Abstract
Introduction
Intracranial teratomas are rare germ cell neoplasms that contain tissues derived from all three germ cell layers and most commonly occurring during childhood. This is the first report of pineal region mixed mature teratoma and germinoma in two fraternal brothers of fraternal triplets.
Case presentation
We report the case of a mixed mature teratoma and germinoma of the pineal region in two brothers of fraternal triplets. Older brother was initially diagnosed at the age of 11 years with the pure teratoma of the pineal region but the review of the pathology 3 years after initial surgery revealed the mixed mature teratoma with 5% germinomatous component. The younger brother was diagnosed at the age of 13 years with the mixed mature teratoma with 10% germinomatous component tumor of the pineal region. Younger brother has been treated with adjuvant chemo-radiotherapy and older brother was treated without adjuvant therapy. Both brothers had no recurrence.
Conclusion
Pineal mature teratomas have a good prognosis, in contrast to their immature or mixed counterparts. A rigorous histological examination of the tumor samples is mandatory, in order to not omit a mixed contingent within the tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germ cell tumors (GCTs) of the central nervous system (CNS) are heterogeneous group of lesions that are divided into germinomas, teratomas, embryonal carcinomas, yolk sac tumors, and choriocarcinomas.
Teratomas are extremely rare intracranial neoplasms classified into mature, immature, and teratoma with malignant transformation. Most commonly, central nervous teratoma occurs in the pediatric patients and accounts for 2–4% of all intracranial tumors in children with higher incidence in Japanese, Korean, and Taiwanese children [14, 7].
Central nervous system GCTs rarely develop in the members of the same family and only a handful of cases have been so far published [2, 8, 12, 13, 16]. Even though mixed mature teratoma and germinoma of pineal region has been published, this is the first report of mixed mature teratoma and germinoma of the pineal region in two brothers of fraternal triplets [17].
Case report
Patient 1
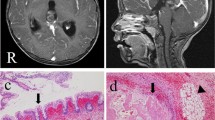
An 11-year-old patient presented in local emergency department due to bilateral headache for 3 weeks, papilledema, dorsal midbrain syndrome (DMS), and sixth nerve palsy. The magnetic resonance imaging (MRI) of the brain showed the acute hydrocephalus due to mass in the pineal region (Fig. 1). Serum and cerebrospinal fluid (CSF) markers for beta-human chorionic gonadotropin (beta-hCG) and alpha-fetoprotein (AFP) were negative. The external ventricular drain was placed to treat the acute hydrocephalus. The patient underwent left occipital craniotomy and occipital transtentorial approach for total resection of the pineal region tumor. Due to persistent hydrocephalus after the surgery, the patient underwent ventriculoperitoneal shunt placement 10 days after tumor resection. Postoperatively, his papilledema, DMS, and sixth nerve palsy completely resolved. Pathological examination revealed dense fibro-connective tissue, skin with adnexal structures, mature cartilage, adipose tissue, and intestinal- and respiratory-type epithelium-lined glands/ducts. No immature or malignant component was identified. Patient did not require any additional treatment. Due to occurrence of the same disease in his fraternal brother, we have revised the pathological examination. Revised pathological examination revealed mature teratoma and germinoma with 95% mature teratoma and 5% germinoma component (Fig. 3a, b). Four years after, the surgery patient did not show any sign of tumor recurrence.
Patient 2
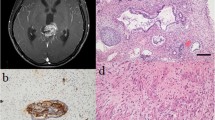
A 13-year-old patient was the younger fraternal twin brother of the above previously described patient who had history of acute hydrocephalus due to a third ventricular arachnoid cyst. The endoscopic fenestration of the third ventricle arachnoid cyst was done in 2008 in outside institution. He was followed with serial MRI of the brain that did not show any cyst recurrence. In December 2014, the patient passed out while attempting to stand up quickly. Computed tomography (CT) and MR scan of the brain revealed well-circumscribed lesion in the left side of the pineal cistern approximately 12 mm in width (Fig. 2). Serum and CSF markers for beta-hCG and AFP were negative. The tumor was gross totally removed through left occipital transtentorial approach. Postoperative patient developed Parinaud syndrome, which completely resolved in early postoperative period.
Pathological examination revealed components of all three germ cell layers. Immunostains showed large cells with clear cytoplasm that were positive for OCT 4 and CD117. Pathology was consistent with a mixed mature teratoma and germinoma with 90% mature teratoma and 10% germinoma component (Fig. 3c–f).
a–e Hematoxylin-eosin staining (×100) (a), hematoxylin-eosin staining (×400) (b), hematoxylin-eosin staining (×200) (c), hematoxylin-eosin staining (×400) (d), CD117 staining (e), and OCT4 staining (f) of the pineal tumor tissue showed squamous-lined cyst with adnexal structures (ectoderm), columnar-lined tissue with goblet cells (endoderm), fibromuscular stroma (mesoderm), and cellular proliferation of large cells with ample clear to esosinophilic cytoplasm (a). Germ cell tumor component was seen (b, d). Positive CD117 staining (e) and OCT4 staining (f) were positive
Due to mixed tumor pathology, patient was subjected for the additional chemotherapy and external radiation therapy. No genetic testing was performed on the resected tissue.
Discussion
According to the World Health Organization classification and the germ cell theory, intracranial GCTs are divided into five histological subtypes: germinomas, teratomas, embryonal carcinomas, yolk sac tumors, and choriocarcinomas. Therapy is dependent on histology subtypes; germinomas are treated with chemoradiation therapy, mature teratomas are treated with gross total resection, and immature and malignant teratomas are treated with gross total resection with adjuvant chemoradiation therapy, while choriocarcinomas, embryonal carcinomas, and yolk sac tumors are treated with chemotherapy and radiation therapy with surgical resection of residual tumor. In cases of mixed germ cell tumor, the treatment of choice is dependent on the most malignant component [10].
Intracranial teratomas are very rare CNS neoplasm and account for approximately 0.5–1% of primary adult intracranial tumors with higher incidence in children where they account for 2–4% of intracranial tumors and with 2 to 10 [6, 14]. The incidence of the intracranial teratoma in Japan in pineal region is 2 to 10 [3]. They are extremely rare intracranial neoplasms most commonly occurring in males, children, and Asian population, and only a few clinical case series have been published so far [11, 14, 17, 6].
These tumors are derived from all three primitive germ layers (endoderm, mesoderm, and ectoderm) and are classified into mature, immature, and teratoma with malignant transformation [6, 14]. Intracranial teratomas tend to involve the midline structures of the CNS and most commonly involve the area of the pineal gland as they have presented in our case but they can involve the wall of the third ventricle, quadrigeminal plate, suprasellar region, and cerebellar vermis [11, 17].
Germ cell tumors may secrete detectable levels of beta-hCG and AFP proteins into the blood and/or CSF, and these proteins can be reliably used in diagnosis and monitor the response to a given therapy. Mature teratomas do not secrete AFP and beta-hCG, but germinomas may occasionally secrete low levels of beta-hCG that can be detected in serum or CSF [1]. In our case, serum and CSF markers for beta-hCG and AFP were negative.
In the current literature, there are only several cases of familiar GCTs occurring in the siblings. Aoyama et al. and Nitta et al. published cases of siblings with germinoma [2, 13]. Kido et al. published a case of three brothers with pineal region tumors that included two brothers with germinomas and the third brother developed embryonal carcinoma [8]. Nakasu et al. published a case of two sisters with yolk sac tumor [12] and Wakai et al. published a case of two brothers with teratoma in pineal region [16].
As far as we know, the present case is the first report of mixed pineal mature teratoma and germinoma in two brothers of the fraternal triplets without hereditary syndromes. Their twin sister’s brain MRI did not reveal any abnormalities.
Limited information is available in current literature on the underlying genomic, epigenetic, and biological alterations of intracranial GCTs. Most recent research revealed that germinomas coexpressed Oct4 and Kit and showed extensive global demethylation suggesting that these tumors display a demethylated nuclear DNA similar to primordial germ cells in early development [15]. In addition, these tumors show mutational activation of Kit, Ras/Raf/Erk, and Akt pathways indicating these pathways might be potential targets for chemotherapy [15]. The genetic aspect of familial intracranial germ cell tumor in twins/triplets is unknown and it has not been further investigated in our case.
All familiar GCT cases including the current one had the same common findings, occurring most commonly in young boys in the pineal region. Since the tumors occur most commonly in pineal region, the first symptom of the tumor is clinical signs of increased intracranial pressure due to hydrocephalus.
Surgical removal with gross total resection is the main treatment for mature teratomas with 5-year survival rate ranging from 87 to 100% while teratomas with malignant component after chemo-radiotherapy and surgery have survival rate from 33 to 71% [4, 14].
Based on the literature recommendations, patients with mixed GCTs are treated based on the most malignant component of the tumor. In general, germinoma shows high radiosensitivity and has excellent prognosis. However, the superiority of combined modality therapy over treatment with radiation alone in non-germinomatous GCTs has been proposed by several landmark studies such as the SIOP-CNS-GCT 96 trial and the Japanese cooperative study [5, 9]. Chemotherapy is one of the pillars in the treatment of intracranial GCT and mixed GCTs but there is no standardized chemotherapeutic protocol which includes the fine details about pathologic subtype, dosage, treatment duration, and temporal sequence with radiation therapy in the literature and varies from institution to institution. Younger brother underwent chemo and radiation therapy after surgical resection due to 10% germinoma component of the mixed GCS tumor and he is now disease free 2 years after completion of the treatment. Older brother has been disease free for the last 4 years after surgical resection even though he has not received any additional treatment except surgical resection for his mixed GCSs with 5% germinoma component. Diagnosis of the 5% of the germinoma component in the older brother has been made after additional pathology review 4 years after initial diagnosis of mature teratoma, and since the 4-year follow up did not reveal the tumor recurrence, neuro-oncology panel decision was not to treat the patient with additional adjuvant therapy.
The cause of familiar central nervous germ cell tumor is unknown due to the extreme rarity of these lesions and maybe the genetic testing might give inside of the natural history of these rare intracranial lesions.
It is interesting that the lesion developed in the later course of the life in the younger brother gives us the clue that the tumor cells were most likely in dormant state for many years and started growing in the later course of life. This raises the question should their twin sister be followed by serial imaging or not?
References
Allen J, Chacko J, Donahue B, Dhall G, Kretschmar C, Jakacki R, Holmes E, Pollack I (2012) Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer 59:1180–1182. doi:10.1002/pbc.24097
Aoyama I, Kondo A, Ogawa H, Ikai Y (1994) Germinoma in siblings: case reports. Surg Neurol 41:313–317
Araki C, Matsumoto S (1969) Statistical reevaluation of pinealoma and related tumors in Japan. J Neurosurg 30:146–149. doi:10.3171/jns.1969.30.2.0146
Brandes AA, Pasetto LM, Monfardini S (2000) The treatment of cranial germ cell tumours. Cancer Treat Rev 26:233–242. doi:10.1053/ctrv.2000.0169
Bromberg JE, Baumert BG, de Vos F, Gijtenbeek JM, Kurt E, Westermann AM, Wesseling P (2013) Primary intracranial germ-cell tumors in adults: a practical review. J Neuro-Oncol 113:175–183. doi:10.1007/s11060-013-1114-6
Goyal N, Kakkar A, Singh PK, Sharma MC, Chandra PS, Mahapatra AK, Sharma BS (2013) Intracranial teratomas in children: a clinicopathological study. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 29:2035–2042. doi:10.1007/s00381-013-2091-y
Goyal N, Singh PK, Kakkar A, Sharma MC, Mahapatra AK (2012) Mature teratoma in association with neural tube defect (occipital encephalocele): series of four cases and review of the literature. Pediatr Neurosurg 48:67–72. doi:10.1159/000339090
Kido G, Takeuchi T, Tsukiyama T, Nakamura S, Tsubokawa T, Henmi A (1984) Tumor of the pineal region in three brothers. No shinkei geka Neurological surgery 12:975–980
Kochi M, Itoyama Y, Shiraishi S, Kitamura I, Marubayashi T, Ushio Y (2003) Successful treatment of intracranial nongerminomatous malignant germ cell tumors by administering neoadjuvant chemotherapy and radiotherapy before excision of residual tumors. J Neurosurg 99:106–114. doi:10.3171/jns.2003.99.1.0106
Kyritsis AP (2010) Management of primary intracranial germ cell tumors. J Neuro-Oncol 96:143–149. doi:10.1007/s11060-009-9951-z
Lee YH, Park EK, Park YS, Shim KW, Choi JU, Kim DS (2009) Treatment and outcomes of primary intracranial teratoma. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 25:1581–1587. doi:10.1007/s00381-009-0974-8
Nakasu S, Handa J, Hazama F, Hirakawa K (1983) Suprasellar yolk-sac tumor in two sisters. Surg Neurol 20:147–151
Nitta N, Fukami T, Nozaki K (2013) Germinoma in two brothers: case report. Neurol Med Chir 53:703–706
Noudel R, Vinchon M, Dhellemmes P, Litre CF, Rousseaux P (2008) Intracranial teratomas in children: the role and timing of surgical removal. J Neurosurg Pediatr 2:331–338. doi:10.3171/PED.2008.2.11.331
Schulte SL, Waha A, Steiger B, Denkhaus D, Dorner E, Calaminus G, Leuschner I, Pietsch T (2016) CNS germinomas are characterized by global demethylation, chromosomal instability and mutational activation of the Kit-, Ras/Raf/Erk- and Akt-pathways. Oncotarget. doi:10.18632/oncotarget.10392
Wakai S, Segawa H, Kitahara S, Asano T, Sano K, Ogihara R, Tomita S (1980) Teratoma in the pineal region in two brothers. Case reports. J Neurosurg 53:239–243. doi:10.3171/jns.1980.53.2.0239
Zygourakis CC, Davis JL, Kaur G, Ames CP, Gupta N, Auguste KI, Parsa AT (2015) Management of central nervous system teratoma. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 22:98–104. doi:10.1016/j.jocn.2014.03.039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Grahovac, G., Alden, T. & Nitin, W. Mixed pineal mature teratoma and germinoma in two brothers of the fraternal triplets. Childs Nerv Syst 33, 859–863 (2017). https://doi.org/10.1007/s00381-017-3349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3349-6