y, Teramoto C, Ban C, Pooh RK (2012) Diagnosis, treatment, and long-term outcomes of fetal hydrocephalus. Semin Fetal Neonatal Med 17:330–335
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Morota, N. (2019). Prenatal Hydrocephalus. In: Cinalli, G., Özek, M., Sainte-Rose, C. (eds) Pediatric Hydrocephalus. Springer, Cham. https://doi.org/10.1007/978-3-319-27250-4_48
Download citation
DOI: https://doi.org/10.1007/978-3-319-27250-4_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27248-1
Online ISBN: 978-3-319-27250-4
eBook Packages: MedicineReference Module Medicine