Abstract
Purpose
The purpose of this study is to describe common indications and technique for the application of chronic invasive electrodes in the pediatric patient suffering from medically intractable epilepsy.
Methods
This chapter was prepared based on a retrospective review of the literature and personal experience based from a large tertiary epilepsy center.
Conclusions
Invasive subdural recordings are a safe and efficacious tool to identify the epileptogenic zone and its relationship to functional cortex in highly selected patients with medically refractory epilepsy. The ability to localize the EZ approaches 90 to 100 %, but seizure-free outcome is more complex depending greatly on the experience of the surgical team and the extent of resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first cortical electrical stimulation studies in humans can be traced back to a work done by Robert Bartholow in Ohio. In 1874, he had in his care a patient with a large cranial defect with exposed cerebral cortex. Needles were inserted into the dura mater and, upon closing the circuit, right arm and leg muscular contractions were observed [1, 2]. In 1893, Krause performed the first documented case of intraoperative electrical stimulation of the human cerebral cortex to determine locations of cerebral function and epileptogenic foci as a guide to cortical resection [1]. Foerster and Altenburger produced the first intraoperative electrocorticogram in 1934. By the early 1950s, direct measurement of electrical activity from the human cerebral cortex during surgery was extensively used to define the irritative zone and guide surgical resection [1]. However, the use of chronic intracranial recordings was not reported until 1939 when Wilder Penfield placed epidural electrodes in a patient with an old left temporo-parietal fracture [3]. The use of subdural grid electrodes became more popular after several publications in the 1980s and demonstrated both safety and efficacy in defining the amount of human cortex necessary to be removed to stop seizures [4–6].
Materials
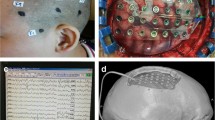
Subdural electrode grids consist of stainless steel or platinum contacts embedded in a thin matrix of biologically inert but flexible material such as Silastic® or Teflon® (Fig. 1). By design, each contact and its connecting wire are electrically isolated from the rest so as to provide precise anatomic localization of seizure foci [7, 8]. These wires extend to insulated cables that attach to an extra-cranial amplifier. The shape and size of the subdural electrodes vary from simple strips consisting of a single row with usually 4 to 11 contacts to rectangular or square arrays of 16 to 64 electrodes. The distance between electrodes is approximately 10 mm. The diameter of the electrode contacts varies between 2 and 5 mm. It is very important that the material in which the electrodes are embedded is flexible and thin permitting the array to adopt the shape of the brain it is covering while minimizing mass effect [9]. The embedding material should be clear in order to facilitate its placement over specific brain areas and also to define the relationship of the contacts with the underlying vessels and other anatomical landmarks such as sulci and gyri.
Variability in the shape and size of the electrodes permits tailoring their use to the specific clinical situation. Custom-designed arrays of subdural electrodes have been configured for placement in specific anatomical locations. For example, to record from interhemispheric brain regions, rows of electrodes arranged in curvilinear fashion were designed to follow the curvature of the corpus callosum. The plate is designed with contacts on both sides to record from the ipsilateral mesial cortex and the contralateral mesial region through the falx [7].
Indications and advantages
Epilepsy surgery is based on the principle that resection of an epileptogenic focus can result in seizure freedom. The epileptogenic zone (EZ) is defined as the area of brain necessary and sufficient to generate seizures [9]. Accordingly, accurate localization of the EZ and its relationship to eloquent cortex is crucial for the success of epilepsy surgery [10–18]. In defined and specific clinical situations, invasive electrode recordings allow for accurate localization of the EZ and mapping of functional cortical regions. This allows for a meaningful informed consent discussion with the patient and family prior to undergoing surgical resection. With application in both pediatric and adult patient populations, its use opens the door for epilepsy surgery for many patients who would otherwise not be surgical candidates.
The use of subdural grids in the pediatric population is limited by age to those more than 2 years old due to the size of the grids themselves and the relative fullness of the infant brain. At our center, subdural grid recordings are used sparingly in the pediatric adolescent population mainly to identify the relationship of eloquent cortex to the suspect EZ. This is especially true for language localization where the adolescent patient is not able to undergo awake craniotomy. Finally, the surgeon must take into account the size of the patient as two surgeries are required and blood loss can be significant enough to require transfusion in the smaller patient.
The most common indications for intracranial electrodes include lateralization or localization of an epilepsy and localization of functional/eloquent cortical information. This is especially true in pediatric patients where intraoperative localization of eloquent motor function can be difficult. In the first case, preoperative non-invasive studies and semiology often suggest a focal epilepsy, but scalp electroencephalography (EEG) is unable to adequately localize or lateralize the epileptogenic zone [15, 19]. Subdural grids have particular advantages: they can be in place long enough to record both spontaneous seizures and interictal activity during various stages of arousal and they have applicability for mapping of cerebral function extra-operatively as well as defining epileptogenic zones over wide areas [9, 20]. These characteristics allow tailored cortical resections around areas of higher function while minimizing the risk of permanent neurological deterioration [21, 22]. Intraoperative electrocorticography and functional mapping, as compared with chronically implanted subdural grids, is a limited option because it only provides information of interictal activity and is time sensitive. Additionally, it requires a cooperative patient that can tolerate awake surgery under local anesthesia for definitive mapping; this is particularly difficult in the pediatric population.
Indications for invasive video-EEG monitoring can be divided into two overlapping groups (Table 1) [13–16, 18].
Disadvantages and limitations
Invasive monitoring is inherently costly, risky, and not devoid of limitations. It requires two surgical procedures during one hospital stay, the first to implant the electrodes and the second for removal of the grids plus/minus resection of the defined epileptogenic zone. Also, in order to adequately implant the electrodes, brain exposure is more extensive, increasing the risk for complications from craniotomy. Subdural grid evaluation increases the length of stay and has the potential of causing intracranial mass effect and infection. It is therefore prudent to consider its use in each particular patient. Invasive monitoring should not be undertaken as an “exploratory procedure” (the non-invasive evaluation needs to provide an approximate location of the epileptogenic zone). Subdural grids should be used if it is believed that their use will alter the ultimate surgical strategy and outcome. An attempt must be made to place adequate electrodes so that the predicted site of seizure origin and its boundaries are sampled.
Limitation in both the area of cortex to be covered and distinct regions of cortex exists. In areas such as the interhemispheric and basal temporal and basal surfaces of the brain, the grids are placed without direct visualization. This makes precise cortical coverage difficult. The use of intraoperative stereotaxis can make this more accurate by displaying the grid’s position on the three-dimensional reconstruction of the cortical surface. The relatively common presence of bridging veins in these regions is also a limiting factor for a safe implantation of grids. Additionally, in the interhemispheric region, it is common to find adhesions between the cortex and dura that makes grid placement difficult. Finally, subdural grid coverage of the mesial temporal lobe structures is not optimal since the grids/strips have to be placed deeply to the subtemporal region without direct visualization and likely cover the parahippocampal gyrus and not the hippocampus [23].
Surgical technique
Placement of subdural grid electrodes is carried out by means of a craniotomy in a standard fashion under general anesthesia [20]. The technique is similar in both the adult and pediatric populations. Our preference is to use careful head positioning, intravenous steroids (dexamethasone), and hyperventilation in order to obtain adequate brain relaxation and sufficient subdural space for the placement of the electrodes. Before prepping, the skin incision should be marked. Localization can be performed with simple craniometric measurement in accompaniment of MRI, or frameless navigation can be used as an aid [24]. The scalp and bone flaps should be of generous proportions, exposing areas of cortex needing coverage and taking into account the requirements of any definitive surgical procedure [25]. The goal of surgery is to maximize the chance of fully documenting seizure foci and to be able to identify functionally important cortex by brain mapping techniques. Thus, the location of electrodes is a synthesis of the preoperative information and the surgical limitations on the amount of cortex that can or needs to be covered [26].
The plates are inserted with a smooth bayonet forceps, directing them towards the desired cortical region using a steady stream of irrigation allowing it to slide smoothly over the surface of the brain and preventing trauma. The grids can be “slid” beyond the edges of the craniotomy to cover adjacent areas, including basal temporal, basal frontal, and interhemispheric regions. Areas of resistance may include bridging cortical veins or adhesions that should be avoided to prevent hemorrhage. Whenever possible, the electrodes should be placed under direct visualization to prevent this complication. Occasionally, when large plates are used over the convexity, there is a tendency for them to buckle. This can be overcome by dividing the grid along lines of electrodes to give a better fit over the brain. If the grids are cut to a smaller size to fit in the region of interest, edges should be trimmed to avoid cortical laceration [27]. Care must also be taken to ensure that the edges of large grids do not compress and impede the outflow of major draining vessels as they enter the dural venous sinuses [28]. Care should be taken when implanting electrodes in patients with mass lesions, in re-operations (there is usually adhesions of the dura to the cortex), and in areas of encephalomalacia (grids may be difficult to secure). Once in place, the electrode cables are secured to the dura with suture. A digital photograph of the brain is taken; this provides a reference between gyral anatomy and electrode placement that cannot be obtained with three-dimensional reconstructed scans (Fig. 2). A watertight dural closure around the electrode cables reduces the possibility of cerebrospinal fluid leakage. Electrode leads are tunneled a minimum of 10 cm from the craniotomy margin and attached to the skin with a purse-string suture. The bone flap is replaced and secured to minimize the risk of electrode movement during seizures.
Computer software has been developed to aid the surgeon intraoperatively in the placement of the subdural grids. A preoperative stereotactic volume acquisition MRI with scalp fiducial markers in place is obtained, and the patient’s head position at the time of surgery is co-registered. The stereotactic pointing tool is then used to register the positions of as many exposed electrodes on a grid as possible, and a mathematical model of the subdural grid can be calculated and displayed as pseudocolored spheres in conjunction with the surface reconstruction. This allows the surgeon to have immediate intra-operative feedback demonstrating the anatomic position of the subdural grid [29, 30].
Postoperative management
The patient is sent to a pediatric intensive care unit for the first postoperative night. The next day, the patient is transferred to the pediatric epilepsy monitoring unit where the electrodes are connected for continuous digital EEG recording along with video imaging. Intravenous antibiotics are prescribed throughout the entire monitoring period. A cephalosporin is the agent of choice for most patients. Following removal of the subdural grids, at least one of the plates is sent for culture (even in the absence of signs of infection). In cases of positive cultures, antibiotics are continued based on these results. Steroids are administered during the first 48 h after surgery to avoid increases in intracranial pressure and allow brain compliance. In some patients, severe headache can develop following surgery requiring the use of intravenous analgesics. Postoperatively, any change in the level of consciousness or neurological function is evaluated with computed tomographic (CT) scanning. The period of implantation is variable with a range between 9 and 26 days and an average of 12 days [21].
Traditionally, the method of localizing implanted electrodes is based on a skull X-ray after implantation, and from this, an electrode map is drawn on a standard hard-copy template of the brain. In this map, electrodes involved in interictal epileptiform activity, ictal onset, and spread patterns can be marked along with the functional brain map obtained during cortical stimulation and evoked potentials (Fig. 3). The main limitation of this method is the difficulty correlating the electrode positions to the actual sulcal and gyral surface anatomy. The clinical value of subdural electrodes can be further enhanced by postimplantation CT of the brain and co-registration to the preoperative MRI [31]. This allows a more precise understanding of the anatomic relationships between the ictal onset zone, eloquent cortex, and the underlying brain anatomy and allows more accurate discussions of the proposed operative resection and its risks/benefits. For this, a stereotactic CT scan is obtained immediately after the surgery to verify grid placement and to supplement drawings or photographs made intraoperatively. The preoperative MRI is fused with the postoperative CT scan in order to obtain a surface reconstruction of the brain and the relationship of the grids with the particular anatomy of the patient [32]. Routinely, postoperative MRI is not utilized because it has been shown that the oscillating magnetic field can induce electrical currents in any metallic implants and potentially cause heating and/or electrical damage to the brain cortex [33]. Unfortunately, CT scan images often show extensive streak artifact which is especially pronounced with alloy grids, as compared with MRI imaging where the artifact is more localized. MR imaging allows for superior evaluation of the plates and possible complications; in this case, however, the risks outweigh the benefits [8].
At time of re-operation for cortical resection, the critical sites over which the electrodes lie has traditionally been marked by the surgeon by small pieces of numbered paper. The precise positioning of these markers may be difficult intraoperatively and may be displaced during the operation. This can be overcome with the use of image-guided technology displaying a representation of the electrode position based on postimplantation imaging [34].
Complications
Subdural monitoring with grids has historically been shown to have low permanent morbidity (0–3 %) compared with intracerebral electrodes (3–6 %) [35]. Adverse events caused by subdural grid implantation can be categorized as either surgical or neurological. For purposes of this chapter, emphasis is placed on the surgical and neurological complications related to the implantation of the grids separate from the resective stage. Resections performed after the monitoring period can also cause neurological complications, either transient or permanent. These sequelae can be variable depending on the amount of cortex resected and are predicted based on the information obtained during the stimulation period. Risk factors for complications related to the invasive recordings include a greater number of electrodes implanted, longer duration of monitoring, dominant side grid insertion, and earlier age at time of monitoring [36].
One of the most common complications is cerebrospinal fluid leakage. Transient cerebrospinal fluid leakage through the electrode exit site has been reported to be between 13 and 31 % of the patients despite careful watertight dural closures, adequate subcutaneous tunneling, and tight skin closure [28, 37]. This occurs most frequently after motor seizures or bouts of vomiting. Use of a lumbar subarachnoid drain has been reported to significantly reduce the incidence of CSF leak [38]. However, since no conclusive correlation has been made between the incidence of transient CSF leakage and infection, we do not support the routine use of lumbar drains. More recently, dural sealants have been available. We now routinely use these in subdural grid implantation cases to help reduce the incidence of postoperative CSF leaking around the electrode exit site. Finally, one must take into consideration that some CSF leaking may help to reduce the intracranial pressure in the first few days after surgery and actually help to avoid more serious complications.
Infectious complications are also reported in several invasive monitoring series. Postoperative wound infections after clean neurosurgical procedures have been quoted to be 0.6 to 11 %. Meningitis has been reported in 0.34 to 2 % of patients [39]. Considering these percentages, a 6 to 8 % rate of infection is not unrealistic for a procedure that leaves several foreign bodies implanted with cables exiting the scalp for seven or more days. The duration of recording following implantation is also found to correlate significantly with infection rate; duration exceeding 14 days is more likely to result in an infectious complication [37]. This can occur acutely during the monitoring period or chronically after removal of the grids. During the invasive video-EEG evaluation period wound infection, meningitis or epidural abscess can appear. Except for cases with superficial localized wound infection, the grids should be removed and aggressive intravenous antibiotic treatment initiated immediately. Subsequently, the treatment plan is modified based on culture results and clinical response. Osteomyelitis is a rare complication that has been described in 3 % of the patients; it usually is a late infection, occurring weeks or months after the initial surgery that requires removal of the bone flap and treatment with intravenous antibiotics [28]. Brain abscess has been described as a potential complication, but its incidence is very low [40]. Bacteria that have been isolated in patients with clinically relevant infections have included different species of Staphylococcus, Streptococcus, Bacillus, and Diptheroid mainly [36].
Cerebral edema and mass effect of the grids is a potentially serious complication. Factors that might favor its development are patients with multiple plates, tumoral lesions, and pediatric patients. Its precise incidence is not well defined because many times the only manifestation is headache with or without nausea that is successfully treated with analgesics and antiemetics. Severe cases in which the cerebral edema causes somnolence or stupor, focal neurological deficit, brain shift, or impending herniation are infrequent and require immediate removal of the electrodes. Large duraplasties and hinging the bone with sutures has been reported to prevent clinically significant cerebral edema in patients with risk factors. Also, some centers report to leave the bone flap out during the monitoring period to avoid this complication [41].
One of the most concerning complications with invasive monitoring is intracranial hemorrhage. Unlike intracerebral electrodes, intracerebral bleeding is a rare occurrence during placement of subdural grids. It is related more to venous occlusion or laceration of cortex caused by the edges of the plates. Subdural hematomas can be more frequently encountered with an incidence around 8 %; they can lie between the cortex and grids or superficial to the electrodes [40]. Infrequently, the subdural hematoma can cause significant mass effect with clinical symptoms or deterioration requiring removal.
As a result of the requirement for large craniotomies and multiple grid implantation, these patients have a significant risk for blood loss during surgery and may require blood transfusion. This risk is increased in the pediatric patient whose smaller blood volume makes them vulnerable to the blood loss that occurs during the two craniotomies necessary to carry out invasive recordings.
In a small number of patients, neurological deficits or neuropsychological disturbances after subdural grid placement have been observed. These can be caused by increased intracranial pressure, cerebral edema, intracranial hemorrhage, or infection (empyema or cerebral abscess). Hemiparesis, mild aphasia, Gerstmann syndrome, and visual field deficits have been described. Left-sided (dominant hemisphere) and bilateral subdural grid insertion appears to be associated with a higher rate of these neurological deficits. If present before resection, these symptoms are transient. New seizure patterns can develop due to cortical compression or contusion and if not properly identified lead to mis-localization of the epileptogenic focus; this may be related to subdural hematoma under the plates [42, 43].
Accidental removal or displacement of grids is a potential complication that can be seen more commonly in patients with severe motor seizures or during periods of postictal confusion where the exiting cables can be pulled. If there is any suspicion of displacement, an immediate skull X-ray and a CT scan should be performed in order to evaluate movement of the grids or possible hemorrhage. When the information obtained during the monitoring shows that the ictal onset zone is at the edge of a grid or is suspected to be in an area not covered by the grids, repositioning of the plates may be necessary in order to obtain adequate coverage of the area of interest. Sometimes, an invasive evaluation fails to localize seizure origin. In selected patients where invasive monitoring fails to identify the site of seizure origin, reinvestigation can achieve localization of the seizure onset and allow a successful surgical treatment [44–46]. Due to the relatively prolonged period of reduced activity in these patients, other serious complications such as deep venous thrombosis, pulmonary embolism, pneumonia, and other sequelae of immobility can develop. Appropriate diagnostic and therapeutic measures should be initiated.
Outcomes
In evaluating outcomes after the application of subdural grids, several important factors should be mentioned. It has been reported that patients selected for intracranial monitoring with subdural grids are less likely to have excellent outcomes because of their inherent complexity (non-concordant preoperative data, non-lesional MRI, or close relationship with eloquent cortex) [47–51]. This population of patients is heterogeneous, and the analysis process itself is dynamic and changes over time. Additionally, the invasive monitoring is frequently a combination of subdural grids and strips, depth electrodes, and scalp electrodes making comparison of techniques difficult [52, 53]. Finally, the success of any invasive recording operation should be measured by the localization of the EZ and not a seizure-free outcome, as perhaps the most important variable in obtaining a seizure free outcome is the surgical resection following invasive EEG identification of the epileptogenic zone. Successful localization of the EZ with the application of subdural grid recordings is achieved in the vast majority of cases, ranging from 83 to 100 % [54–57]. In a recent paper, Bulacio et al. reported that 91 % of 414 patients had the EZ successfully localized [54]. Interestingly, this paper also failed to demonstrate a difference in outcome between the lesional and non-lesional groups [54, 55]. Factors important in outcome following invasive recording include a focal onset of ictal activity within the margins of the grids and completeness of resection of ictal onset electrodes [58].
Surgical resection is a key factor in deciding seizure freedom after invasive recordings. This is highly dependent on the skill and experience of the surgical team. A consistent factor in the literature is the extent of resection with more tissue removal correlating with improved outcomes [54, 59]. The recent paper by Chung demonstrated significantly improved outcomes when the ictal onset plus early spread cortex (within 5 s) was resected as compared with the ictal onset cortex alone [59].
Despite adequate and prolonged invasive monitoring with subdural grids, up to 10 % of the patients are found not to be candidates for surgical resections. In most of these cases, the epileptogenic zone is overlapping with eloquent cortex that if resected will cause neurological deficit that is not acceptable to the patient or family. At other times, it is found that the ictal onset zone is widespread or cannot be localized thus impeding surgical treatment.
Conclusion
Invasive subdural recordings are a safe and efficacious tool to identify the epileptogenic zone and its relationship to functional cortex in highly selected patients with medically refractory epilepsy. The ability to localize the EZ approaches 90 to 100 %, but seizure-free outcome is more complex depending greatly on the experience of the surgical team and the extent of resection. For these reasons, the application of subdural grid recordings should be undertaken only at those centers experienced in the surgical treatment of epilepsy.
References
Luders JC, Luders HO (2000) Contributions of Fedor Krause and Otfrid Foester to epilepsy surgery. In: Luders H, Comair Y (eds) Epilepsy surgery. Lippincott Williams and Wilkins, Philadelphia, pp 23–33
Wolf W (2000) History of epilepsy surgery: introduction. In: Luders H, Comair Y (eds) Epilepsy surgery. Lippincott Williams and Wilkins, Philadelphia, pp 19–21
Almeida AN, Martinez V, Feindel W (2005) The first case of invasive EEG monitoring for the surgical treatment of epilepsy: historical significance and context. Epilepsia 46(7):1082–1085
Levy WJ, Hahn JH, Lueders H, Lesser R (1982) Chronic cortical electrode array for seizure investigation. Childs Brain 9:48–52
Luders H, Hahn J, Lesser RP, Dinner DS, Morris HH 3rd, Wyllie E, Friedman L, Friedman D, Skipper G (1989) Basal temporal subdural electrodes in the evaluation of patients with intractable epilepsy. Epilepsia 30:131–142
Wyllie E, Luders H, Morris HH, Lesser RP, Dinner DS, Rothner AD, Erenberg G, Cruse R, Friedman D, Hahn J, Estes ML (1988) Subdural electrodes in the evaluation for epilepsy surgery in children and adults. Neuropediatrics 19:80–86
Ebner A, Luders HO (2000) Subdural electrodes. In: Luders HO, Comair YG (eds) Epilepsy surgery. Lippincot Williams and Wilkins, Philadelphia, pp 593–596
Silberbusch MA, Rothman MI, Bergey GK, Zoarski GH, Zagardo MT (1998) Subdural grid implantation for intracranial EEG recording: CT and MRI appearance. Am J Neuroradiol 19:1089–1093
Lesser RP, Gordon B, Fisher R (1992) Subdural grid electrodes in surgery of epilepsy. In: Luders HO (ed) Epilepsy surgery. Raven, New York, pp 399–408
Carreno M, Luders HO (2000) General principles of presurgical evaluation. In: Luders H, Comair Y (eds) Epilepsy surgery. Philadelphia, Lippincott Williams and Wilkins, pp 185–199
Cukiert A, Buratini JA, Machado E, Sousa A, Vieira JO, Argentoni M, Forster C, Baldauf C (2001) Results of surgery in patients with refractory extratemporal epilepsy with normal or nonlocalizing magnetic resonance findings investigated with subdural grids. Epilepsia 42:889–894
Cukiert A, Sousa A, Machado E, Buratini JA, Vieira J, Ferreira V, Forster C, Argentoni M, Frayman L (2000) Paradigms for subdural grids’ implantation in patients with refractory epilepsy. Arq Neuropsiquiatr 58:630–636
Duchowny MS, Jayakar P (2000) Indications for invasive video electroencephalographic evaluation: special considerations in children. In: Luders HO, Comair YG (eds) Epilpesy surgery. Lippincott Williams and Wilkins, Philadelphia, pp 567–572
Engel J Jr, Wieser HG, Spencer D (1997) Overview: surgical therapy. In: Engel J Jr, Pedley TA (eds) Epilepsy a comprehensive textbook. Lippincot-Raven Publishers, Philadelphia, pp 1673–1676
Hamer HM, Morris HH (2000) Indications for invasive video-electroencephalographic monitoring. In: Luders HO, Comair YG (eds) Epilepsy surgery. Lippincot Williams and Wilkins, Philadelphia, pp 559–566
Luders HO, Awad I (1992) Conceptual considerations. In: Luders HO (ed) Epilepsy surgery. Raven, New York, pp 51–62
Uematsu S, Lesser R, Fisher R, Krauss G, Hart J, Vining EP, Freeman J, Gordon B (1990) Resection of the epileptogenic area in critical cortex with the aid of a subdural electrode grid. Stereotact Funct Neurosurg 54:34–45
Wyllie E, Luders HO, Morris HH, Lesser RP, Dinner DS, Hahn J, Estes ML, Rothner AD, Erenberg G, Cruse R, Friedman D (1987) Clinical outcome after complete or partial cortical resection for intractable epilepsy. Neurology 37:1634–1641
Najm IM, Bingaman WE, Luders HO (2002) The use of subdural grids in the management of focal malformations due to abnormal cortical development. Neurosurg Clin N Am 37:87–92
Fabinyi G (2000) Operative diagnostic methods in the treatment of epilepsy. In: Kaye AH, Black PML (eds) Operative neurosurgery. Churchill Livingston, New York
Behrens E, Zetner J, van Roost D, Hufnagel A, Elger CE, Schramm J (1994) Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir (Wien) 128:84–87
Swartz BE, Rich JR, Dwan PS, DeSalles A, Kaufman MH, Walsh GO, Delgado-Escueta AV (1996) The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol 46:87–93
Shimizu H, Suzuki I, Ohta Y, Ishijima B (1992) Mesial temporal subdural electrode as a substitute for depth electrode. Surg Neurol 38:186–191
Murphy M, O’Brien TJ, Morris K, Cook MJ (2001) Multimodality image-guided epilepsy surgery. J Clin Neurosci 8:534–538
Adelson PD, Black PM, Madsen JR, Kramer U, Rockoff MA, Riviello JJ, Helmers SL, Mikati M, Holmes GL (1995) Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr Neurosurg 22:174–180
Rosenow F, Luders H (2001) Presurgical evaluation of epilepsy. Brain 124:1683–1700
Bruce DA, Bizzi JW (2000) Surgical technique for the insertion of grids and strips for invasive monitoring in children with intractable epilepsy. Childs Nerv Syst 16:724–730
Onal C, Otsubo H, Araki T, Chitoku S, Ochi A, Weiss S, Elliott I, Snead OC 3rd, Rutka JT, Logan W (2003) Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg 98:1017–1026
Hadar EJ, LaPresto EL, Bingaman WE (2002) Image-guided epilepsy surgery. In: Germano IM (ed) Advanced techniques in image-guided brain and spine surgery. Thieme, New York, pp 156–161
Murphy MA, O’Brien TJ, Cool MJ (2002) Insertion of depth electrodes with or without subdural grids using frameless stereotactic guidance systems—technique and outcome. Br J Neurosurg 16:119–125
Winkler PA, Vollmar C, Krishnan KG, Pfluger T, Brückmann H, Noachtar S (2000) Usefulness of 3-D reconstructed images of the human cerebral cortex for localization of subdural electrodes in epilepsy surgery. Epilepsy Res 41:169–178
Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, Nair D, Bingaman W, Prayson R, Lüders H (2003) Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia 44(1):69–76
Davis LM, Spencer DD, Spencer SS, Bronen RA (1999) MRI imaging of implanted depth and subdural electrodes: is it safe. Epilepsy Res 35:95–98
Morris K, O’Brien TJ, Cook MJ, Murphy M, Bowden SC (2004) A computer-generated stereotactic Virtual Subdural Grid to guide resective epilepsy surgery. Am J Neuroradiol 25:77–83
Rydenhag B, Silander HC (2001) Complications of epilepsy surgery after 654 procedures in Sweden, September 1990–1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 49:51–57
Hamer HM, Morris HH, Mascha EJ, Karafa MT, Bingaman WE, Bej MD, Burgess RC, Dinner DS, Foldvary NR, Hahn JF, Kotagal P, Najm I, Wyllie E, Lüders HO (2002) Complications of invasive video-EEG monitoring with subdural grid electrodes. Neurology 58:97–103
Simon SL, Telfeian A, Duhaime AC (2003) Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg 38:47–52
Weinand ME, Oommen KJ (1993) Lumbar cerebral spinal fluid drainage during long-term electrocorticographic monitoring with subdural strip electrodes: elimination of cerebro spinal fluid leak. Seizure 2:133–136
Wiggins GC, Elisevich K, Smith BJ (1999) Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res 37:73–80
Lee W, Lee JK, Lee SA, Kang JK, Ko TS (2000) Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol 54:346–351
Vives KP, Lee S, McCarthy K (2003) Intracranial monitoring. In: Winn HR (ed) Youmans neurological surgery. Saunders, Philadelphia, pp 2551–2563
Fountas KN, King DW, Jenkins PD, Smith JR (2004) Nonhabitual seizures in patients with implanted subdural electrodes. Stereotact Funct Neurosurg 82:165–168
Wennberg R, Gross D, Quesney F, Gross R, Olivier A, Lozano A (1999) Transient epileptic foci associated with intracranial hemorrhage in patients with subdural and epidural electrode placement. Clin Neurophysiol 110:419–423
Awad IA, Nayel M, Luders H (1991) Second operation after the failure of previous resection of epilepsy. Neurosurgery 28:510–518
Schwartz TH, Spencer DD (2001) Strategies for reoperation after comprehensive epilepsy surgery. J Neurosurg 95:615–623
Siegel AM, Roberts DW, Thadani VM, McInerney J, Jobst BC, Williamson PD (2000) The role of intracranial electrode reevaluation in epilepsy patients after failed initial invasive monitoring. Epilepsia 41:571–580
Benbadis S, Wyllie E, Bingaman WE (2000) Intracranial electroencephalography and localization studies. In: Wyllie E (ed) The treatment of epilepsies, 3rd edn. Philadelphia, Lippincott Williams & Wilkins, pp 1067–1075
Benbadis SR (2000) Invasive EEG. In: Luders HO (ed) The epileptic seizure: pathophysiology and semiology. WB Saunders, New York, pp 49–53
Cukiert A, Buratini JA, Machado E, Sousa A, Vieira J, Forster C, Argentoni M, Baldauf C, Frayman L (2001) Seizure’s outcome after cortical resections including the face and tongue rolandic areas in patients with refractory epilepsy and normal MRI submitted to subdural grids’ implantation. Arq Neuropsiquiatr 59:717–721
Jayakar P (1999) Invasive EEG monitoring in children: when, where, and what? J Clin Neurophysiol 16:408
Lesser RP, Arroyo S (2005) Subdural electrodes. In: Niedermeyer E, Lopes da Sliva F (eds) Electroencephalography: basic principles, clinical applications, and related fields, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 777–790
Brekelmans GJ, van Emde BW, Velis DN, Lopes da Silva FH, van Rijen PC, van Veelen CW (1998) Comparison of combined versus subdural or intracerebral electrodes alone in presurgical focus localization. Epilepsia 39:1290–1301
Mihara T, Baba K (2000) Combined use of subdural and depth electrodes. In: Luders HO, Comair YG (eds) Epilepsy surgery. Lippincot Williams and Wilkins, Philadelphia, pp 613–621
Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, Najm I, Bingaman W (2012) Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia 53:1722–1730
Chapman K, Wyllie E, Najm I, Ruggieri P, Bingaman W, Lüders J, Kotagal P, Lachhwani D, Dinner D, Lüders HO (2005) Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry 76:710–713
Fountas KN, Smith JR (2007) Subdural electrode-associated complications: a 20-year experience. Stereotact Funct Neurosurg 85:264–272
Morace R, Di Gennaro G, Picardi A, Quarato PP, Sparano A, Mascia A, Meldolesi GN, Grammaldo LG, De Risi M, Esposito V (2012) Surgery after intracranial investigation with subdural electrodes in patients with drug-resistant focal epilepsy: outcome and complications. Neurosurg Rev 35:519–526
See SJ, Jehi LE, Vadera S, Bulacio J, Najm I, Bingaman W (2013) Surgical outcomes in patients with extratemporal epilepsy and subtle or normal magnetic resonance imaging findings. Neurosurgery 73:68–76
Wook KD, Kim KH, Lee SK, Chu K, Chung CK (2010) Extent of neocortical resection and surgical outcome of epilepsy: intracranial EEG analysis. Epilepsia 51:1010–1017
Matsumoto R, Nair D, LaPresto E, Najm I, Bingaman W, Shibasaki H, Lüders HO (2004) Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain 127:2316–2330
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bingaman, W.E., Bulacio, J. Placement of subdural grids in pediatric patients: technique and results. Childs Nerv Syst 30, 1897–1904 (2014). https://doi.org/10.1007/s00381-014-2534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2534-0