Abstract
Purpose
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in children. Preventing secondary injury by controlling physiological parameters (e.g. intracranial pressure [ICP], cerebral perfusion pressure [CPP] and brain tissue oxygen [PbtO2]) has a potential to improve outcome. Low PbtO2 is independently associated with poor clinical outcomes in both adults and children. However, no studies have investigated associations between low PbtO2 and neuropsychological and behavioural outcomes following severe pediatric TBI (pTBI).
Methods
We used a quasi-experimental case-control design to investigate these relationships. A sample of 11 TBI patients with a Glasgow Coma Scale score ≤8 who had PbtO2 and ICP monitoring at the Red Cross War Memorial Children’s Hospital underwent neuropsychological evaluation ≥1 year post-injury. Their performance was compared to that of 11 demographically matched healthy controls. We then assigned each TBI participant into one of two subgroups, (1) children who had experienced at least one episode of PbtO2 ≤ 10 mmHg or (2) children for whom PbtO2 > 10 mmHg throughout the monitoring period, and compared their results on neuropsychological evaluation.
Results
TBI participants performed significantly more poorly than controls in several cognitive domains (IQ, attention, visual memory, executive functions and expressive language) and behavioural (e.g. externalizing behaviour) domains. The PbtO2 ≤ 10 mmHg group performed significantly worse than the PbtO2 > 10 mmHg group in several cognitive domains (IQ, attention, verbal memory, executive functions and expressive language), but not on behavioural measures.
Conclusion
Results demonstrate that low PbtO2 may be prognostic of not only mortality but also neuropsychological outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between secondary brain injury and poor outcome in adults and children following traumatic brain injury (TBI) is well known [1–4]. Therefore, preventing, limiting, or managing secondary injuries like ischemia is crucial to improving outcome following TBI [5, 6]. Ischemia or hypoxia following TBI is significantly associated with an unfavourable outcome [7, 8]. Early identification and intervention is therefore critical to preventing secondary injury, and therefore [5, 9], methods of improving outcome are largely focused on preventing ischemic injury [10].
Prevention of ischemia following TBI traditionally involves methods aimed at improving cerebral perfusion pressure (CPP) and controlling intracranial pressure (ICP). Current TBI management focuses on maintaining ICP and CPP within recommended thresholds. However, the use/extrapolation of these thresholds in pediatric TBI is based on weak evidence [11]; maintaining ICP and CPP within these thresholds is not a strong indicator of acceptable levels of brain oxygenation [5, 12–16]. Up to one third of children with severe TBI may experience episodes of low brain oxygenation tension, even when recommended treatment targets for ICP and CPP are achieved [5].
In children, determining what represents adequate ICP and CPP control is more complex than that in adults, given that children of different ages have different and less well-established normative thresholds for ICP and blood pressure [5]. To date, there are no age-based recommendations for these thresholds, and so treatment and injury thresholds are extrapolated from adult studies [11, 17]. This strategy is suboptimal given that children are physiologically different from adults. Therefore, a measure of the adequacy of brain oxygenation, rather than using ICP or CPP values as a proxy measures is preferable [5].
Brain tissue oxygen tension (PbtO2) monitors have therefore been proposed as a complementary tool to ICP monitoring to detect the adequacy of brain perfusion and oxygenation. These monitors are used in both adults and children at risk of cerebral ischemia and so are being utilized increasingly in the management of patients with severe TBI [5, 9, 18–20]. The aim of PbtO2 monitoring is, ideally, to maintain PbtO2 values greater than 15–20 mmHg. PbtO2 values less than 20 mmHg suggest progressively increased tissue hypoxia or ischemia, and values less than 10 mmHg are deemed critical, as this appears to approximate an ischemic threshold [18].
Low PbtO2 post-TBI is common and is associated with increased mortality and morbidity after severe TBI in adults [9, 18]. PbtO2-directed treatment appears to be associated with reduced mortality in adults [20, 21]. Although fewer studies of this kind have been conducted with children [4, 5, 12, 15], emerging data are consistent with observations made in adult studies. In a study that included a large pTBI sample (N = 52), low PbtO2 was independently associated with poor outcome (as defined by the Glasgow Outcome Score [GOS] and Pediatric Cerebral Performance Category Scale [PCPCS]) and was a stronger predictor than other factors traditionally associated with outcome [13]. Furthermore, low PbtO2 was not predicted by measures of initial injury severity, suggesting that the contribution of low PbtO2 to poor outcome represents secondary brain injury that is, at least in theory, amenable to treatment.
There is some limited evidence from the adult literature that low PbtO2 is associated with poor performance in the domains of general intellectual functioning and memory [22], but to date, there have been no published studies on the relationship between PbtO2 and specific neuropsychological and behavioural outcomes in children. In fact, TBI-related neuropsychological outcome studies rarely consider neurosurgical monitoring variables such as PbtO2 levels, and neurosurgical outcome studies rarely include neuropsychological outcome variables. In this study, we aimed to investigate the relationship between PbtO2 levels and neuropsychological and behavioural functioning following severe pediatric TBI (pTBI). Specifically, we investigated whether PbtO2 levels that are maintained above the ischemic threshold (PbtO2 > 10 mmHg) are associated with more favourable outcomes for children who have sustained severe TBIs. We hypothesized that TBI patients who experienced at least one episode of brain hypoxia as measured by PbtO2 < 10 mmHg would perform more poorly on the administered tests than those who did not experience an episode of brain hypoxia.
Materials and methods
Research design
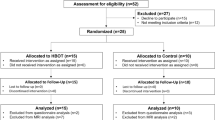
This was a case-control study. The study design was quantitative, retrospective and cross-sectional. It included two between-group comparisons. The first comparison was between a group of children who had sustained severe TBIs and who underwent PbtO2 monitoring and a healthy matched control group. The second between-group comparison involved dividing the pTBI group into two subgroups, one including those who had experienced at least one episode of PbtO2 lower than 10 mmHg (i.e. they reached the ischemic threshold) during monitoring (hypoxia group) and the other including those for whom PbtO2 had remained above 10 mmHg throughout the monitoring period (no-hypoxia group).
Participants
The patient sample included 11 children admitted to Red Cross War Memorial Children’s Hospital (RCCH), in Cape Town, South Africa, for severe TBI with an admission post-resuscitation Glasgow Coma Scale (GCS) score ≤8, or who deteriorated to this level after admission. All patients underwent PbtO2 monitoring and were managed according to institutional protocol described elsewhere [13].
Only children in whom monitoring was started within the first 24 h were considered for inclusion in the study. Of those for whom complete monitoring data were available, we selected for inclusion only those (a) who were English and/or Afrikaans speaking, (b) who were admitted for closedFootnote 1 severe TBI, (c) who were at least 1 year post-injury,Footnote 2 (d) who were aged 6–16 years at the time of assessment and (e) for whom informed consent and assent were granted.
We recruited a group of healthy controls (n = 11) against which to compare the performance of the TBI participants. Control participants were recruited by (1) identifying potential participants from the schools that the TBI participants attended and (2) consulting with other local researchers who were conducting pediatric neuropsychological studies and who had included healthy participants in their studies. These control participants were matched as closely as possible to TBI participants on a range of demographic variables, including age, sex, language, socioeconomic status (SES) and race. Exclusion criteria for all participants included previous head injuries that resulted in hospitalization or loss of consciousness and any formally diagnosed learning, psychiatric, neurological or developmental disorders.
Measures
The cognitive assessment tools were the Wechsler Abbreviated Scale of Intelligence [26] (WASI), the Rey Complex Figure Test [27] (RCFT), and selected subtests from the Children’s Memory Scale [28] (CMS), the Test of Everyday Attention for Children [29] (TEA-Ch), and the NEPSY-II [30]. Hence, the assessment battery covered a range of cognitive domains, including general intellectual functioning (verbal IQ [VIQ], performance IQ [PIQ], and full-scale IQ [FSIQ]), verbal and visual memory, attention, executive functions, and visuospatial functioning. The behavioural assessment tools were the Child Behaviour Checklist [31] (CBCL; Achenbach, 1991) and the Behaviour Rating Inventory of Executive Functions [32] (BRIEF; Gioia, Isquith, Guy and Kenworthy, 2000).
These tests and questionnaires were all originally published in English. To facilitate administration to those participants with a home language of Afrikaans, the test instructions and relevant stimuli were translated by the University of Stellenbosch Language Laboratory (Cape Town, South Africa) who carried out forward and back translations and authentication.
We also used a questionnaire designed to acquire socio-demographic information about the participants which captured details about parental education, occupation and income, as well as information about the home living environment (e.g. the type of dwelling and participants’ residence and neighbourhood).It also included an index of material resources in the household [33] as well as more traditional measures of SES.
Procedure
TBI participants: physiological monitoring
Following the local TBI management protocol, intracranial catheters for ICP (Codman, Raynham, MA, USA) and PbtO2 (Licox; Integra Neurosciences, Plainsboro, NJ) were inserted into normal appearing white matter in the right frontal lobe or on the side of the greatest cerebral swelling or most significant lesion (as per admission head computed tomography [CT] scan). The accurate positioning of the monitors was confirmed on follow-up CT scan. The treatment threshold for PbtO2 threshold was 20 mmHg [4, 13]. Physiological data were continuously recorded using a high-frequency computerized software recording system (ICMPlus®, Cambridge University, UK).
TBI and healthy control participants: neuropsychological and behavioural testing
Parents/caregivers were contacted via telephone and invited to participate in the study. Each participant was tested individually. The duration of testing was approximately 3 h. Parents completed the sociodemographic questionnaire and the BRIEF and CBCL forms during that time.
Scoring procedures and statistical analyses
Identifying and measuring episodes of low PbtO2
Patient physiological data were examined to identify episodes of low PbtO2 including (a) the lowest PbtO2 reading that persisted for at least 30 min during the entire monitoring period and (b) the cumulative time that PbtO2 was less than thresholds of 20, 10 and 5 mmHg. Data from the first 2 h of PbtO2 catheter stabilization were excluded from analyses. Although PbtO2 was treated at 20 mmHg, this represented a ‘softer’ target for interventions with more aggressive interventions being used when PbtO2 fell below 10 mmHg. Because adult and pediatric data suggest a stronger association with outcome when PbtO2 is below 10 mmHg, the 10–20 mmHg range likely represents a region of oligemia rather than ischemia if cerebral blood flow restriction is the cause of the decreased PbtO2. For this reason, the 10 mmHg threshold was used for analysis in the current study.
Scoring procedures for neuropsychological and behavioural data
For each (sub)test in the battery, we followed the conventional scoring procedures described in the respective test administration manuals. We converted all raw scores to age-adjusted scaled scores.
Statistical procedures
We used SPSS version 22.0 and set the threshold for statistical significance (α) at 0.05. For each analysis, we calculated the appropriate effect size estimate.
TBI cases vs. controls
For demographic data, we used one-way ANOVAs or Mann-Whitney U tests to assess between-group differences on continuous variables, depending on whether or not assumptions of normality and homogeneity were upheld, and chi-square or Fisher’s exact test to assess between-group differences on categorical variables. We used the latter statistical procedure in instances where the sample was small and where the cells of the variables in the analyses had expected counts of less than 5.
For the neuropsychological data, there were a large number of dependent variables (32) in proportion to the sample size (N = 22) for the comparison between TBI and control groups. Therefore, we used a standardized set of procedures to reduce the number of dependent variables. The resulting ten outcome variables included three IQ measures, WASI nerbal IQ, performance IQ and full-scale IQ, and seven composite measures covering the domains of basic and higher-order attention, verbal and visual memory, executive function, visuospatial ability and expressive language. We created these composites using a hybrid method [34, 35].
We then used one-way ANOVAs or Mann-Whitney U tests to investigate between-group differences in neuropsychological test scores and behavioural measures. Despite the fact that we conducted multiple comparisons, we did not apply the Bonferroni (or similar) correction to the results of these analyses. Although one might typically control for the risk of type I error using a conservative measure such as this, in other public health research contexts (e.g. pediatric exposure to neurotoxins), researchers are more concerned about missing important effects (type II errors) than about the strict control of alpha values [36]. This concern might also be extrapolated to TBI research: employing an adjustment to control for type I error may result in an underestimation of the effects of TBI on neuropsychological and behavioural outcomes.
Hypoxia vs. no-hypoxia
We compared outcome on the (a) demographic and injury variables, (b) SES data, (c) physiological variables and (d) neuropsychological and behavioural variables for the hypoxia and no-hypoxia groups. We repeated the steps outlined above in terms of checking assumptions, deriving composites, between-group comparisons of demographic, neuropsychological and behavioural data and non-use of Bonferroni adjustment.
Ethical considerations
Ethical approval for this study was obtained from the University of Cape Town’s Faculty of Health Sciences Human Research Ethics Committee. Permission to include the school learners and to use the school facilities for testing was obtained from the Western Cape Education Department. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The parents of all participants and controls provided informed consent for participation in this study.
Results
TBI cases vs. controls
There were 16 boys and 6 girls in the sample, with a mean age at assessment of 10.87 years (SD = 31.49). Most participants (n = 20) were mixed race, with the rest (n = 2) being White. Most (n = 16) reported they spoke English and Afrikaans equally well; the rest (n = 6) spoke Afrikaans only. The two groups did not differ significantly in terms of age at assessment (p = 0.936) and were evenly matched in terms of sex, race and home language. There were also no significant between-group differences for any of the SES measures.
Table 1 presents the results of the between-group comparisons on measures of IQ and neuropsychological test performance. The control group performed significantly better than the pTBI group on the following: PIQ, FSIQ, basic attention composite, higher-order attention composite, visual memory composite, executive functions composite, visuospatial skills composite and expressive language composite. Moderate-to-large effect sizes were associated with each of these comparisons.
Table 2 presents results from the between-group comparisons of behavioural data. The groups differed significantly on all of the BRIEF indices, with the controls reportedly scoring better than the pTBI participants. Large effect sizes were associated with these comparisons.
The groups also differed significantly on the CBCL anxious/depressed, withdrawn depressed, rule-breaking and aggression syndrome scales and on the externalizing behaviour syndrome grouping, with the controls reportedly scoring better than the pTBI participants. Again, moderate-to-large effect sizes were associated with these comparisons.
Hypoxia vs. no-hypoxia
Table 3 shows that there were no significant differences between the hypoxia and the no-hypoxia groups in terms of the demographic or injury related variables (admission GCS, mechanisms of injury). The only significant between-group difference was that the hypoxia group had experienced a longer time since injury than those in the no-hypoxia group. There was a large effect size associated with this finding (r = 0.68).
There were no significant between-group differences on any of the SES measures or for the categorical physiological variables (pupils on admission, initial systemic hypoxia, initial SBP < 90 mmHg, polytrauma, and ICU risk of mortality score ≥0.50 (p = 0.286–1.00).
Table 4 presents the results of between-group comparisons for the continuous physiological variables. Besides the significant difference between the groups in terms of the number of episodes of PbtO2 < 10 and the lowest PbtO2 value reached during the monitoring period, the groups also differed significantly on the following variables: mean ICP > 20, highest ICP, lowest CPP and lowest PaO2. The effect size estimates suggest, however, that PbtO2 accounted for greater between-group variance than the ICP, CPP and PaO2 variables.
Table 5 presents the results for the between-group comparisons on measures of IQ and neuropsychological test performance. There were significant between-group differences, in favour of the no-hypoxia group, on the following: VIQ, FSIQ, basic attention composite, higher-order attention composite, verbal memory composite, visual memory composite, executive functions composite, visuospatial skills composite and expressive language composite. There were large effect sizes associated with each of these comparisons.
Table 6 shows that the hypoxia and the no-hypoxia groups did not differ significantly on any of the BRIEF indices, on any of the CBCL syndrome scales or on the internalizing and externalizing syndrome groupings.
Discussion
Although a growing body of research demonstrates the association between decreased brain oxygenation and increased mortality and morbidity in both adults and children, there is a dearth of investigations of this nature in children. The few pediatric studies of PbtO2 that have been conducted previously have not included neuropsychological outcome measures. The inclusion of both neuropsychological and behavioural outcome measures is therefore novel in this study.
Summary of results
TBI cases vs. controls
There were no significant between-group differences on any of the sociodemographic variables. These factors can impact on neuropsychological test performance [e.g. see 23, 37]. Hence, it is important that the groups were matched as closely as possible.
The groups did, however, differ significantly on measures of IQ, and on composite indices of basic attention, expressive language, visual memory, visuospatial skills, higher-order attention and executive functions; in each case, the TBI participants performed more poorly than their matched controls. These outcomes are consistent with published literature describing expected neuropsychological sequelae following pTBI [38–47].
In terms of the behavioural measures, the groups also differed significantly on all of the BRIEF indices, with the parents of controls reporting better functioning than the parents of pTBI participants. Regarding the CBCL, the groups differed significantly on the externalizing behaviour syndrome grouping. They also differed on both of the syndrome profiles (rule-breaking and aggressive behaviour) included in this grouping, as well as on the anxious/depressed and withdrawn/depressed profiles of the internalizing behaviour syndrome grouping. The pTBI group reportedly showed more problems in these domains than the controls.
These results from the BRIEF are consistent with those from the neuropsychological tests. Executive functions are subserved primarily by the frontal lobes, and in particular, by the prefrontal cortex. The frontal lobes are especially vulnerable to the effects of TBI owing to their anatomical position and the kinds of biomechanical forces acting on the skull in many TBIs [48]. In terms of the CBCL, behavioural and emotional problems (particularly internalizing and externalizing problems) are reported to be the main reason that children who have sustained TBIs are referred to mental health and rehabilitation professionals [49]. Hence, the syndrome profiles on which significant between-group differences were detected are commonly reported behavioural sequelae following pTBI [49–53].
Although the scores of the pTBI group were not significantly different from controls on the internalizing behaviour syndrome grouping, their mean score fell within the clinical range, whereas the mean score for the healthy controls fell within the normal range. Although there is a small effect size associated with this comparison, these results show a trend in the expected direction. Somatic complaints are not necessarily suggestive of emotionally based problems. Therefore, a non-significant finding here does not conflict with reports of emotional and behavioural problems commonly associated with TBI.
In summary, the current data confirmed that participants who have sustained severe TBI perform more poorly than matched healthy controls on neuropsychological and behavioural measures.
Hypoxia vs. no-hypoxia groups
Although participants in the hypoxia group had had a longer time since injury to evaluation, the significant difference in time since injury is not expected to have an effect on the outcome. Even if the overall time since injury differed significantly between the two groups, all of the participants in both groups were at least 1 year post-injury. It has long been reported that improvement in outcome post-TBI is more limited and plateaus after 1-year post injury, particularly in children who have sustained severe TBIs [24, 25, 54].
Between-group differences in neuropsychological outcomes
The hypoxia group performed significantly more poorly on measures of IQ, as well as on the composite measures of basic and higher-order attention, verbal and visual memory, executive functioning, visuospatial ability and expressive language. These findings suggest that secondary injury effects, such as exposure to episodes of hypoxia, create further unfavourable outcomes in children who have sustained severe TBI. Overall, these data are consistent with literature on neuropsychological sequelae following hypoxia-ischemia [23, 55, 56].
Between-group differences in behavioural outcomes
The results show that the experience of one or more hypoxic episodes does not, however, seem to be directly related to outcome on any of the behavioural measures. The hypoxia and no-hypoxia groups did not differ significantly on any of the BRIEF or CBCL outcome variables. Despite this pattern of data, there were moderate effect sizes associated with the MI index of the BRIEF, and the withdrawn depressed, rule-breaking and aggressive behaviour syndrome scales and externalizing behaviour syndrome grouping of the CBCL, suggesting that with a larger sample size, these comparisons could potentially reach significance.
The literature on predictors of cognitive and behavioural outcomes post-TBI suggests a divide in terms of these two areas of outcome. Although cognitive outcomes are strongly determined by injury-related variables, a combination of injury-related factors (e.g. severity) and environmental factors (e.g. family functioning and psychosocial adversity), rather than injury-related factors on their own, is strongly predictive of behavioural outcome [23, 57–60]. Hence, there is a wider range of predictors for behavioural outcomes than for cognitive outcomes. Researchers view behavioural outcomes following TBI as complex and as a product of a range of interacting factors that are not only limited to injury severity but also extend to the family environment and to resources both prior to and after the injury [61–63]. There is a dose-response relationship between the predictors of behaviour and associated outcome, such that more marked and persistent post-injury behavioural difficulties are associated with more severe TBI and poorer family environments [64].
Brain hypoxia-ischemia is a secondary injury-related factor and not an environmental factor. In line with the argument above, it is not surprising that PbtO2 on its own would not predict behavioural functioning, at least not independently of environmental factors (e.g. constraints that our children with TBI face with poor schooling, low parental education levels, poor rehabilitation facilities and special schooling post-injury) or certainly not as strongly as the cognitive outcomes. That being the case, in light of the aforementioned literature on predictors of cognitive and behavioural outcomes, the fact that episodes of PbtO2 < 10 mmHg may be associated more strongly with cognitive rather than behavioural outcomes is consistent with the literature.
An alternative explanation might be that the patients from both groups came from lower SES backgrounds, and the two groups did not differ significantly in terms of sociodemographic factors. Therefore, the same factors that could contribute to poor behavioural outcomes were present in both groups.
Finally, one might also consider how behaviour is typically measured (i.e. via self- or other-report) in this field. In contrast, there are objective measures for cognition. Hence, reports on behaviour might be less accurate than data collected for cognition.
Significant differences on other physiological parameters
Besides significant differences between the groups on the PbtO2 variables (number of episodes when PbtO2 < 10 mmHg and lowest PbtO2 value), the basis on which the groups were formed, the hypoxia and no-hypoxia groups also differed significantly on variables relating to raised ICP, low CPP and lowest PaO2. The hypoxia group experienced higher ICP values and lower CPP and PaO2 values. Hence, perturbations in variables other than PbtO2 might also contribute to the differences found between hypoxia and no-hypoxia groups on measures of neuropsychological test performance.
There was a large effect size associated with the between-group comparison on lowest CPP. This suggests that this physiological parameter accounts for a substantial proportion of the total variance in neuropsychological test performance between the two groups. Very limited literature exists exploring the relationship between CPP and cognitive outcomes. Lannoo et al. [65] did not find a definite association between CPP and ICP in combination and cognitive outcomes. However, Lannoo et al. included both CPP and ICP measures in combination and focused on an older sample (15–65 years). Hence, follow-up studies are required to investigate the specific relationship between CPP and cognition.
Limitations and directions for future research
The small sample size limits the strength of the conclusions that can be drawn and the generalizability of these results. However, effect sizes are large and suggestive of real between-group differences. We will aim to increase the sample size in follow-up studies.
Implementing a three-group comparison (i.e. a pTBI/Hypoxia group, a pTBI/no-hypoxia group and a healthy control group) might have been most ideal for the questions we attempted to answer in this study. The ultimate aim in implementing this design would be to tease apart TBI and hypoxic effects, both independently and combined. One way to do this legitimately, however, would be in a regression model where one could partial out the two effects and look at an interaction effect. This design was not implemented, however, due to the limited sample of eligible participants.
The sample’s broad age range might be interpreted as another limitation, due to the fact that a great degree of neurodevelopment can occur during the years covered by that range. An increased sample size would not only give the study better power but would also allow the detection of developmental trends across more age bands (7–8, 9–10, 11–12 etc.).
A final possible limitation is that the measures of behavioural outcomes included in this study were all self-report measures, and hence, the fidelity of the behavioural results depends solely on reliable reporting by parents. There are obvious limitations to using these self-report measures, including (a) the possibility of social desirability biases and, with that, (b) under- or over-reporting of behaviours, (c) problems related to accessing data on moods and behaviours retrospectively, which can lead to inaccurate reporting, or (d) potential lack of information from respondents on the wide range of behaviours surveyed in the questionnaires, rendering the data incomplete [66–68]. Administering teacher, as opposed to just parent versions, of each of the behavioural measures would have strengthened the power of these results.
Summary and conclusion
The data reported here suggest that reaching a critical PbtO2 threshold of ≤10 mmHg may be detrimental to cognitive outcomes following pTBI. Therefore, over and above the effects of the TBI, which lead to poor neuropsychological and behavioural outcomes, there may also be additional post-TBI hypoxic effects that contribute to even worse cognitive outcomes.
In spite of the outlined limitations associated with this study, the findings presented here and their potential implications warrant attention and further inquiry. This study is an important first step in discerning the prognostic value of low PbtO2 in determining neuropsychological outcomes post-pTBI. However, although the conclusions that may be drawn from these results are noteworthy and could have important implications, they are tentative at this stage, requiring replication in studies with larger samples.
Notes
References
Chambers IR, Jones PA, Lo TYM, Forsyth RJ, Fulton B, Andrews PJD, Mendelow AD, et al. (2006) Critical thresholds of intracranial pressure and cerebral perfusion pressure related to age in paediatric head injury. J Neurol Neurosurg Psychiatry 77(2):234–240. doi:10.1136/jnnp.2005.072215
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, et al. (1993) The role of secondary brain injury in determining outcome from severe head injury. The Journal of Trauma 34(2):216–222
Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, Mann NC (2000) Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. The Journal of Trauma 49(4):658–659
Figaji AA, Zwane E, Thompson C, Fieggen AG, Argent AC, Le Roux PD, Peter JC (2009b) Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 2: relationship with clinical, physiological, and treatment factors. Childs Nerv Syst 25(10):1335–1343. doi:10.1007/s00381-009-0821-y
Figaji AA, Zwane E, Fieggen AG, Peter JC, Leroux PD (2008) Acute clinical grading in pediatric severe traumatic brain injury and its association with subsequent intracranial pressure, cerebral perfusion pressure, and brain oxygenation. Neurosurg Focus 25(4):E4. doi:10.3171/FOC.2008.25.10.E4
Tang ME, Lobel DA (2009) Severe traumatic brain injury: maximizing outcomes. Mt Sinai J Med 76(2):119–128. doi:10.1002/MSJ
Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, et al. (2004) The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 114(3):805–816. doi:10.1542/peds.2004-0227
Hopkins RO, Haaland KY (2004) Neuropsychological and neuropathological effects of anoxic or ischemic induced brain injury. J Int Neuropsychol Soc 10(7):957–961
Rohlwink UK, Figaji AA (2010) Methods of monitoring brain oxygenation. Childs Nerv Syst 26(4):453–464. doi:10.1007/s00381-009-1033-1
Greve MW, Zink BJ (2009) Pathophysiology of traumatic brain injury. Mt Sinai J Med 76(2):97–104. doi:10.1002/MSJ
Kochanek PM, Carney N, Adelson PD, et al. (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-second edition. Pediatr Crit Care Med 13(1 Suppl):S1–S82
Narotam PK, Burjonrappa SC, Raynor SC, Rao M, Taylon C (2006) Cerebral oxygenation in major pediatric trauma: its relevance to trauma severity and outcome. J Pediatr Surg 41(3):505–513. doi:10.1016/j.jpedsurg.2005.11.069
Figaji AA, Zwane E, Thompson C, Fieggen AG, Argent AC, Le Roux PD, Peter JC (2009a) Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: relationship with outcome. Childs Nerv Syst 25(10):1325–1333. doi:10.1007/s00381-009-0822-x
Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E, Bloom S, et al. (2005) Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg 103(5):805–811. doi:10.3171/jns.2005.103.5.0805
Stiefel MF, Udoetuk JD, Storm PB, Sutton LN, Kim H, Dominguez TE, Helfaer M, et al. (2006) Brain tissue oxygen monitoring in pediatric patients with severe traumatic brain injury. J Neurosurg 105(Suppl. 4):281–286. doi:10.3171/ped.2006.105.4.281
Van den Brink WA, Van Santbrink H, Steyerberg EW, Avezaat CJ, Suazo JA, Hogesteeger C, Jansen WJ, et al. (2000) Brain oxygen tension in severe head injury. Neurosurgery 46(4):868–876
Adelson PD, Bratton SL, Carney NA, Chesnut RM, Du Coudray HEM, Goldstein B, Kochanek PM (2003a) World Federation of Pediatric and Critical Care Societies .Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr Crit Care Med 4(3, Suppl.):S2–S4. doi:10.1097/01.CCM.0000066600.71233.01
Maloney-Wilensky E, Gracias V, Itkin A, Hoffman K, Bloom S, Yang W, Christian S, et al. (2009) Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med 37(6):2057–2063. doi:10.1097/CCM.0b013e3181a009f8
Nortje J, Gupta AK (2006) The role of tissue oxygen monitoring in patients with acute brain injury. Br J Anaesth 97(1):95–106. doi:10.1093/bja/ael137
Spiotta AM, Stiefel MF, Gracias VH, Garuffe AM, Kofke WA, Maloney-Wilensky E, Troxel AB, et al. (2010) Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg 113(3):571–580. doi:10.3171/2010.1.JNS09506
Narotam PK, Morrison JF, Nathoo N (2009) Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg 111(4):672–682. doi:10.3171/2009.4.JNS081150
Meixensberger J, Renner C, Simanowski R, Schmidtke A, Dings J, Roosen K (2004) Influence of cerebral oxygenation following severe head injury on neuropsychological testing. Neurol Res 26(4):414–417
Anderson V, Catroppa C, Haritou F, Morse S, Pentland L, Rosenfeld J, Stargatt R (2001) Predictors of acute child and family outcome following traumatic brain injury in children. Pediatr Neurosurg 34(3):138–148
Ginstfeldt T, Emanuelson I (2010) An overview of attention deficits after paediatric traumatic brain injury. Brain Inj 24(10):1123–1134. doi:10.3109/02699052.2010.506853
Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N (2002) A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology 16(4):514–523. doi:10.1037//0894-4105.16.4.514
Wechsler D (1974) Wechsler intelligence scale for children—revised. Psychological Corporation, New York
Osterreith PA (1944) Filitest de copie d‘une figure complex: contribution e l‘etude de la perception et de la memoire [the test of copying a complex figure: a contribution to the study of perception and memory]. Archives de Psychology 30:286–356
Cohen MJ (1997) Children’s memory scale. The Psychological Corporation, San Antonio, TX.
Manly T, Robertson IH, Anderson V, Nimmo-Smith I (1999) Test of everyday attention for children (TEA-Ch). Harcourt Assessment, London
Korkman M, Kirk U, Kemp S (2007) NEPSY-II. Psychological Corporation, San Antonio, TX.
Achenbach TM (1991) Manual for the child behaviour checklist/4–18 and 1991 profile. Department of Psychiatry, University of Vermont, Burlington, VT
Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000) The behaviour rating inventory of executive function. Psychological Assessment Resources, Odessa, FL
Myer L, Stein DJ, Grimsrud A, Seedat S, Williams DR (2008) Social determinants of psychological distress in a nationally-representative sample of South African adults. Soc Sci Med (1982) 66(8):1828–1840. doi:10.1016/j.socscimed.2008.01.025
Ferrett HL, Carey PD, Thomas KGF, Tapert SF, Fein G (2010) Neuropsychological performance of South African treatment-naïve adolescents with alcohol dependence. Drug Alcohol Depend 110(1–2):8–14
Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF (2007) Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc 13(5):807–820
Jacobson JL, Jacobson SW (2005) Methodological issues in research on developmental exposure to neurotoxic agents. Neurotoxicol Teratol 27(3):395–406. doi:10.1016/j.ntt.2005.01.009
Max JE, Roberts MA, Koele SL, Lindgren SD, Robin DA, Arndt S, Smith WL, et al. (1999) Cognitive outcome in children and adolescents following severe traumatic brain injury: influence of psychosocial, psychiatric, and injury-related variables. J Int Neuropsychol Soc 5(1):58–68
Babikian T, Asarnow R (2009) Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 23(3):283–296. doi:10.1037/a0015268
Beauchamp M, Catroppa C, Godfrey C, Morse S, Rosenfeld JV, Anderson V (2011) Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Dev Neuropsychol 36(5):578–595. doi:10.1080/87565641.2011.555572
Catroppa C, Anderson V, Godfrey C, Rosenfeld JV (2011) Attentional skills 10 years post-paediatric traumatic brain injury (TBI). Brain Inj 25(9):858–869
Catroppa C, Anderson V (2007) Recovery in memory function, and its relationship to academic success, at 24 months following pediatric TBI. Child Neuropsychology 13(3):240–261. doi:10.1080/09297040600837362
Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME (1997) Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc 3(6):581–591
Levin HS (1995) Neurobehavioral outcome of closed head: implications for clinical trials. J Neurotrauma 12(4):601–610
Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP (2005) Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. J Int Neuropsychol Soc 11(5):645–653. doi:10.1017/S1355617705050769
Sullivan JR, Riccio CA (2010) Language functioning and deficits following pediatric traumatic brain injury. Appl Neuropsychol 17(2):93–98. doi:10.1080/09084281003708852
Van Heugten CM, Hendriksen J, Rasquin S, Dijcks B, Jaeken D, Vles JHS (2006) Long-term neuropsychological performance in a cohort of children and adolescents after severe paediatric traumatic brain injury. Brain Inj 20(9):895–903. doi:10.1080/02699050600832015
Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, Drotar D (2005) Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psychiatry 44(6):574–584. doi:10.1097/01.chi.0000159947.50523.b4
Stuss DT (2011) Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Curr Opin Neurol 24(6):584–589. doi:10.1097/WCO.0b013e32834c7eb9
Dooley JJ, Anderson V, Hemphill SA, Ohan J (2008) Aggression after paediatric traumatic brain injury: a theoretical approach. Brain Inj 22(11):836–846. doi:10.1080/02699050802425444
Cook LG, Chapman SB, Levin HS (2008) Self-regulation abilities in children with severe traumatic brain injury: a preliminary investigation of naturalistic action. NeuroRehabilitation 23(6):467–475
Schachar R, Levin HS, Max JE, Purvis K (2004) Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: do preinjury behaviour and injury severity predict outcome? Dev Neuropsychol 25:179–198
Cole WR, Gerring JP, Gray RM, Vasa RA, Salorio CF, Grados M, Christensen JR, et al. (2008) Prevalence of aggressive behaviour after severe paediatric traumatic brain injury. Brain Inj 22(12):932–939. doi:10.1080/02699050802454808
Max JE, Keatley E, Wilde EA, Bigler ED, Schachar RJ, Saunders AE, Ewing-Cobbs L, et al. (2012) Depression in children and adolescents in the first 6 months after traumatic brain injury. Int J Dev Neurosci 30(3):239–245. doi:10.1016/j.ijdevneu.2011.12.005
Chadwick O, Rutter M, Brown G, Shaffer D, Traub MU (1981) A prospective study of children with head injuries: II. Cognitive sequelae. Psychol Med 11(1):49–61
Caine D, Watson JD (2000) Neuropsychological and neuropathological sequelae of cerebral anoxia: a critical review. J Int Neuropsychol Soc 6(1):86–99
Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS (2004) Neuropsychological assessment, 4th edn. Oxford University Press, New York
Anderson VA, Catroppa C, Haritou F, Morse S, Rosenfeld JV (2005) Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J Neurol Neurosurg Psychiatry 76(3):401–408. doi:10.1136/jnnp.2003.019174
Anderson VA, Morse SA, Catroppa C, Haritou F, Rosenfeld JV (2004) Thirty month outcome from early childhood head injury: a prospective analysis of neurobehavioural recovery. Brain 127(12):2608–2620. doi:10.1093/brain/awh320
Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing-Cobbs L (2011) Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J Int Neuropsychol Soc 17(4):663–673. doi:10.1017/S1355617711000464
Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N (2004) Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc 10(3):412–426
Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM (1990) Behavioral changes after closed head injury in children. J Consult Clin Psychol 58(1):93–98
Kinsella G, Ong B, Murtagh D, Prior M, Sawyer M (1999) The role of the family for behavioral outcome in children and adolescents following traumatic brain injury. J Consult Clin Psychol 67(1):116–123
Rutter M (1982) Developmental neuropsychiatry: concepts, issues and problems. J Clin Neuropsychol 4:91–115
Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N (2002) A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology 16(1):15–27. doi:10.1037//0894-4105.16.1.15
Lannoo E, Colardyn F, De Deyne C, Vandekerckhove T, Jannes C, De Soete G (1998) Cerebral perfusion pressure and intracranial pressure in relation to neuropsychological outcome. Intensive Care Med 24(3):236–241
Holden R, Troister T (2009) Developments in self-report assessments of personality and psychopathology in adults. Can Psychol 50(3):120
Hunsley J (2009) Introduction to the special issue on developments in psychological measurement and assessment. Can Psychol 50(3):117–119. doi:10.1037/a0016686
Williamson A (2007) Using self-report measures in neurobehavioural toxicology: can they be Trusted? Neurotoxicology 28(2):227–234
Acknowledgments
The South African National Research Foundation, the University of Cape Town’s University Research Committee and the A. W. Mellon Foundation supported this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schrieff-Elson, L.E., Thomas, K.G.F., Rohlwink, U.K. et al. Low brain oxygenation and differences in neuropsychological outcomes following severe pediatric TBI. Childs Nerv Syst 31, 2257–2268 (2015). https://doi.org/10.1007/s00381-015-2892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2892-2