Abstract
Purpose
Atypical teratoid rhabdoid tumors (AT/RT) are rare, aggressive, central nervous system neoplasms that typically affect children under 3 years of age and have a very poor prognosis. Early case series consistently demonstrated rapid recurrence with progression to death, but more recent experience has shown significant improvements in progression free and overall survival.
Methods
A retrospective analysis of the clinical data of children diagnosed with AT/RT at the Ann & Robert H. Lurie Children’s Hospital of Chicago (formerly Children’s Memorial Hospital) between 2000 and 2014 was performed. Overall survival (OS) was used to describe outcome. Our small sample size and the utilization of different adjuvant regimens over the study period precluded a detailed statistical analysis.
Results
Eight children with AT/RT of the posterior fossa were included in our report. Gross total resection (GTR) was achieved in five children (63 %), two children underwent subtotal resection (25 %), and there was one who underwent biopsy. Patients were treated with various combinations of chemotherapy with or without conformal radiation therapy (RT). Median overall survival was 5 months (range 1 to 107 months) with two patients achieving sustained responses to 45 and 107 months.
Conclusions
Our experience is in line with prior reports that show that children diagnosed with AT/RT of the posterior fossa have a poor prognosis, but that long-term survival is possible. These tumors provide many challenges, but contemporary series are beginning to show improvements in survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical teratoid rhabdoid tumors are rare, aggressive, central nervous system neoplasms that typically affect children under 3 years of age [1–4]. Since first being defined in 1987 [5], distinct morphological, immunohistochemical, and cytogenetic features have been recognized. While the histiogenesis of these tumors remains undefined, the presentation and radiographic features of AT/RT are similar to other more common CNS tumors such as primitive neuroectodermal tumor (CNS-PNET) and medulloblastoma (MB).
AT/RTs are highly malignant tumors with a poor prognosis whose optimal treatment has yet to be determined. The earliest descriptions [2, 6] of the cases of AT/RT indicated that most patients developed rapid disease recurrence and death due to tumor progression with median survival in the range of 6 to 11 months [2, 6, 7]. Recent reports describing strategies including maximal safe resection coupled with various regimens of high-dose chemotherapy, intrathecal chemotherapy, and radiation therapy (RT) have begun to show improved outcomes with long-term survival now possible [8–12].
We describe our experience with a retrospectively collected series of AT/RTs of the posterior fossa and present a review of the literature to better understand the epidemiology of AT/RT, to review the role of extent of surgical resection, and to describe the evolving role of aggressive, multi-modality therapy on survival.
Methods
After authorization was obtained from the institutional review board of the Ann & Robert H. Lurie Children’s Hospital of Chicago (formerly Children’s Memorial Hospital), a retrospective review of our institution’s Falk Brain Tumor Center database was performed. We identified 19 children diagnosed with central nervous system AT/RT between June 2000 and July 2014 including 8 children with AT/RT of the posterior fossa who underwent their primary resective surgery at our institution. We then gathered demographic information and clinical data regarding initial presentation, tumor location, presence of dissemination at diagnosis as assessed by the Chang system [13], extent of surgical resection, and postoperative chemotherapy and/or radiation therapy features and survival.
Age at diagnosis, progression, and survival data are reported in months. Extent of surgical resection was defined as biopsy only, subtotal (>90 % tumor removal), or gross total resection (no detectable tumor) based on operative reports and post-operative imaging analysis. Overall survival (OS) was measured from the time of diagnosis to the date of death or the date of last contact. Kaplan–Meier analysis was applied to the collected data.
Clinical features
The clinical characteristics of the patients are shown in Table 1. There were 7 female patients and 1 male patient in the study. Age at diagnosis ranged from 1 to 43 months with a median age of 5.5 months. All of our patients, but one, were under the age of 24 months (87.5 %). The oldest patient in our series was 43 months old at the time of diagnosis.
The most common symptoms at presentation were related to increased intracranial pressure and included macrocephaly (4 patients), vomiting (2 patients), abnormal eye movements (2 patients), and abnormal posturing. Behavioral changes encountered included irritability and regression of developmental milestones. Focal neurological signs included abducens and facial nerve palsies and hearing loss.
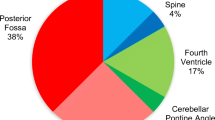
Seven patients (87.5 %) were noted to have acute hydrocephalus at the time of presentation. In two patients, the tumor was located entirely within the fourth ventricle. Two children presented with tumors that appeared to originate within the superior medullary velum and extend superiorly into the cerebellomesencephalic fissure and quadrigeminal cistern. In the two cases, the tumors arose primarily not only within the cerebellopontine angle (CPA), but also involved portions of the lateral cerebellar hemisphere. The two remaining cases had tumor in the fourth ventricle, foramen of Luschka, and the CPA. Five children (63 %) had evidence of multifocal disease at the time of presentation. Three children had multifocal intracranial disease, and one of these patients had evidence of both intracranial and spinal dissemination.
Initial maximal resection of the dominant posterior fossa tumor was attempted in all but one case. Case 1 underwent biopsy and placement of an external ventricular drain (EVD) at presentation. Case 4 was treated with biopsy and ventriculoperitoneal shunt insertion at another center 1 month prior to undergoing gross total resection at our hospital. Overall, the gross total resection was achieved in 5 patients (63 %) and subtotal resection in 2 patients (25 %).
The diagnosis of AT/RT was made based on the pathologic and immunohistochemical features of the tumors according to the World Health Organization (WHO) classification of central nervous system tumors [14]. BAF47/SMARCB1 immunohistochemistry was performed using a BAF47 mouse monoclonal antibody on seven patients. In case 1, the testing was not performed and the diagnosis was made based on histopathological features alone. The seven remaining cases all showed loss of BAF47/SMARCB1 protein expression.
Seven patients received some type of chemotherapy. Cases 1 and 2 were treated using a modified Baby-POG regimen consisting of vincristine, cisplatin, cytoxan, and methotrexate. Cases 3, 4, 5, and 8 were treated with a modified Intergroup Rhabdomyosarcoma Study—III (IRS-III) regimen consisting of vincristine, dactinomycin, cyclophosphamide, cisplatin, doxorubicin, temozolamide and intrathecal methotrexate, cytarabine, and hydrocortisone. Case 6 died from medical complications prior to starting chemotherapy. Case 7 was treated according to the ACNS0333 regimen with vincristine, methotrexate, etoposide, cyclophosphamide, and cisplatin. Three children received focal radiation therapy (RT) using intensity-modulated delivery of 180 cGy fractions as part of the IRS-III regimen. Cases 3 and 5 received a total dose of 5,400 cGy. Case 8 received a total dose of 5,220 cGy.
Median overall survival was 5 months (range 1 to 107 months; Fig. 1). Two patients achieved sustained responses and are alive at 45 and 107 months. Both were treated on the modified IRS-III regimen and received focal radiation therapy. Of the three remaining children treated on the modified IRS-III regimen, the care of case 4 was transferred to another facility at the family’s request. This patient died of sepsis during pre-irradiation induction therapy and did not receive radiation. During the post-RT maintenance chemotherapy, case 5 suffered a posterior fossa hemorrhage and died 1 month later. One child (case 6) died from gastrointestinal hemorrhage and respiratory failure 1 month after undergoing a gross total resection and did not receive any other tumor directed therapy. Of the two patients treated using a modified Baby-POG regimen, case 1 progressed 4 months after starting treatment and died of disease. Case 2 progressed 6 months after starting the same regimen and died of disease 8 months after diagnosis. The child treated under the ACNS0333 protocol died of septic complications 3 months after her surgical resection during the third cycle of her chemotherapy. None of our cases required reoperation.
Discussion
Incidence
Atypical teratoid rhabdoid tumor is a highly malignant, aggressive, central nervous system tumor that primarily affects infants and children under 3 years of age [1, 3, 15]. These tumors were first recognized in 1987 [5] then later defined [2, 16] by Rorke and colleagues. In 2000, AT/RT was included in the World Health Organization (WHO) classification of central nervous system tumors for the first time [17]. It is generally believed that prior to these initial descriptions and the identification of AT/RT as a distinct histology, tumors with histological characteristics similar to primitive neuroectodermal tumor (CNS-PNET)-medulloblastoma (MB), but with more aggressive biological features, may have been incorrectly classified as CNS-PNET/MB or choroid plexus carcinoma.
While the true incidence of AT/RT is unknown, population-based registry data provide some insight. Ostrom and colleagues [15] described the incidence and relative survival of AT/RT in the USA using population-based registry data from the Central Brain Tumor Registry of the United States (CBTUS) for newly diagnosed AT/RT. This database captured brain tumor incidence for over 98 % of the US population from 2001 to 2010. In their report, they identified 586 cases of AT/RT in children aged 0–19 years at diagnosis, accounting for 1.6 % of all brain and CNS tumors. AT/RT represented 10.6 % of all primary brain and CNS tumors in the CBTRUS database for persons less than 1 year during this time period. The median age at diagnosis was 1 year and 65.7 % of cases were <2 years old. Tumors (35.8 %) occurred supratentorially; 28.3 % were entirely infratentorial. There were 27.8 % in overlapping regions of the brain and 4.6 % were intraspinal. Overall, there was no significant difference in incidence by sex, race, or Hispanic ethnicity. From CBTRUS data, AT/RT represented 4.4 % of all CNS tumors diagnosed in children aged 0–5 years with an average annual incidence of 0.07 per 100,000 population [15].
From a nationwide Austrian population-based brain tumor registry, Woehrer [1] and colleagues found 19 cases of AT/RT between 1996 and 2006. The reported median age at diagnosis was 1.4 years and AT/RT represented 6.1 % of all CNS tumors in patients less than 7 years old. In their analysis, the incidence was higher in children under 3 years (68.4 %). Lafay-Cousin and colleagues [3] reported their analysis of the Canadian Pediatric Brain Tumor Consortium experience in a retrospective review in 2012. They identified 50 cases of AT/RT diagnosed between 1995 and 2007 that included 31 males and 19 females. The median age at diagnosis was 16.7 months (range 1 day to 187.9 months). Thirty-four percent of the tumors were diagnosed in children less than 1 year old and 24 % of the patients were older than 36 months.
Presentation
As with most central nervous system tumors, the signs and symptoms that occur in children with AT/RT at presentation are consistent with the location of the tumor and the age of the patient. Since AT/RT occurs more commonly in very young children, common presenting symptoms include macrocephaly, irritability, regression of developmental milestones, failure to thrive, and head tilt. In older children, common symptoms include headache, vomiting, and ataxia. If the diagnosis is delayed, lethargy due to hydrocephalus or brainstem compression can occur. Since many AT/RT occur in the cerebellopontine angle, cranial nerve palsies can occur.
Neuroimaging features
The neuroimaging features are nonspecific, and there is considerable overlap with those found in CNS-PNET/MB. These tumors often appear large at presentation. On computed tomography (CT), these tumors are frequently hyperdense with areas of calcification, necrosis, intense enhancement, and occasional cyst formation. With magnetic resonance imaging, these tumors appear iso- to hyperintense on T1-weighted sequences. On T2-weighted imaging, the solid portions of these tumors are usually isointense to hyperintense. Patterns of contrast enhancement vary from minimal or no enhancement to heterogenously or densely enhancing. With diffusion weighted imaging (DWI), AT/RT are hyperintense relative to normal brain signifying restricted diffusion, an indicator of high cellular density within the tumor (Figs. 2 and 3). Jin and Feng [18] suggested several features that may help differentiate AT/RT from CNS-PNET/MB. They noted that AT/RT usually grows from the cerebellum and into the adjacent space of the CPA. They also tend to occur off midline whereas CNS-PNET/MB arise more commonly in the midline. Intratumoral hemorrhage was also noted more frequently in AT/RT. They also note that it is difficult to differentiate AT/RT from CNS-PNET/MB with DWI. This paper’s senior author has previously described four cases of AT/RT that originated within the superior medullary velum [19] and presented with a mass in the fourth ventricle that extended into the cerebellomesencephalic fissure and quadrigeminal cistern (Fig. 4).
Case 8. a Preoperative unenhanced CT demonstrates mildly hyperdense tumor with intratumoral calcification. b Contrast-enhanced sagittal T1-weighted image shows heterogenous enhancement of the tumor. c Axial T2-weighted image shows isointense fourth ventricular mass. d Diffusion weighted image shows hyperintense mass indicating restricted diffusion
Case 1. a Contrast-enhanced axial T1-weighted image shows primary fourth ventricular lesion with evidence of intracranial dissemination. b Contrast-enhanced sagittal T1-weighted image shows primary fourth ventricular lesion with evidence of intracranial dissemination. c Contrast-enhanced sagittal T1-weighted lumbar spine image shows multiple enhancing nodules indicating dissemination
Case 7. a T1-weighted sagittal image showing a midline infratentorial ATRT with obstructive hydrocephalus. b Contrast-enhanced sagittal T1-weighted image shows minimal enhancement. Note that the quadrigeminal plate is flattened and displaced anteriorly and the vermis is displaced posteriorly by the tumor. c Coronal T2-weighted image demonstrating extension of the slightly hyperintense tumor extending into the cerebellomesencephalic fissure and quadrigeminal cistern. d Diffusion weighted image shows hyperintense mass indicating restricted diffusion extending into the quadrigeminal cistern
Pathological features
Histology and immunohistochemistry
Rorke and colleagues in 1996 [2] coined the term atypical teratoid rhabdoid tumor to reflect the “unusual combination of mixed cellular elements similar to but not typical of teratomas” that were characteristic of this group of tumors. AT/RTs frequently grow as solid sheets of non-cohesive tumor cells. They may display a spectrum of histopathologic features including primitive neuroectodermal, mesenchymal, epithelial, and rhabdoid components. The rhabdoid phenotype is characterized by large cells with abundant eosinophilic cytoplasm, frequent globular cytoplasmatic inclusions that correspond to aggregates of intermediate filaments, eccentrically placed nuclei with vesicular chromatin, and a prominent nucleolus. The primitive neuroectodermal component is characterized by undifferentiated small round blue cells. The mesenchymal component is usually represented by spindle cells within a basophilic background. The epithelial component, which is rarely detected, can be observed as squamous, papillary, adenomatous, or ribbon-like structures. AT/RTs are polyphenotypic tumors that express markers of divergent differentiation.
Immunohistochemical lack of expression of SMARCB1 (Fig. 5i) is the most useful marker to distinguish AT/RT from other malignant CNS tumors [20, 21]. The SMARCB1 gene is also known as hSNF5, BAF47, and INI1. The classic immune profile of AT/RT consistently shows diffuse expression of smooth muscle actin (SMA), vimentin, and epithelial membrane antigen (EMA; Fig. 5d). Consistent with their histological complexity, these markers may be associated with variable expression of other antigens such as neuron-specific enolase (NSE), protein S100 (Fig. 5f), glial fibrillary acidic protein (GFAP; Fig. 5e), and cytokeratins (Fig. 5g). Absence of expression of muscle markers such as desmin and myogenin are also a frequent feature.
Morphological and immunohistochemical aspects of posterior fossa AT/RT. a Cytology (smear preparation) shows mild to moderate anisonucleosis, eccentrically placed nuclei with occasional prominent nucleoli and eosinophilic-globoid cytoplasmic inclusion (arrow). b Areas of primitive undifferentiated component (UN) side by side with areas containing cells with rhabdoid features (RB) (hematoxylin and eosin, H&E, ×20). c Areas of rhabdoid phenotype containing rhabdoid cells with eccentric nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm with cytoplasmic inclusions. Arrow indicates atypical mitosis (hematoxylin and eosin, H&E, ×40). Immunohistochemical reactions show diffuse expression of d epithelial membrane antigen (EMA, ×40). e Glial fibrillary acidic protein (GFAP, ×40), f protein S100 (S100, ×40), g and cytokeratin CAM 5.2 (CAM 5.2, ×40). h Ki-67 immunostaining shows proliferation index (Ki-67 labeling index) of 30 % (Ki-67, ×40). i Absence of SMARCB1 nuclear staining in tumor cells. Note the retained expression in endothelial cells of intra-tumor vasculature and infiltrating tumor lymphocytes as internal positive control (SMARCB1, ×40)
AT/RTs are highly proliferative tumors. This intense proliferative activity is reflected by a high mitotic count, frequent atypical mitoses (Fig. 5c), and the high percentage of tumor cells with Ki-67 nuclear expression as detected by immunohistochemistry [22, 23] (Fig. 5h).
Documentation of loss of SMARCB1 (hSNF5/BAF47/INI1) protein expression is a highly sensitive test for the detection of alterations in the SMARCB1 gene, making it particularly useful in characterizing and diagnosing these tumors. Loss of SMARCB1 staining is noted in tumor cells but staining is retained in non-neoplastic cells like vascular endothelial cells and inflammatory cells permeating the tumors (Fig. 5i). Tumors that not only retain the expression of SMARCB1, but also exhibit features of a malignant rhabdoid tumor, should be tested for loss of SMARCA4 protein expression by immunohistochemistry [24]. Such tumors may be classified amongst a very recently described group of SMARCA4-mutated rhabdoid tumors [24].
It is important to recognize that loss of SMARCB1 protein expression in a CNS tumor is not unique to AT/RT. A growing number of tumor types with loss of SMARCB1 expression have been described [22, 25]. These tumors include chordomas [26, 27], the newly described cribriform neuroepithelial tumor (CRINET) [28–31], primitive neuroectodermal tumors (CNS-PNET) [20, 32], choroid plexus carcinomas [33], and meningiomas [34, 35]. Because AT/RT histology is fairly variable and indistinctive pathologic features can make the diagnosis challenging, the aforementioned tumors should be considered within the differential diagnoses when there is loss of SMARCB1 expression.
Genetics and biology
Despite its multiple histologic and immunistochemical phenotypic aspects, it is currently recognized that AT/RT has a “remarkably simple genome” with inactivation of the SMARCB1 tumor suppressor gene being the primary recurrent genetic event involved in rhabdoid tumor development [36, 37]. While inactivation of the SMARCB1 gene is recognized as the single critical genetic alteration in AT/RT, the mechanisms that drive AT/RT formation are still poorly understood. SMARCB1 is an ubiquitously expressed nuclear protein member of the ATPase-dependent SWI/SNF (switch and sucrose non-fermenting) chromatin remodeling complex [38]. Although its exact function in tumorigenesis is not known, it is well recognized that the SMARCB1 protein is recruited to various chromatin regions including gene promoters that regulate cell cycle, growth, and differentiation. The evolutionary highly conserved SMARCB1 gene is located on chromosome 22q11.2. SMARCB1 abnormalities in rhabdoid tumors are characterized as biallelic inactivating mutations within tumors with or without a predisposing germline mutation. It is estimated that about one third of rhabdoid tumor patients present with a germline mutation as exhibited in rhabdoid tumor predisposition syndrome (RTPS) [39–41].
Recently, a second core element of the SWI/SNF complex, the SMARCA4 (BRG1, SNF2, or BAF190) gene located at 19p13.2, was found to be inactivated in rare cases of rhabdoid tumors that retained SMARCB1 expression [42, 43].
Treatment
The optimal treatment of AT/RT of the posterior fossa has yet to be determined. As experience with these aggressive tumors continues to accumulate, the role of surgery, chemotherapy, and radiotherapy remains undefined. Aside from the aggressive biological nature of these tumors, some of the difficulty in determining the ideal management lies in the previously inconsistent diagnostic methodology and criteria and the lack of a consistent therapeutic approach. In many cases, a complete resection is not possible, and since patients with AT/RT are usually very young, there has been a reluctance to utilize radiation therapy. It has also been difficult to evaluate existing treatments due to the small size of many of the series and inconsistencies in the regimens used. Additionally, there is little data specific to AT/RTs that arise within the posterior fossa because these cases have been combined with cases of AT/RT that occur throughout the neuroaxis in all published series. In recent years, substantial progress has been made in our understanding of AT/RT, but no consensus regarding optimal therapy exists.
Surgery alone is not curative. In Rorke’s series [2], the nine children who did not receive any treatment after surgery were all dead within 1 month. A meta-analysis of 143 patients performed by Athale and colleagues [44] identified 11 patients who received no further therapy after surgery. Three children with GTR survived for 6 to 9 months and eight children with biopsy or partial resection were dead soon after surgery.
The role of surgery, and more specifically, the impact of the extent of surgical resection in AT/RT, has not been studied prospectively. Like other posterior fossa tumors, AT/RTs are amenable to resection, and tumor location, size, and patient age have important implications. Since posterior fossa AT/RTs frequently present with a component of the tumor within the CPA, involvement of cranial nerves may make complete resection less likely. Large size [18, 45, 46] is another common feature of AT/RT that can affect the surgeon’s ability to achieve complete resection, especially in very young children. Early reports suggested that total or near-total resection was possible in only one third of cases [6], but data from St. Jude Children’s Research Hospital suggested otherwise. In a series of 31 cases that included 14 posterior fossa tumors, Tekautz and colleagues [8] reported 21 patients (68 %) who underwent GTR or near total resection (NTR) and ten patients (32 %) who underwent STR. No analysis correlating extent of resection with survival was reported.
In a summary of the results of therapy of 42 children enrolled in the AT/RT registry in the USA from 2004, Hilden and colleagues [4] reported that a large majority of patients underwent surgical resection, with only two patients undergoing biopsy. Twenty patients had a GTR with an event-free survival (EFS) of 14 months (range 1.5 to 72 months) and a median survival of 20 months (range 3–76 months). At the time of the report, 10 of these patients (50 %) were free of disease 9.5 to 76 months from diagnosis. Of the 22 patients with partial resection or biopsy, the median survival was 15.25 months and the median EFS was 9.25 months with four patients remaining free of disease. In their analysis, they found that surgery was the strongest factor associated with survival and recommended an aggressive surgical approach in children with AT/RT with consideration of second look surgery, when possible, to achieve GTR.
In a report of 20 children with newly diagnosed AT/RT treated with intensive multimodality therapy, including 9 children with posterior fossa AT/RT, Chi and colleagues [12] identified 10 patients (50 %) who underwent gross total resection, including 6 (66 %) of the posterior fossa tumors. Extent of resection of all cases significantly influenced both PFS and OS. Children with GTR had a 2-year OS of 91 %. Median OS for those with less that GTR was 18 months. At the time of the report, four of the children with posterior fossa tumors were alive with no evidence of disease (NED; range 1.5 to 2.9 years) and two were alive with disease (1.5 and 2.8 years).
In Athale’s [44] meta-analysis, they identified 138 cases in which surgical details were available. Thirty-two percent of the tumors were located infratentorially. GTR was achieved in 61 (44 %), whereas 15 (10.9 %) underwent biopsy only. Children who underwent GTR had longer survival compared to those with subtotal resection or biopsy but the difference was not statistically significant (19.0 vs. 14.6 months). Nicolaides and colleagues [47] reported their experience with nine children with AT/RT including four posterior fossa cases. Six children underwent GTR including two (50 %) of the posterior fossa cases, but the small number of cases precluded any statistical analysis.
Data from the Canadian Paediatric Brain Tumor Consortium experience was reported by Lafay-Cousin and colleagues [3] in 2012. Fifty cases of AT/RT were identified across 10 centers without a description of tumor location. Fifteen patients underwent GTR (30 %), 18 had STR (36 %) and 17 had partial resection or biopsy (34 %). Patients who had a GTR had improved overall survival with a 2-year OS of 60 % compared to 21 % for those with less than a GTR (p = 0.03). As with all studies that attempt to estimate the impact of extent of resection, this type of data must be interpreted with some degree of caution due to the bias that these studies include. For example, some children reported as having had a STR may have had a large tumor that was much less amenable to resection or they may have had widespread dissemination at the time of diagnosis and the surgeon elected only to perform a limited resection.
Since the majority of AT/RTs occur in young children, there has been considerably more experience with chemotherapy than RT. Even though various chemotherapeutic regimens have been utilized and the number of patients treated with any given regimen remains small, objective responses have been achieved and chemotherapy remains the main adjuvant therapy for AT/RT. More recently, intensive regimens that utilize a combination of high-dose chemotherapy, intrathecal chemotherapy, and radiation therapy have been shown to prolong survival in some patients.
In general, the chemotherapeutic regimens that have been used have either been identical to those used in the treatment of other CNS tumors, for example the Baby POG or the Headstart regimens, or have used similar approaches to those used for parameningeal rhabdomysarcoma or have used high-dose chemotherapy with stem cell transplantation with or without the use of intrathecal (IT) chemotherapy. Reddy [48] reported on the results of 33 patients with AT/RT treated on the Pediatric Oncology Group (POG) 9923 trial. Sixty-nine percent progressed between 12 and 24 months on therapy with a median survival of 193 days. Children treated on the CCG 9921 trial were randomly assigned to receive one of two five-cycle induction chemotherapy regimens followed by a uniform regimen of maintenance chemotherapy for 56 months as a means of delaying radiation therapy in infants with brain tumors. The event-free survival for 28 children with AT/RT under 3 years was 32 % at 1 year [48] and 14 % at 2 years [49]. Early responses were seen in both studies followed by rapid disease progression and failure was predominantly local (45 %) or local and metastatic (29 %) [48]. Tekautz et al. [8] reported an 11 % 2-year EFS for 22 children under 3 years of age at diagnosis treated with various high-dose alkylator-based chemotherapy. They observed that patients under three were more likely to have disseminated disease at diagnosis and tended to develop disease progression and/or recurrence with a higher frequency and earlier than older children. In our series, cases 1 and 2 were treated with a modified Baby-POG chemotherapy regimen and were dead of disease at 5 and 8 months.
Intensive chemotherapeutic regimens that include high-dose systemic chemotherapy, intrathecal chemotherapy, and RT have shown increased survival in some series. In data from the Headstart II protocol which used multi-agent chemotherapy, including systemic methotrexate during induction, Gardner and colleagues [50] reported 3 of 6 patients with AT/RT who had no evidence of disease at 12, 34, and 46 months after diagnosis. In Headstart I, methotrexate was not used and all six AT/RT patients died of disease suggesting some role for methotrexate in the treatment of AT/RT [48].
Applying rhabdomyosarcoma-like therapy has been another approach applied to the treatment of AT/RT. In 1995, Olson et al. [51] used intensive systemic multi-agent chemotherapy augmented with triple intrathecal chemotherapy along with craniospinal irradiation in patients with disseminated CNS malignant rhabdoid tumors. Their regimen, which was based on a protocol for children with rhabdomyosarcoma with parameningeal extension (Intergroup Rhabdomyosarcoma Study—III [IRS-III, Regimen 36), achieved prolonged remission in their three cases with two long-term survivors. Zimmerman and colleagues [52] reported their experience with treating two newly diagnosed and two recurrent AT/RT patients using IRS-III based therapy with three of the four patients alive with no evidence of disease a median of 6.5 years after completion of therapy. Based on this initial data, a multi-institutional prospective phase II clinical trial was conducted to test the efficacy of an aggressive IRS-III derived approach on children with AT/RT. From the report of Chi and colleagues [12], 25 patients were enrolled and with data from 20 cases available for evaluation. Median age was 26 months (range 2.4 months to 19.5 years) and GTR was achieved in 11 patients. 14 patients were M0, one patient had M2 disease and five patients had M3 disease. Eleven patients received focal radiation and four received craniospinal radiation. Achieving a complete response was an important factor in their patient’s survival. The reported 2-year progression-free and overall survival rates of 53 and 70 % suggested that radiation therapy in conjunction with IRS-III therapy in patients less than 3 years of age may be an effective strategy in the treatment of AT/RT.
Slavc and colleagues [9] reported the Medical University of Vienna experience with AT/RT in 2013. Nine patients were correctly diagnosed with AT/RT at the time of initial diagnosis and treated using a similar strategy (cohort A). The remaining 13 patients were evaluated for SMARCB1/INI1 expression in a retrospective manner (cohort B) after undergoing treatment for their original respective diagnosis. Treatment in cohort A comprised of doxorubicin, cyclophosphamide, vincristine, ifosfamide, cisplatin, etoposide, and methotrexate followed by high-dose chemotherapy with stem cell rescue and focal radiotherapy. At the time of the report, cohort A patients had a 5-year OS of 100 % and EFS was 89.5 % with all 9 patients alive for a median of 76 months.
The efficacy of radiation therapy in children with AT/RT has been poorly studied, but there is some evidence that RT has a positive impact on survival. From the report of the AT/RT registry [4], 13 patients (31 %) underwent RT as part of their initial therapy. Nine of these children received focal RT to the tumor bed and the remaining four children received RT to the tumor bed and craniospinal axis. The median survival of 48 months in the RT group compared favorably to the median survival of 16.5 months in the entire group. In children with supratentorial tumors, three of the eight irradiated patients survived compared to five of 18 who were not irradiated. Five of the 16 patients with infratentorial tumors received RT and all survived compared to the one survivor among the 11 patients not irradiated. From the prospective study reported by Chi et al. [12], 15 of the 20 patients evaluated received RT. Of the 11 patients who received conformal RT, eight received RT before the age of 3 years and two patients (18 %) experienced relapse. Of the four children who received craniospinal RT, three (75 %) experienced a relapse at 1.8 to 2.2 years postdiagnosis.
Conclusion
Atypical teratoid rhabdoid tumors are rare, aggressive CNS tumors that primarily affect young children. The typical presentation encountered in children with AT/RT is consistent with other posterior fossa tumors, particularly CNS-PNET/MB, and many tumors are amenable to surgical resection. Documentation of loss of SMARCB1 (hSNF5/BAF47/INI1) protein expression is a highly sensitive test for the detection of alterations in the SMARCB1 gene, making it particularly useful in characterizing and diagnosing these tumors, many of which show loss of chromosome region 22q11.2.
Multiple therapeutic regimens have been utilized in the past with dismal results. RT appears to play an important role in the treatment of children with AT/RT, but in many cases its use is not an option because of the potential long-term negative impact of the radiation in very young children. The role of focal, conformal RT needs to be elucidated. There is some evidence that high-dose chemotherapy with stem cell rescue can be effective, and recent experience with high-dose chemotherapy with stem-cell rescue and intensified multimodal therapy including RT has shown improved survival. However, despite recent advances, the overall prognosis of patients with AT/RT remains poor. While cases of long-term AT/RT survivors have been described, indicating a clinical and genetic heterogeneity among these tumors, significant toxicities and side effects associated with the current treatment protocols are of great concern. Going forward, it appears that some combination of surgery, chemotherapy, and radiotherapy will be required to cure patients with this aggressive tumor, and multidisciplinary, cooperative trials are greatly needed.
References
Woehrer A, Slavc I, Waldhoer T, Heinzl H, Zielonke N, Czech T, Benesch M, Hainfellner JA, Haberler C, Austrian Brain Tumor R (2010) Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996–2006. Cancer 116:5725–5732
Rorke LB, Packer RJ, Biegel JA (1996) Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 85:56–65
Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, Jabado N, Scheinemann K, Eisenstat D, Fryer C, Fleming A, Mpofu C, Larouche V, Strother D, Bouffet E, Huang A (2012) Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer 48:353–359
Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, Walter AW, Rorke LB, Biegel JA (2004) Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol 22:2877–2884
Lefkowitz IBRLB, Packer RJ, Sutton LN, Siegel KR, Katnick RJ (1987) Atypical teratoid tumor of infancy: Definition of an entity. Ann Neurol 22:448–449
Packer RJ, Biegel JA, Blaney S, Finlay J, Geyer JR, Heideman R, Hilden J, Janss AJ, Kun L, Vezina G, Rorke LB, Smith M (2002) Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol 24:337–342
Burger PC, Yu IT, Tihan T, Friedman HS, Strother DR, Kepner JL, Duffner PK, Kun LE, Perlman EJ (1998) Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol 22:1083–1092
Tekautz TM, Fuller CE, Blaney S, Fouladi M, Broniscer A, Merchant TE, Krasin M, Dalton J, Hale G, Kun LE, Wallace D, Gilbertson RJ, Gajjar A (2005) Atypical teratoid/rhabdoid tumors (AT/RT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol 23:1491–1499
Slavc I, Chocholous M, Leiss U, Haberler C, Peyrl A, Azizi AA, Dieckmann K, Woehrer A, Peters C, Widhalm G, Dorfer C, Czech T (2014) Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992–2012. Cancer Med 3:91–100
Ginn KF, Gajjar A (2012) Atypical teratoid rhabdoid tumor: current therapy and future directions. Front Oncol 2:114
Finkelstein-Shechter T, Gassas A, Mabbott D, Huang A, Bartels U, Tabori U, Janzen L, Hawkins C, Taylor M, Bouffet E (2010) Atypical teratoid or rhabdoid tumors: improved outcome with high-dose chemotherapy. J Pediatr Hematol Oncol 32:e182–e186
Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, Rorke-Adams LB, Fisher MJ, Janss A, Mazewski C, Goldman S, Manley PE, Bowers DC, Bendel A, Rubin J, Turner CD, Marcus KJ, Goumnerova L, Ullrich NJ, Kieran MW (2009) Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 27:385–389
Chang CH, Housepian EM, Herbert C Jr (1969) An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93:1351–1359
Judkins AR, Eberhart CG, Wesseling P (2007) Atypical teratoid/rhabdoid tumor. In: Ohgaki H, Wiestler OD, Cavenee WK (eds) Louis DN. WHO Classification of Tumors of the Central Nervous System. International Agency for Research on Cancer Press, Lyon, pp 147–149
Ostrom QT, Chen Y, MdB P, Ondracek A, Farah P, Gittleman H, Wolinsky Y, Kruchko C, Cohen ML, Brat DJ, Barnholtz-Sloan JS (2014) The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001–2010. Neuro Oncol 16:1392–1399
Rorke LB, Packer R, Biegel J (1995) Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol 24:21–28
Kleihues PLDN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2000) Tumours of the Nervous System: World Health Organization Classification of Tumors. International Agency for Research on Cancer Press, Lyon
Jin B, Feng XY (2013) MRI features of atypical teratoid/rhabdoid tumors in children. Pediatr Radiol 43:1001–1008
Tomita T, Frassanito P (2013) Tumors of the superior medullary velum in infancy and childhood: report of 6 cases. J Neurosurg Pediatr 11:52–59
Haberler C, Laggner U, Slavc I, Czech T, Ambros IM, Ambros PF, Budka H, Hainfellner JA (2006) Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol 30:1462–1468
Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA (2004) Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol 28:644–650
Hollmann TJ, Hornick JL (2011) INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol 35:e47–e63
Margol AS, Judkins AR (2014) Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genet 207:358–364
Hasselblatt M, Gesk S, Oyen F, Rossi S, Viscardi E, Giangaspero F, Giannini C, Judkins AR, Fruhwald MC, Obser T, Schneppenheim R, Siebert R, Paulus W (2011) Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am J Surg Pathol 35:933–935
Agaimy A (2014) The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol 21:394–410
Yadav R, Sharma MC, Malgulwar PB, Pathak P, Sigamani E, Suri V, Sarkar C, Kumar A, Singh M, Sharma BS, Garg A, Bakhshi S, Faruq M (2014) Prognostic value of MIB-1, p53, epidermal growth factor receptor, and INI1 in childhood chordomas. Neuro Oncol 16:372–381
Mobley BC, McKenney JK, Bangs CD, Callahan K, Yeom KW, Schneppenheim R, Hayden MG, Cherry AM, Gokden M, Edwards MS, Fisher PG, Vogel H (2010) Loss of SMARCB1/INI1 expression in poorly differentiated chordomas. Acta Neuropathol 120:745–753
Arnold MA, Stallings-Archer K, Marlin E, Grondin R, Olshefski R, Biegel JA, Pierson CR (2013) Cribriform neuroepithelial tumor arising in the lateral ventricle. Pediatr Dev Pathol 16:301–307
Hasselblatt M, Oyen F, Gesk S, Kordes U, Wrede B, Bergmann M, Schmid H, Fruhwald MC, Schneppenheim R, Siebert R, Paulus W (2009) Cribriform neuroepithelial tumor (CRINET): a nonrhabdoid ventricular tumor with INI1 loss and relatively favorable prognosis. J Neuropathol Exp Neurol 68:1249–1255
Park JY, Kim E, Kim DW, Chang HW, Kim SP (2012) Cribriform neuroepithelial tumor in the third ventricle: a case report and literature review. Neuropathology 32:570–576
Ibrahim GM, Huang A, Halliday W, Dirks PB, Malkin D, Baskin B, Shago M, Hawkins C (2011) Cribriform neuroepithelial tumour: novel clinicopathological, ultrastructural and cytogenetic findings. Acta Neuropathol 122:511–514
Miller S, Ward JH, Rogers HA, Lowe J, Grundy RG (2013) Loss of INI1 protein expression defines a subgroup of aggressive central nervous system primitive neuroectodermal tumors. Brain Pathol 23:19–27
Zakrzewska M, Wojcik I, Zakrzewski K, Polis L, Grajkowska W, Roszkowski M, Augelli BJ, Liberski PP, Rieske P (2005) Mutational analysis of hSNF5/INI1 and TP53 genes in choroid plexus carcinomas. Cancer Genet Cytogenet 156:179–182
van den Munckhof P, Christiaans I, Kenter SB, Baas F, Hulsebos TJ (2012) Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 13:1–7
Christiaans I, Kenter SB, Brink HC, van Os TA, Baas F, van den Munckhof P, Kidd AM, Hulsebos TJ (2011) Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet 48:93–97
Jackson EM, Sievert AJ, Gai X, Hakonarson H, Judkins AR, Tooke L, Perin JC, Xie H, Shaikh TH, Biegel JA (2009) Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res 15:1923–1930
Lee RS, Stewart C, Carter SL, Ambrogio L, Cibulskis K, Sougnez C, Lawrence MS, Auclair D, Mora J, Golub TR, Biegel JA, Getz G, Roberts CW (2012) A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122:2983–2988
Roberts CW, Biegel JA (2009) The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther 8:412–416
Sredni ST, Tomita T (2015) Rhabdoid tumor predisposition syndrome. Pediatr Dev Pathol 18:49–58
Bourdeaut F, Lequin D, Brugieres L, Reynaud S, Dufour C, Doz F, Andre N, Stephan JL, Perel Y, Oberlin O, Orbach D, Bergeron C, Rialland X, Freneaux P, Ranchere D, Figarella-Branger D, Audry G, Puget S, Evans DG, Pinas JC, Capra V, Mosseri V, Coupier I, Gauthier-Villars M, Pierron G, Delattre O (2011) Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res 17:31–38
Eaton KW, Tooke LS, Wainwright LM, Judkins AR, Biegel JA (2011) Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56:7–15
Schneppenheim R, Fruhwald MC, Gesk S, Hasselblatt M, Jeibmann A, Kordes U, Kreuz M, Leuschner I, Martin Subero JI, Obser T, Oyen F, Vater I, Siebert R (2010) Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet 86:279–284
Fruhwald MC, Hasselblatt M, Wirth S, Kohler G, Schneppenheim R, Subero JI, Siebert R, Kordes U, Jurgens H, Vormoor J (2006) Non-linkage of familial rhabdoid tumors to SMARCB1 implies a second locus for the rhabdoid tumor predisposition syndrome. Pediatr Blood Cancer 47:273–278
Athale UH, Duckworth J, Odame I, Barr R (2009) Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol 31:651–663
Meyers SP, Khademian ZP, Biegel JA, Chuang SH, Korones DN, Zimmerman RA (2006) Primary intracranial atypical teratoid/rhabdoid tumors of infancy and childhood: MRI features and patient outcomes. AJNR Am J Neuroradiol 27:962–971
Warmuth-Metz M, Bison B, Dannemann-Stern E, Kortmann R, Rutkowski S, Pietsch T (2008) CT and MR imaging in atypical teratoid/rhabdoid tumors of the central nervous system. Neuroradiology 50:447–452
Nicolaides T, Tihan T, Horn B, Biegel J, Prados M, Banerjee A (2010) High-dose chemotherapy and autologous stem cell rescue for atypical teratoid/rhabdoid tumor of the central nervous system. J Neurooncol 98:117–123
Reddy AT (2005) Atypical teratoid/rhabdoid tumors of the central nervous system. J Neurooncol 75:309–313
Gottardo NG, Gajjar A (2008) Chemotherapy for malignant brain tumors of childhood. J Child Neurol 23:1149–1159
Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J (2008) Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer 51:235–240
Olson TA, Bayar E, Kosnik E, Hamoudi AB, Klopfenstein KJ, Pieters RS, Ruymann FB (1995) Successful treatment of disseminated central nervous system malignant rhabdoid tumor. J Pediatr Hematol Oncol 17:71–75
Zimmerman MA, Goumnerova LC, Proctor M, Scott RM, Marcus K, Pomeroy SL, Turner CD, Chi SN, Chordas C, Kieran MW (2005) Continuous remission of newly diagnosed and relapsed central nervous system atypical teratoid/rhabdoid tumor. J Neurooncol 72:77–84
Conflict of interest
The authors have no conflicts of interest to disclose.
Author’s contributions
Arthur J. DiPatri, Jr., MD
Conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critical revision of the article, and final approval of the version to be published
Simone Treiger Sredni, MD, PhD
Drafting the article, critical revision of the article, and final approval of the version to be published
Tadanori Tomita, MD
Conception and design, analysis and interpretation of data, critical revision of the article, and final approval of the version to be published
Gordan Grahovac, MD
Acquisition of data and final approval of the version to be published
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DiPatri, A.J., Sredni, S.T., Grahovac, G. et al. Atypical teratoid rhabdoid tumors of the posterior fossa in children. Childs Nerv Syst 31, 1717–1728 (2015). https://doi.org/10.1007/s00381-015-2844-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2844-x